Research Article, Jceog Vol: 9 Issue: 4
Safe Combination of Cisplatin and Metformin Reverts the Malignant Ascites in a Mouse Model to a Solid Tumor by Downregulation of ΔNp63 and Induces Tumor Dormancy via m TOR/ p21 Mechanism
Sara Gebril*, Om-Ali Elkhawaga
Department of Chemistry and Biochemistry, Faculty of Science, Mansoura University, Mansoura, Egypt
*Corresponding Author: Sara Gebril
Department of Chemistry and Biochemistry, Faculty of Science, Mansoura University, Mansoura, Egypt
Tel: 201019219068
E-mail: sara_gebril@mans.edu.eg
Received: February 17, 2020 Accepted: July 23, 2020 Published: July 30, 2020
Citation: Gebril S, Elkhawaga O (2020) Safe Combination of Cisplatin and Metformin Reverts the Malignant Ascites in a Mouse Model to a Solid Tumor by Downregulation of ΔNp63 and Induces Tumor Dormancy via m TOR/ p21 Mechanism. J Clin Exp Oncol 9:4. doi: 10.37532/jceog.2020.9(4).246
Abstract
Currently, combination therapy has become the cornerstone of cancer treatment. The combination of different anti-cancer mechanisms can induce tumor cell quiescence. However, toxicity to normal tissue is the major limitation of existing combined drugs. In this study, Ehrlich ascites carcinoma (EAC) inoculated into mice was targeted with just one dose of cisplatin and later doses of metformin, a safe anti-diabetic drug with an anti-cancer effect, to maintain EAC cells in the quiescent state and secure a longer survival time without tumor recurrence. The group that underwent dual therapy had developed a delayed solid tumor instead of a malignant ascites. Induction of chemo-quiescence in the EAC cells was proven by downregulation of mechanistic target of rapamycin (mTOR) and upregulation of cyclin- dependent kinase inhibitor 1 (p21) expressions. Intriguingly, the conversion of free neoplastic cells into a solid tumor was associated with a significant decrease in ΔNp63 immunostaining in EAC cells. Taken together, a single dose of cisplatin followed by metformin doses could overcome the aggressiveness of malignant ascites by the conversion into a solid tumor, induction of chemo-quiescence and extension of survival time
Keywords: Chemo-quiescence; Ehrlich ascites carcinoma; mTOR; Metformin; Cisplatin; ΔNp63.
Introduction
By 2040, 27.5 million new malignancy cases are expected every year worldwide if the recent rate of cancers incidence and population development continues in the future [1]. In many types of malignancy, ascites is a prognostic sign of advanced stage, with only 11% of patients survive to more than six months. [2]. Combination of therapeutic modalities has become the cornerstone of cancer therapy [3]. Basically, the combined agents work in a synergistic or additive effect, and thus, the required therapeutic dose of each agent is low [4]. The treatment with multiple drugs increases the opportunity of targeting all cancer cells including cancer stem cells that accused of drug resistance and cancer recurrence [5]. In contrast, the treatment with a constant single compound activates alternative salvage pathways in the cancer cell which confers a subsequent drug resistance [6]. Unfortunately; the majority of existent combined chemotherapeutic drugs for cancer treatment are still limited by their toxicity to healthy cells [7]. Another challenge is that many chemotherapeutic drugs induced tumor dormancy, where cancer cells are in the quiescent state, i.e. G0. They tend to resume proliferation when the general environment becomes available, resulting in tumor relapse. To overcome these problems, there is a strategy called “locked-in”, which pharmacological agents can be used to maintain cancer cells in the quiescence (G0) state to prevent further tumor growth, recurrence, and/or metastasis throughout the lifetime of a patient [8]. The pursuit of safe and alternative chemo-quiescence adjuvants work in different anti-cancer mechanisms for combination therapy becomes necessary.
Currently, there is a tendency to using a category of pharmaceutical agents as anti-cancer drugs although they are primarily used for other therapeutic purposes [9]. Fortunately, a great benefit is associated with such an approach because existing drugs would have already undergone FDA procedures of drug safety and have identified pharmacokinetic properties [10]. Metformin, the first line drug for diabetes type 2, can acts as a safe anti-cancer agent through decreasing glucose utilization, the fuel for tumor initiation and growth [11]. Also, it suppresses mTOR activity, a major regulator of cell growth, proliferation, and survival, which is highly expressed in malignant tissue [12,13]. There are initial indications of activating cellular quiescence by metformin, the mechanism that reduces glucose uptake by malignant cells leading to cell cycle arrest [14].
Cisplatin is the alkylating agent that has been used for decades to treat many cancers such as ovary, neck, lung, testis, head, and breast cancer. However, it causes toxicity to bone marrow, hair, stomach [15]. The main dose-limiting toxicity of cisplatin is nephrotoxicity [16]. Furthermore, drug resistance has been observed in many cisplatintreated patients who have relapsed in later years after remission [17]. Correspondingly, the combination therapy of cisplatin has become the mainstream of several cancer treatments to reach the desired therapeutic effect with low toxicity and resistance possibility [18].
Combating cancer cells with serial non-toxic therapeutic agents that differ in the mechanisms of action enhances the treatment efficacy with a low chance of tumor recurrence in later years and extending the survival rate. Herein, we targeted EAC- bearing mice with one dose of cisplatin and subsequent doses of metformin, to induce EAC quiescence and improve the survival time.
Materials and Methods
Drugs and chemical reagents
Cisplatin was purchased from Mylan (10 mg/10 mL vial; Saint- Priest, France). Metformin from (Sigma-Aldrich, St. Louis, MO, USA) was dissolved in sterile water to make a solution of a concentration of 0.15 M to be used in the experiment. All other chemicals/reagents were of analytical grade.
Animal care and handling
A total of 75 female Swiss albino mice aged 6–8 weeks and weighed 18–25 g was purchased from National Research Center (Cairo, Egypt). In wire cages, mice were undergone to one-week acclimatization at identical conditions (27 ± 2 °C; 70–80 % humidity; 12-h light/darkness cycle) and supplied with standard pellet diet and water ad libitum. All performed experiments followed the guidelines for the care and use of laboratory animals approved by the University Animal Ethical Committee.
Tumor cell line
Ehrlich ascites carcinoma (EAC) is developed from a high grade of malignant mouse breast adenocarcinoma. As the mice were acclimatizing, EAC cells (1 × 106 cells) obtained from National Cancer Institute (Cairo, Egypt) were transplanted into the peritoneal cavity of a mouse to propagate. After 10 days, ascitic fluid containing EAC cells had developed and cells viability was testedfor in vivo experiments by trypan blue dye exclusion method and counted by a hemocytometer. The percentage of viable cells = [(total number of cells – number of trypan blue positive cells)/total number of cells] *100 [19].
Tumor transplantation and Experimental Design
On day zero, all mice were divided into five groups, 15 mice/ group. Four groups were inoculated with 2.5 ×1 06 EAC cells (0.2 ml PBS/mouse) and one group was left as a normal healthy group and injected intraperitoneally by 200 μL saline for 14 days. 24 hour later, the inoculated groups with Ehrlich cells were classified according to the treatment mode as following: EAC control group was tumorized mice. EAC+Cis group was given one dose of cisplatin. EAC+Met group treated with metformin for three consecutive days. EAC+Cis+Met was represented the group received the dualcombination treatment which involved one dose of cisplatin then two days interval followed by metformin doses for the next three days. All tested therapeutic agents are injected intraperitoneally. Cisplatin was injected at dose of 3.5 mg/kg while the metformin dose was 200 mg/ kg. All groups treated daily with 0.2 ml saline solution after 24 hourss of the last therapeutic dose until 14th day post tumor inoculation..
