Research Article, J Vet Sci Med Diagn Vol: 11 Issue: 3
Safety and Therapeutic Efficacy of Tolfenamic Acid in Poultry and, Animal in Bangladesh
Md Shafiqul Islam1*, Shahena Akhtar2, Md Rakibul Hasan1, Md Shakil Islam1
1Department of Pharmacology, Faculty of Veterinary Science, Bangladesh Agricultural University, Mymensingh, Bangladesh.
2Department of Agricultural Science, Jainta Degree College, Jaintapur, Sylhet, Bangladesh
Corresponding Author: Md Shafiqul Islam
Department of Pharmacology, Faculty of Veterinary Science, Bangladesh Agricultural University, Mymensingh-2202, Bangladesh
E-mail: shafiqpharma@yahoo.co.uk
Received date: February 17, 2022, Manuscript No: JVSMD-22-12756;
Editor assigned date: February 21, 2022, PreQC No. JVSMD-22-54741 (PQ);
Reviewed date: March 04, 2022, QC No. JVSMD-22-54741;
Revised date: March 14, 2022, Manuscript No. JVSMD-22-54741 (R);
Published date: March 23, 2022, DOI: 10.4172/2325-9590.100308
Citation: Islam MDS (2022) Safety and Therapeutic Efficacy of Tolfenamic Acid in Poultry and Animal in Bangladesh. J Vet Sci Med Diagn 11:3.
Abstract
Objectives: Drug safety and efficacy are the most important characteristics of a drug to be decided for the preventive and therapeutic purposes. A comprehensive research work of Tolfenamic acid has been investigated on poultry, pet, small and large animals before launching the drug in veterinary practices in Bangladesh. In this study, we have investigated the safety and therapeutic efficacy of Tolfenamic acid in poultry and animals.
Methods: Day old broiler chicks were collected, reared and on day 14; chicks were divided into three groups namely control, discriminate and indiscriminate group (n=30 each). The control group was left untreated, whereas, discriminate group was treated one week with Tolfenamic acid in drinking water (200 mg/2 L drinking water) followed by one week withdrawal period. On the other hand, indiscriminate group of chicks were not followed any withdrawal period. On the 28th day, random sampling of 15 birds was sacrificed from each group. On the basis of empirical and final diagnosis of diseases; patients having mastitis, pain, fever and stress condition have been treated with Tolfenamic acid (Tufnil (R) Vet Bolus).
Results: Physical condition, body weight and gross meat quality were