Sampling
On 15th day, six mice from each group were anesthetized, blood was collected via cardiac puncture, and then mice were killed by cervical dislocation. Peritoneal fluids were collected from the groups that had ascitic fluids for immediate measurement of tumor growth parameters (volumes of ascitic fluids and cells viability), and then EAC cells were isolated by centrifugation (2,000 rpm for 10 minutes at 4 °C) and divided for later experiments. Also, organs were preserved in 10% neutral buffer formalin for the histopathological investigation. The remaining mice in each group (n = 9 mice/ group) were left alive to estimate the mean survival time (MST) [20]. On 40th day, 3 mice from the group that had developed a solid tumor were dissected to collect solid tumors for cell cycle analysis, immunohistochemistry and histopathological examination. Total experimental period was 50 days.
QPCR analysis
On the 15th day, 4 × 106 cells were isolated from groups had ascitic fluid, washed and suspended in a cold 1ml PBS for immediate gene expression analysis. Total RNA was extracted from 4 x 106 cells using a TRIzol™ Plus RNA Purification Kit (Invitrogen, Carlsbad, CA, USA, cat. no. 12183555) according to the manufacturer’s instructions. The extracted total RNA was quantified by measuring the optical density at 260 nm using a spectrophotometer. The expression of p21, mTOR mRNA and GAPDH mRNA as a housekeeping gene was estimated according to the manufacturer’s instructions of The One-Step RT-PCR Kit (Power SYBR® Green RNA-to-CTTM 1-Step Kit, Applied Biosystems, USA). The following primer pairs were designed using online Oligoperfect Designer Software (Thermo Fisher Scientific, USA) and their specificity was checked by BLAST analysis. (p21 forward, 5’-ACGGTGGAACTTTGACTTCG-3’ and reverse, 5’-GAGTGCAAGACAGCGACAAG-3’; mTOR forward, 5’-CGTCACAATGCAGCCAACAA-3’ and reverse, 5’-TGCCTTTCACGTTCCTCTCC-3’. GAPDH forward 5’-ATGGTGAAGGTCGGTGTGAAC-3’ and reverse, 5’-TTGATGTTAGTGGGGTCTCGC-3’. The relative expression of the gene amplification product was calculated using the using 2–ΔΔCt method [21].
Cell cycle analysis by flow cytometry
2.5 × 106 EAC cells were collected from groups that had ascitic fluids on the 15th day, besides solid tumors that excised from (EAC+Cis+Met) group on 40th day were fixed in 1ml ice cold absolute alcohol and preserved at +4°C for cell cycle analysis according to the Vindeløv’s method [22]. The samples were run in a FACS Calibur system (BD, Sunnyvale, CA, USA). Data analysis was conducted using DNA analysis program MODFIT (verity software and CELLQuest software (version 3.3; Becton Dickinson).
Immunohistochemical analysis
EAC cells collected on the 15th day and Ehrlich solid tumors excised on the 40th day were fixed in formalin and paraffin embedded for Immunohistochemistry [23]. Three-micron of tissue sections were tested for the immune-histochemical detection of antigens with anti- ΔNp63 antigen (Clone 4A4, BioGenex, USA, cat.no. AM418-5M). Tissue sections visualized with Ultra vision LP detection system: HRP polymer/DAB plus Chromogen (TL-015-HD; Thermo scientific). Images were obtained with a light microscope (Binocular, Olympus Microscope; Shinjuku, Tokyo, Japan) for IHC quantification. The percentage of yellow ΔNp63 staining was evaluated with image J software (version 1.48, 32 bit).
Biochemical assays in serum and tumor homogenate
Serum samples were obtained by centrifugation at 3000 rpm/min for 20 min and stored at −20 C° for biochemical analysis. Creatinine, urea, aspartate aminotransferase (AST), alanine aminotransferase (ALT) and total proteins were estimated in serum using commercial kits (Biodiagnostic Company for Laboratory Services, Giza, Egypt). Also, the levels of glutathione (GSH) and superoxide dismutase (SOD) were measured in the collected EAC cells using assay kits (Biodiagnostic Company for Laboratory Services, Giza, Egypt).
Histopathology analysis
Cytological changes in lungs, kidneys and liver were studied by hematoxylin and eosin staining according to the method of Bancroft [24]. Organs were collected from all mice groups wither those were killed on the 15th or 40th day washed in PBS and to remove the blood. Tissues were then fixed in 10% neutral buffered formalin for 24 hours blocks of tissues were prepared, sectioned, and stained with hematoxylin–eosin then examined with a light microscope (Binocular, Olympus Microscope; Shinjuku, Tokyo, Japan).
Statistical analysis
All data are expressed as mean ± SD. One-way analysis of variance (ANOVA) followed by a Tukey’s test was used to assess significant differences among all groups using GraphPad Prism Software 5 (LaJolla, CA, USA). P values were considered statistically significant at P < 0.05.
Result
Metformin enhances the anti-tumor potential of one dose of Cisplatin on EAC cells
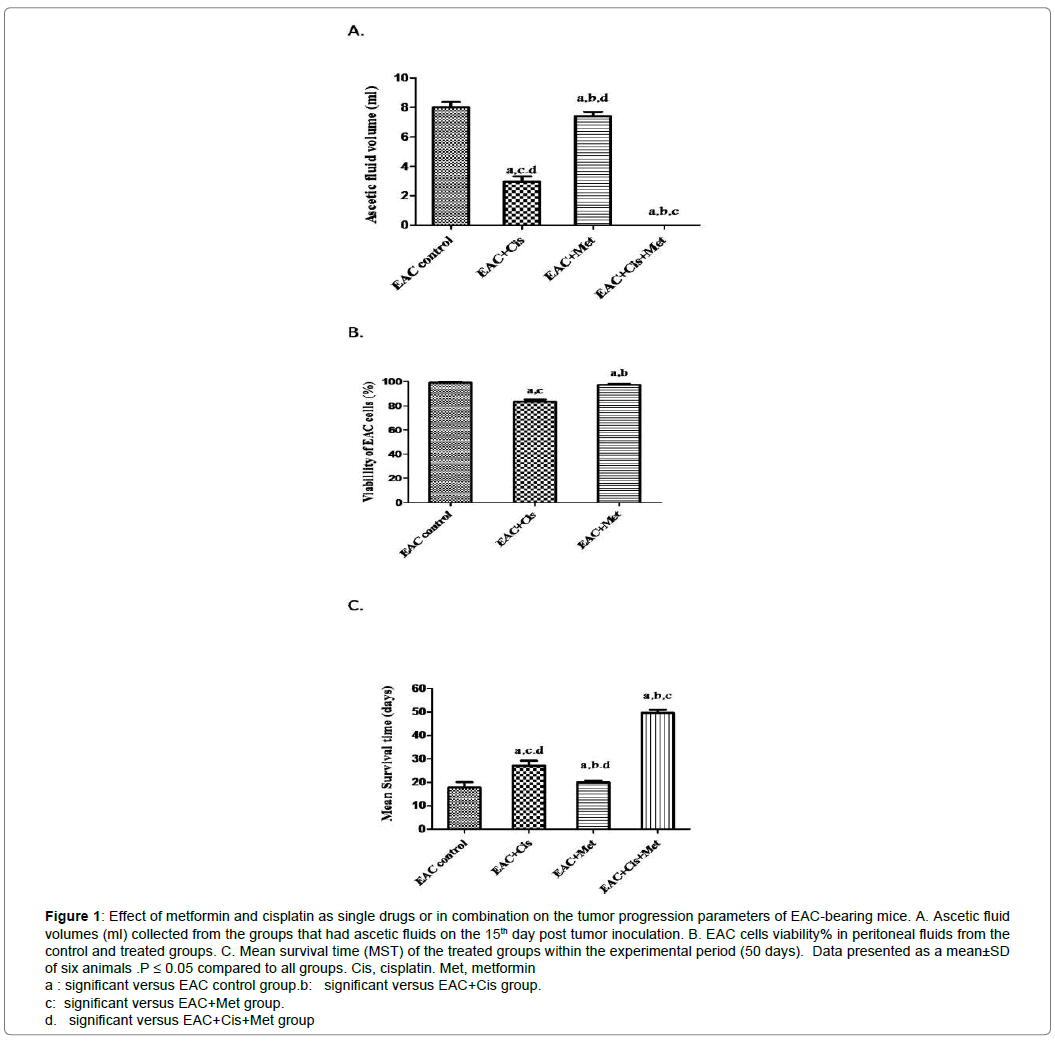
To assess the anti-tumor effects of metformin and cisplatin against EAC cells, changes in tumor volume, tumor cells viability and mean survival time of treated groups were observed Figure 1. EAC-bearing mice treated with the combination therapy showed no ascitic fluid volume on the day 15 post tumor inoculation compared to the other groups Figure 1A. There was a delayed tumor recurrence on day 25 as a solid tumor instead of developing peritoneal fluid containing tumor cells. Regarding cells viability, cisplatin and metformin displayed a significant decrease in EAC cells (P < 0.05) reached to 83.39 ± 1.64% and 97.16 ± 1.15% respectively compared to the EAC control group Figure1B. Subsequently, the treatment with the combination treatment showed a significant increase in MST to 50 days compared to the control and other treated groups.
Figure 1: Effect of metformin and cisplatin as single drugs or in combination on the tumor progression parameters of EAC-bearing mice. A. Ascetic fluid volumes (ml) collected from the groups that had ascetic fluids on the 15th day post tumor inoculation. B. EAC cells viability% in peritoneal fluids from the control and treated groups. C. Mean survival time (MST) of the treated groups within the experimental period (50 days). Data presented as a mean±SD of six animals .P ≤ 0.05 compared to all groups. Cis, cisplatin. Met, metformin
a : significant versus EAC control group.b: significant versus EAC+Cis group.
c: significant versus EAC+Met group.
d. significant versus EAC+Cis+Met group
Effect of metformin and cisplatin on relative expression of mTOR and P21 genes in EAC cells
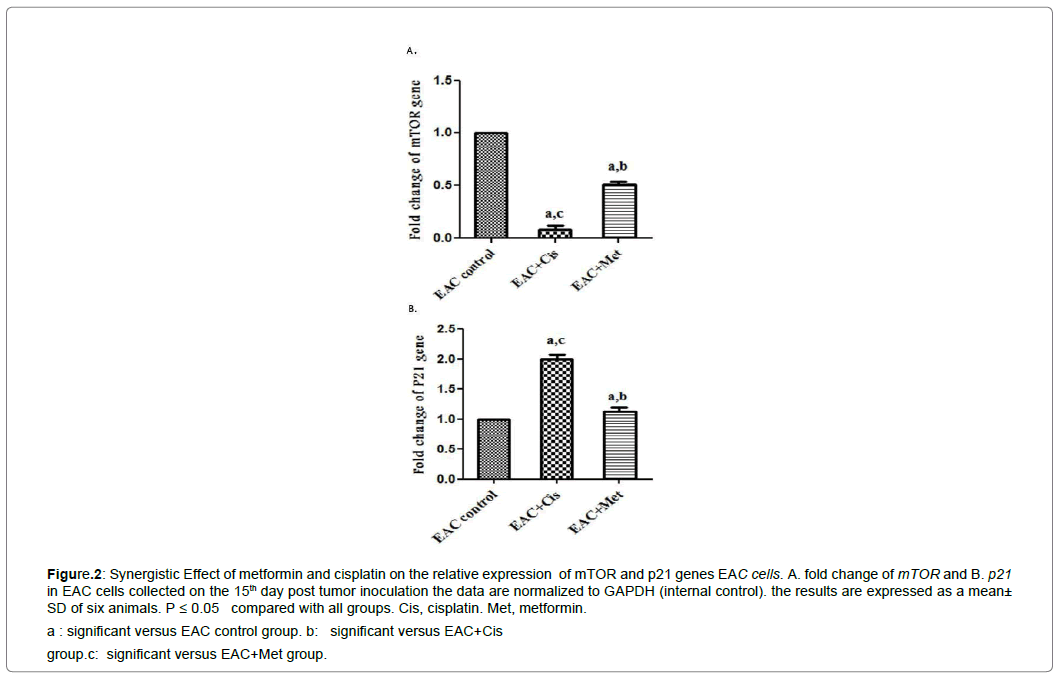
There was a reversal relationship between p21 and mTOR expressions after treatment with cisplatin or metformin. Cisplatin that was injected as one dose and metformin caused a long-term significant increase of p21 expression and a significant decrease in mTOR expression compared to EAC control group Figure 2.
Figure 2: Synergistic Effect of metformin and cisplatin on the relative expression of mTOR and p21 genes EAC cells. A. fold change of mTOR and B. p21 in EAC cells collected on the 15th day post tumor inoculation the data are normalized to GAPDH (internal control). the results are expressed as a mean ± SD of six animals. P ≤ 0.05 compared with all groups. Cis, cisplatin. Met, metformin.
a : significant versus EAC control group. b: significant versus EAC+Cis
group.c: significant versus EAC+Met group.
Metformin and Cisplatin induce cell cycle arrest in G0-G1 phase
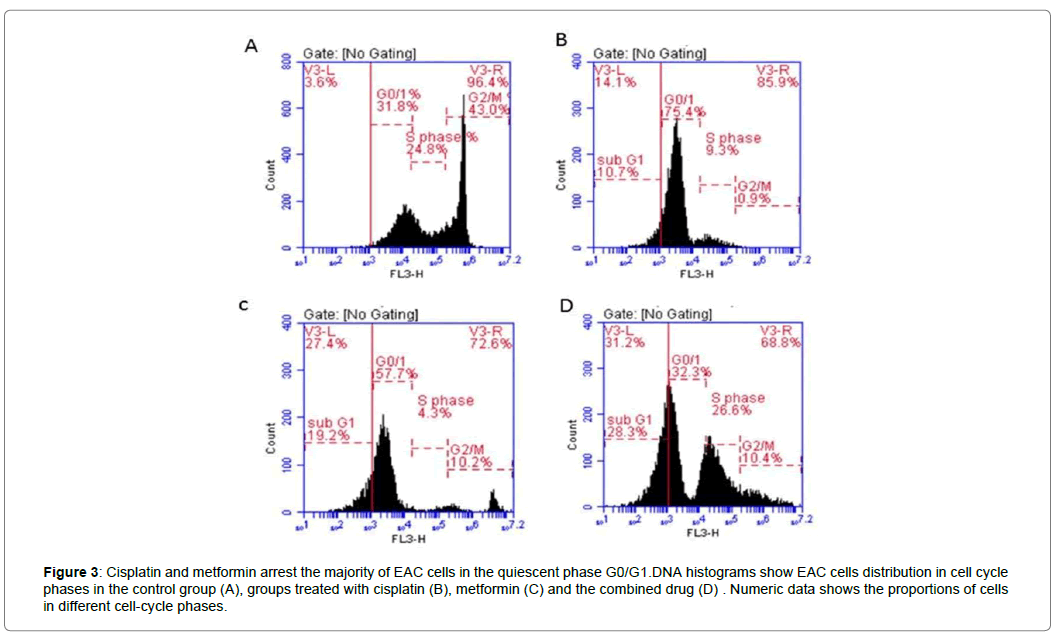
The used treatments significantly increased arrest in the G0/G1 phase compared to EAC control group. Cisplatin arrested 75.4% of EAC cells in G0/G1, while metformin arrested 57.7% of EAC cells and 19.2% of cells were apoptotic cells Figure 3A, B, C. After 40 days of tumor inoculation, the analysis of cell cycle of the solid tumor showed just 32.2% of tumor cells remained in the quiescent state, with 37.2% proliferating cells and highest percentage of apoptotic cells among the other treated groups reached to 28.3% Figure 3D.
Figure 3: Cisplatin and metformin arrest the majority of EAC cells in the quiescent phase G0/G1.DNA histograms show EAC cells distribution in cell cycle phases in the control group (A), groups treated with cisplatin (B), metformin (C) and the combined drug (D) . Numeric data shows the proportions of cells in different cell-cycle phases.
Inhibitory effect of metformin and/or Cisplatin on ΔNp63 level
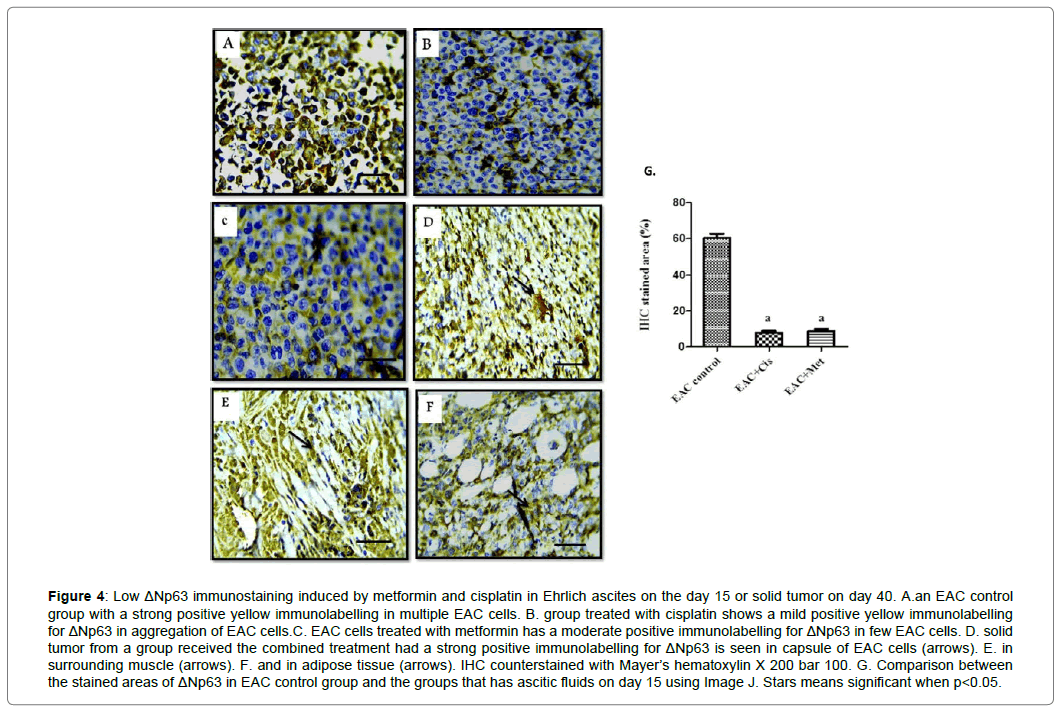
In Figure 4, immunohistostaining analysis of ΔNp63 in EAC cells showed a strong positive yellow immunolabelling for ΔNp63 in multiple EAC control cells Figure 4A. Mild was seen in cisplatintreated cells and moderate in few EAC cells treated with metformin Figure 4B and C. However, after 40 days of tumor inoculation, Cis+Met group showed a strong positive immunolabelling for ΔNp63 in the capsule of the solid tumor Figure 4D, surrounding muscle Figure 4E and adipose tissue Figure 4F.
Figure 4: Low ΔNp63 immunostaining induced by metformin and cisplatin in Ehrlich ascites on the day 15 or solid tumor on day 40. A.an EAC control group with a strong positive yellow immunolabelling in multiple EAC cells. B. group treated with cisplatin shows a mild positive yellow immunolabelling for ΔNp63 in aggregation of EAC cells.C. EAC cells treated with metformin has a moderate positive immunolabelling for ΔNp63 in few EAC cells. D. solid tumor from a group received the combined treatment had a strong positive immunolabelling for ΔNp63 is seen in capsule of EAC cells (arrows). E. in surrounding muscle (arrows). F. and in adipose tissue (arrows). IHC counterstained with Mayer’s hematoxylin X 200 bar 100. G. Comparison between the stained areas of ΔNp63 in EAC control group and the groups that has ascitic fluids on day 15 using Image J. Stars means significant when p<0.05.
Metformin and cisplatin effect on antioxidant enzymes In EAC cells
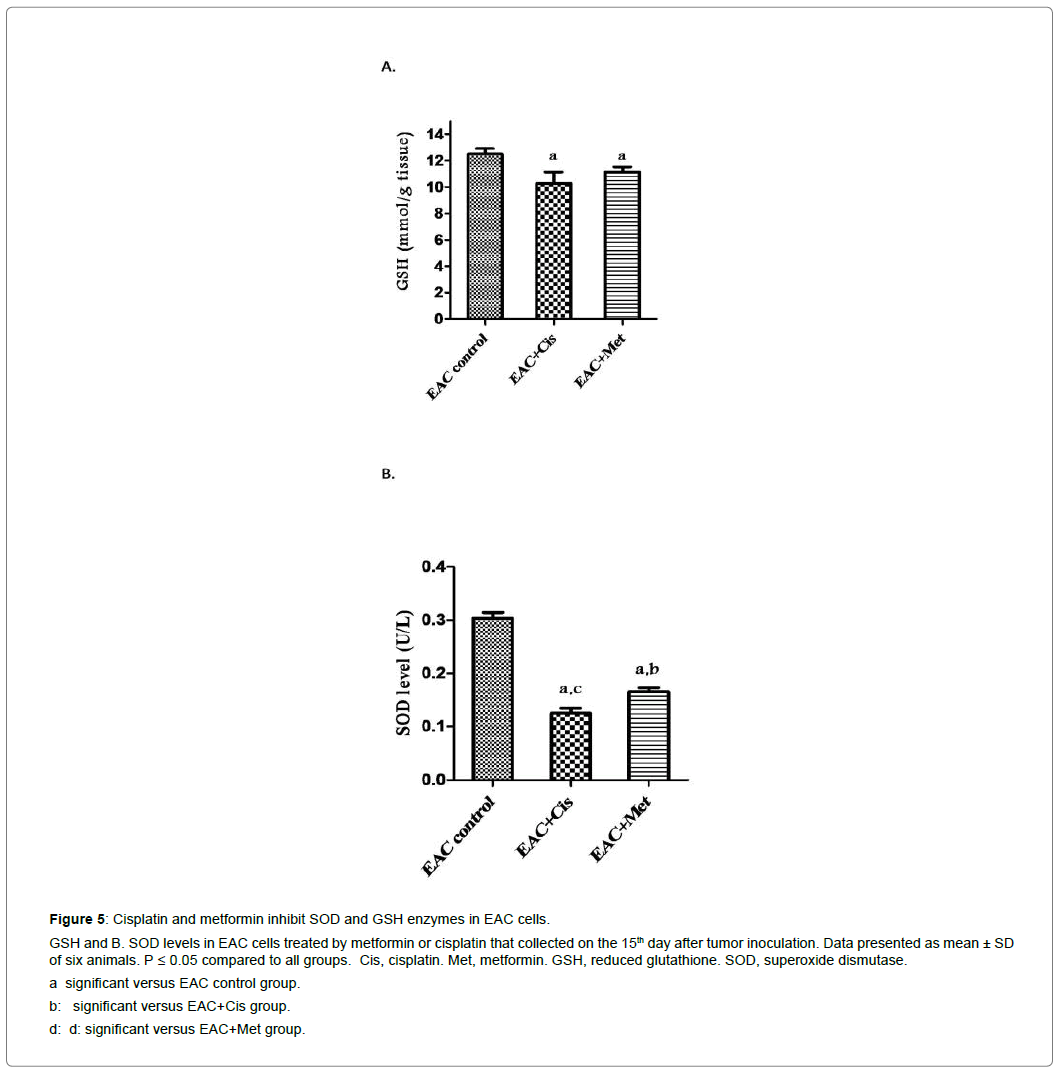
Cisplatin and metformin as single drugs inhibited SOD and GSH levels significantly (P<0.05) in the EAC cells compared to that of EAC control group Figure 5. The effect of cisplatin and metformin as a combination therapy would be higher.
Figure 5: Cisplatin and metformin inhibit SOD and GSH enzymes in EAC cells.
GSH and B. SOD levels in EAC cells treated by metformin or cisplatin that collected on the 15th day after tumor inoculation. Data presented as mean ± SD of six animals. P ≤ 0.05 compared to all groups. Cis, cisplatin. Met, metformin. GSH, reduced glutathione. SOD, superoxide dismutase.
a significant versus EAC control group.
b: significant versus EAC+Cis group.
d: d: significant versus EAC+Met group.
Assessment of kidney and liver functions after treatment with metformin and cisplatin.
Data presented in Table 1 demonstrated that serum of Cis+Met group showed most significant changes in the tested serum parameters compared to other groups. There was an elevation in ALT, AST and creatinine concentrations and a decrease in total proteins and urea (P<0.05) compared to other groups. In general, serum of EACbearing mice showed a significant change compared to the normal mice (p<0.05).
| EAC+Cis+Met | EAC+Met | EAC+Cis | EAC control | Normal | |
|---|---|---|---|---|---|
| 2.79 ± 0.15a,b,c | 2.1 ± 0.07a,b,d | 2.48 ± 0.26a,c,d | 1.53 ± 0.09# | 0.54 ± 0.06 | Creatinine |
| (g/dl) | |||||
| 56.53 ± 0.85 a,b,c | 63.06 ± 0.14b,a,d | 66.65 ± 0.80a,c,d | 62.43 ± 0.56# | 43.76 ± 2.21 | Urea(mg/dl) |
| 3.75 ± 0.12 a,b,c | 4.66 ± 0.07a,b,d | 5.00 ± 0.54a,c,d | 4.18 ± 0.39# | 6.08 ± 0.16 | Total protein (g/dl) |
| 81.58 ± 0.38 a,b,c | 71.00 ± 0.62 a,b,d | 68.27 ± 0.46a,c,d | 76.66 ± 0.46# | 27.44 ± 0.9 | ALT(U/L) |
| 141.30 ± 0.96a,b,c | 130.00 ± 1.73a,b,d | 125.22 ± 1.05a,c.d | 138.7 ± 0.57# | 93.85 ± 0.52 | AST(U/L) |
Table 1: the effect of cisplatin and metformin as single drugs or in combination on the level creatinine, urea, ALT, AST, and total proteins in serum of the different groups.
Histopathology findings
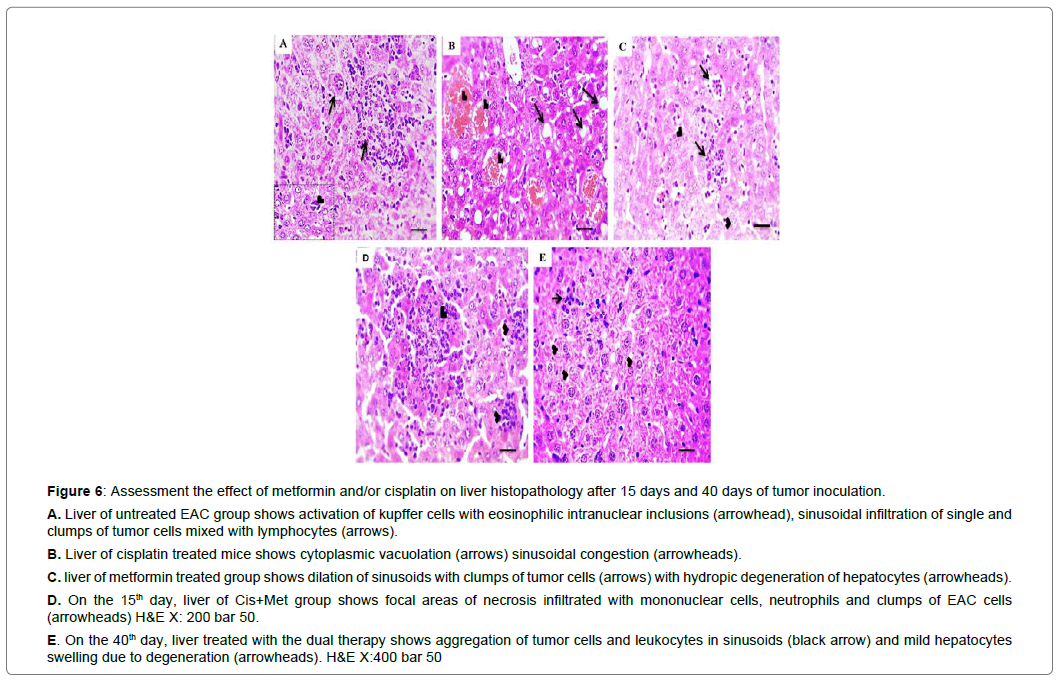
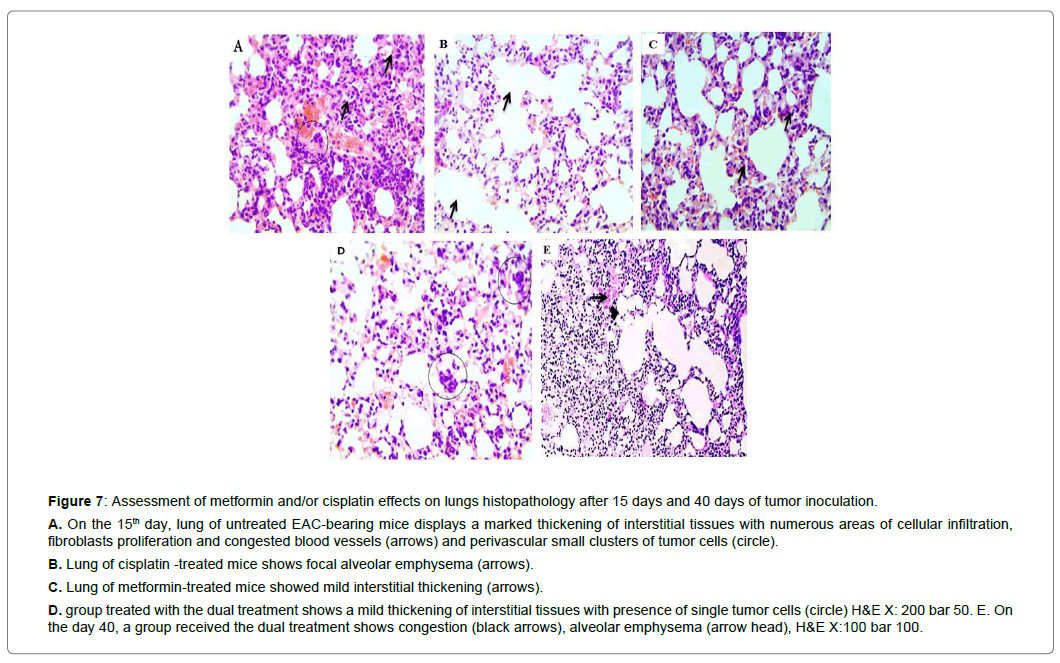
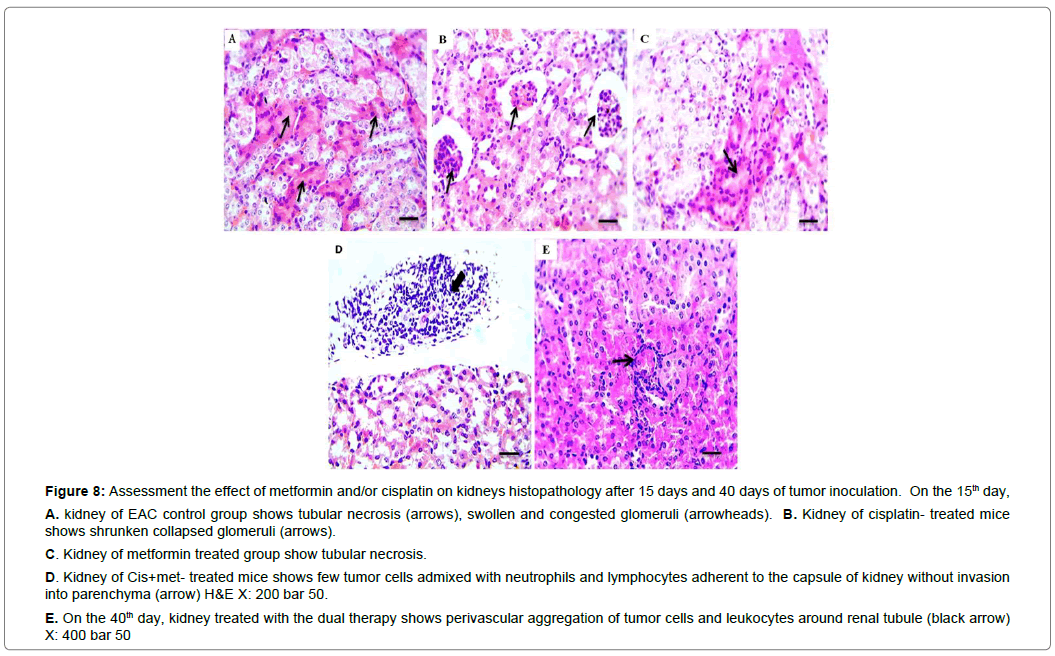
In our histopathological results, as the cytotoxicity of drugs increases, the number of infiltrative tumor cells increase. Cis+Met treated group showed a high EAC metastasis compared to a single drug-treated groups in liver and lungs and kidney of the treated group Figure 6, 7, 8. The other treated groups showed no tumor infiltration. In general, the number of infiltrative tumor cells and damaged tissue structure had not been increased on the 40th day after tumor inoculation.
Figure 6: Assessment the effect of metformin and/or cisplatin on liver histopathology after 15 days and 40 days of tumor inoculation.
A. Liver of untreated EAC group shows activation of kupffer cells with eosinophilic intranuclear inclusions (arrowhead), sinusoidal infiltration of single and clumps of tumor cells mixed with lymphocytes (arrows).
B. Liver of cisplatin treated mice shows cytoplasmic vacuolation (arrows) sinusoidal congestion (arrowheads).
C. liver of metformin treated group shows dilation of sinusoids with clumps of tumor cells (arrows) with hydropic degeneration of hepatocytes (arrowheads).
D. On the 15th day, liver of Cis+Met group shows focal areas of necrosis infiltrated with mononuclear cells, neutrophils and clumps of EAC cells (arrowheads) H&E X: 200 bar 50.
E. On the 40th day, liver treated with the dual therapy shows aggregation of tumor cells and leukocytes in sinusoids (black arrow) and mild hepatocytes swelling due to degeneration (arrowheads). H&E X:400 bar 50
Figure 7: Assessment of metformin and/or cisplatin effects on lungs histopathology after 15 days and 40 days of tumor inoculation.
A. On the 15th day, lung of untreated EAC-bearing mice displays a marked thickening of interstitial tissues with numerous areas of cellular infiltration, fibroblasts proliferation and congested blood vessels (arrows) and perivascular small clusters of tumor cells (circle).
B. Lung of cisplatin -treated mice shows focal alveolar emphysema (arrows).
C. Lung of metformin-treated mice showed mild interstitial thickening (arrows).
D. group treated with the dual treatment shows a mild thickening of interstitial tissues with presence of single tumor cells (circle) H&E X: 200 bar 50. E. On the day 40, a group received the dual treatment shows congestion (black arrows), alveolar emphysema (arrow head), H&E X:100 bar 100.
Figure 8: Assessment the effect of metformin and/or cisplatin on kidneys histopathology after 15 days and 40 days of tumor inoculation. On the 15th day,
A. kidney of EAC control group shows tubular necrosis (arrows), swollen and congested glomeruli (arrowheads). B. Kidney of cisplatin- treated mice shows shrunken collapsed glomeruli (arrows).
C. Kidney of metformin treated group show tubular necrosis.
D. Kidney of Cis+met- treated mice shows few tumor cells admixed with neutrophils and lymphocytes adherent to the capsule of kidney without invasion into parenchyma (arrow) H&E X: 200 bar 50.
E. On the 40th day, kidney treated with the dual therapy shows perivascular aggregation of tumor cells and leukocytes around renal tubule (black arrow)
X: 400 bar 50
Discussion
Malignant ascites develops in last stage of several cancers as a sign of the treatment failure to control cancer progression [25]. Currently, combination therapy is a solution to overcome drug resistance and enhance treatment efficacy [26, 27]. However, the damage to healthy tissue and drug resistance associated by multiple doses of the existing anti-cancer drugs averts the combination therapy benefit [28]. Combining an effective one dose of the anti-neoplastic drug with safe agents that act in different anti-cancer mechanisms may provide the optimum treatment. In our study, just one dose of cisplatin followed by metformin doses could reverse the malignancy of free neoplastic cells in peritoneal ascites fluid to a solid neoplasm leading to extended survival time. Ascitic fluid is the direct source of nutrients for tumor cells and as the tumor cells proliferate rapidly, the volume of ascites fluid increases at the same levels [29]. In the present study, the volume of ascitic fluid reflected the low proliferation rate and viability of cells treated with metformin and cisplatin. The therapeutic potential of metformin and cisplatin in combination was observed in human ovarian cancer cells [30] and hepatocarcinoma cells [31].
Cell quiescence is a reversible G0 phase from which cells may back to the cell cycle under physiological stimuli[32]. The quiescent cancer stem cells protect cancer cells from anti-proliferating agents. Awakening of these dormant cancer cells leads to tumor recurrence which may occur after long periods [33]. It is generally believed that mTOR inhibition maintains quiescence and suppresses senescence (geroconversion). High level of p53 response inhibits mTOR, which favors quiescence over senescence [34]. p21 is a downstream target gene of the tumor suppressor p53 [35]. It promotes quiescent state by blocking G1 progression under serum stimulation [36]. Our RTPCR results revealed that one dose cisplatin alone caused a longterm inhibition of the mTOR expression in malignant ascites cells. In addition, the results demonstrated that cisplatin increases the expression of p21, the matter that confirmed by previous publications [37,38]. Metformin acts as anticancer by its downregulation of mTOR and activation of AMPK [39].
In the present study metformin upregulated p21 expression. This is in agreement with Cai, who observed the upregulation of p53, p27 and p21 in a xenograft model of esophageal squamous cells carcinoma treated with metformin [40]. Thus, the cell cycle arrest at G0/G1 induced by metformin and cisplatin in combination was through inhibition of mTOR and activation of p21. Also, the results provided an explanation for the reason of delayed tumor development by the combined treatment.
ΔNp63 isoforms are a class of p63, which bind to TAp63, p53, p21, and TAp73 and repress their functions, thus acting as oncogenes [41, 42]. Recently, it has been reported that ΔNp63 expression can deregulate tumor cell migration and tumor invasiveness [43,44]. In the present study, the development of solid tumors instead of ascitic fluids confirmed the combined effect of cisplatin and metformin to inhibit ΔNp63, leading to restriction of cancer progression. This is similar to the results of Yun-Feng He et al., that revealed that the ΔNp63 silencing can promote the adhesiveness of the human bladder carcinoma cell line 5637 cells by activating F-actin cytoskeleton synthesis [45].Cancer cells are known for their high level of reactive oxygen species (ROS) which play a pivotal role in cancer progression [46]. However, excessive ROS level can be toxic to cancer cells, the reason behind many trails for developing many ROS generating agents and antioxidant inhibitors [47] .Herein, EAC cells treated with cisplatin had low SOD and GSH levels.
This agreed with previous studies [48,49]. Also, metformin depleted GSH and SOD levels. In accordance with this result, it was found that metformin acts as a pro-oxidant via downregulation of intracellular glutathione, inhibition of proliferation and induction of apoptosis of esophageal squamous cancer cells [50]. Therefore, the combined effect of both drugs caused further accumulation of ROS leading to kill EAC cells.
Previously, it has shown that EAC cells are metastasized to liver, kidney, lungs, spleen, diaphragm, bone, blood and adrenal glands [51]. In this work, metastasis increased among the treated groups as the cytotoxicity of the drug increased EAC cells invasion to liver, kidney, and lungs were marked with the combined therapy treated group. This can be attributed to chemotherapy increasing the invasion of cancer cells [52, 53]. Herein, as the Ehrlich cells metastasized to the surrounding tissues, they lost their malignant capacity. The histopathology results did not show increasing numbers of EAC cells after 40 days post tumor inoculation. Also, it has observed that most EAC cells infiltrating into the liver may die or may become “dormant in the liver [53].
Although cisplatin is known for its nephrotoxicity, which is doselimiting toxicity. In the present investigation, there were no acute toxicity or histopathological complications in the lungs, liver, and kidney even after 40 days. On other words, the tissue damages were reversible and associated with significant changes in biochemical parameters in serum of the dual therapy. These findings supported that the tested combination treatment in the aforementioned doses is safe.
In summary, the value of this work was the restriction of a malignant ascites in a solid tumor in EAC xenografts by inhibition of p63 level, induction of chemo-quiescence by inhibition of mTOR and upregulation of p21expression besides accumulation of ROS by inhibition of antioxidant system. These effects achieved by non-toxic and existing drugs in combination involved one dose of cisplatin and subsequent doses of metformin results in extending survival time.
Conclusion
SLICER score was not validated in our series since it underestimates the RFS. In order to be applied in daily practice, it must be tested and validated on a larger cohort of patients with different demographic and clinical characteristics and for a longer period of time.
References
- International Agency for Research on Cancer (2018), GLOBOCAN 2018 accessed via Global Cancer Observatory. Accessed September.
- Parsons SL, Lang MW, Steele RJ (1996) malignant ascites: a 2-year review from a teaching hospital. Eur. J. Surg. Oncol, 22: 237-239.
- Yap TA. Omlin A, de Bono JS (2013) Development of therapeutic combination targeting major cancer signaling pathways. J of Cli Onc 31: 1592-1605.
- Albain KS, Nag SM, Calderillo-Ruiz G, Jordaan JP, Llombart AC, et al. (2008) Gemcitabine plus paclitaxel versus paclitaxel monotherapy in patients with metastatic breast cancer and prior anthracycline treatment. J of Clini Onc. 26: 3950-3957.
- Wang T, Narayanaswamy R, Ren H, Torchilin VP (2016) Combination therapy targeting both cancer stem-like cells and bulk tumor cells for improved efficacy of breast cancer treatment. Cancer biology & therapy 17: 698-707.
- Gottesman MM, Fojo T, Susan E (2002) Multidrug resistance in cancer: role of ATP–dependent transporters. Nature Reviews Cancer 2: 48-58.
- Liboiron BD, Mayer LD (2014) Nanoscale particulate systems for multidrug delivery: towards improved combination chemotherapy. Therapeutic delivery 5: 149-171.
- Chen W, Dong J, Haiech J, Kilhoffer MC, Zeniou M, et al. (2016) Cancer stem cell quiescence and plasticity as major challenges in cancer therapy. Stem cells international 2016: 1-16.
- Chong CR, Sullivan Jr DJ (2007) New uses for old drugs. Nature 448: 645-646.
- Ashburn TT, Thor KB (2004) Drug repositioning: identifying and developing new uses for existing drugs. Nature reviews Drug discovery 3: 673-683.
- Gong J, Kelekar G, James S, Shen J, Kaur S, et al. (2016) The expanding role of metformin in cancer: an update on antitumor mechanisms and clinical development. Targeted oncology 11: 447-467.
- Paquette M, El-Houjeiri L, Pause A (2018) mTOR pathways in cancer and autophagy. Cancers 10: 18.
- Cristian M (2016) PI3K inhibitors as new cancer therapeutics: implications for clinical trial design. OncoTargets and therapy 7: 203-210.
- Onodera Y, Nam JM, Bissell MJ (2014) Increased sugar uptake promotes oncogenesis via EPAC/RAP1 and O-GlcNAc pathways. The Journal of clinical investigation 124: 367-384.
- Hartmann JT, Lipp HP (2003) Toxicity of platinum compounds. Expert opinion on pharmacotherapy 4: 889-901.
- Sastry J, Kellie SJ (2005) Severe neurotoxicity, ototoxicity and nephrotoxicity following high-dose cisplatin and amifostine. Pediatric hematology and oncology 22: 441-445.
- Gottesman MM, Fojo T, Bates SE (2002) Multidrug resistance in cancer: role of ATP–dependent transporters. Nature Reviews Cancer 2: 48-58.
- Miller RP, Tadagavadi RK, Ramesh G, Reeves WB (2010) Mechanisms of cisplatin nephrotoxicity. Toxins 2: 2490-2518.
- Dasari S, Tchounwou PB (2014) Cisplatin in cancer therapy: molecular mechanisms of action. European journal of pharmacology 740: 364-378.
- Agrawal SS, Saraswati S, Mathur R, Pandey M(2011) Cytotoxic and antitumor effects of brucine on ehrlich ascites tumor and human cancer cell line. Life sciences 89: 147-158.
- Kenneth JL, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 25: 402-408.
- Vindeløv LL (1997) Flow microfluorometric analysis of nuclear DNA in cells from solid tumors and cell suspensions. Virchows Archiv B Cell Pathol. 24: 227-242.
- Jakob S (2008) Detection of apoptosis in vivo using antibodies against caspase-induced neo-epitopes. Methods 44: 255-261.
- Bancnoft JD, Stevens A, Turner DR (1996) Theory and practice of histological technique. 3rEd. Edinburgh London, Melbourne and New York.
- Garrison RN, Lawrence DK, Louis HS, Rebecca HG (1986) Malignant ascites clinical and experimental observations. Annals of surgery 203: 644-651.
- Jack H, Aryal S, Zhang L (2010) Nanoparticle-assisted combination therapies for effective cancer treatment. Therapeutic delivery 1: 323-334.
- Jack HC, Zhang L (2012) Nanoparticle-based combination therapy toward overcoming drug resistance in cancer. Biochemical pharmacology 83: 1104-1111.
- Sariah L, Kurzrock R (2014) Toxicity of targeted therapy: Implications for response and impact of genetic polymorphisms. Cancer treatment reviews 40: 883-891.
- Ding JF (2011) Anticancer activity of an oligopeptide isolated from hydrolysates of sepia ink. Chinese journal of natural medicines 9: 151-155.
- Jian‑Hong D, Jin JJ, Liy XJ, Hu D, Wang J, et al. (2017) Metformin in combination with cisplatin inhibits cell viability and induces apoptosis of human ovarian cancer cells by inactivating ERK 1/2. Oncology letters 14: 7557-7564.
- Dong H, Huang J, Zheng K, Tan D, Chang Q, et al. (2017) Metformin enhances the chemosensitivity of hepatocarcinoma cells to cisplatin through AMPK pathway. Oncology letters 14: 7807-7812.
- Cheung TH, Rando TA (2013) Molecular regulation of stem cell quiescence. Nature reviews Molecular cell biology 14: 329-340.
- Sosa, MS, Bragado P, Aguirre-Ghiso JA (2014) Mechanisms of disseminated cancer cell dormancy: an awakening field. Nature Reviews Cancer 14: 611-622.
- Serrano M (2010) Shifting senescence into quiescence by turning up p53. Cell Cycle 9: 4256-4257.
- Benson EK, Mungamuri SK, Attie O, Kracikova M, Sachidanandam R, et al. (2014) p53-dependent gene repression through p21 is mediated by recruitment of E2F4 repression complexes. Oncogene 33: 3959-3969.
- Heldt FS, Barr AR, Cooper S, Bakal C, Novaik B, et al. (2018) A comprehensive model for the proliferation–quiescence decision in response to endogenous DNA damage in human cells. Proceedings of the National Academy of Sciences 115: 2532-2537.
- Torgovnick A, Heger JM, Liaki V, Herter-Sprie GS, Schumacher B, et al. (2018) The Cdkn1aSUPER Mouse as a Tool to Study p53-Mediated Tumor Suppression. Cell Reports 25: 1027-1039.
- Yip HT, Chopra R, Chakrabarti R (2006) Cisplatin-induced growth arrest of head and neck cancer cells correlates with increased expression of p16 and p53. Archives of Otolaryngology–Head & Neck Surgery 132: 317-326.
- Howell JJ, Hellberg K, TurnerM, SaghatelianA, Shaw RJ, et al. (2017) Metformin inhibits hepatic mTORC1 signaling via dose-dependent mechanisms involving AMPK and the TSC complex. Cell metabolism 25: 463-471.
- Sacco F, Calderone A, Castagnoli L, Cesareni G (2016) The cell-autonomous mechanisms underlying the activity of metformin as an anticancer drug. British journal of cancer 115: 1451-1456.
- Su X, Chakravarti D, Flores ER (2013) p63 steps into the limelight: crucial roles in the suppression of tumorigenesis and metastasis. Nature reviews Cancer 13: 136-143.
- Westfall MD (2003) The ΔNp63α phosphoprotein binds the p21 and 14-3-3σ promoters in vivo and has transcriptional repressor activity that is reduced by Hay-Wells syndrome-derived mutations. Molecular and cellular biology 23: 2264-2276.
- Cheung KJ, Gabrielson E, Werb Z, Ewald EJ (2013) Collective invasion in breast cancer requires a conserved basal epithelial program. Cell 155: 1639-1651.
- He YF, Tian DY, Yi ZJ, Yin ZK, Luo CL, et al. (2012) Upregulation of cell adhesion through delta Np63 silencing in human 5637 bladder cancer cells. Asian journal of andrology 14: 788-792.
- Wu WS (2006) The signaling mechanism of ROS in tumor progression. Cancer and Metastasis Reviews 25: 695-705.
- Trachootham D, Alexandre J, Huang P (2009) Targeting cancer cells by ROS-mediated mechanisms: a radical therapeutic approach?. Nature reviews Drug discovery 8: 579-591.
- Davis, Christopher A, Nick HS, Agarwal A (2001) Manganese superoxide dismutase attenuates cisplatin-induced renal injury: importance of superoxide. Journal of the American Society of Nephrology 12: 2683-2690.
- Borrego A, Zamora ZB, González R, Romay C, Menéndez S, et al. (2004) Protection by ozone preconditioning is mediated by the antioxidant system in cisplatin-induced nephrotoxicity in rats. Mediators of inflammation 13: 13-19.
- Li PD, Liu Z, Cheng TT, Luo WG, Chen J, et al. (2017) Redox-dependent modulation of metformin contributes to enhanced sensitivity of esophageal squamous cell carcinoma to cisplatin. Oncotarget 8: 62057-62068.
- Ambrus JL, Ambrus CM, Byron JW, Goldberg ME, Harrisson JW, et al. (1956) Study of metastasis with the aid of labeled ascites tumor cells. Annals of the New York Academy of Sciences 63: 938-961.
- Eddy RJ, Weidmann MD, Sharma VP, Condeelis JS (2017) Tumor cell invadopodia: invasive protrusions that orchestrate metastasis. Trends in cell biology 27: 595-607.
- Karagiannis GS (2017) Neoadjuvant chemotherapy induces breast cancer metastasis through a TMEM-mediated mechanism. Science translational medicine 9: 397.
- Koike A, Nakazato H, Moore GE (1963) The fate of Ehrlich cells injected into the portal system. Cancer 16, and MOOREG, Effects of tumorinhibitory principle of normal mouse liver homogenate on Ehrlich ascites cells. Cancer Res. 16: 716-720.
 Spanish
Spanish  Chinese
Chinese  Russian
Russian  German
German  French
French  Japanese
Japanese  Portuguese
Portuguese  Hindi
Hindi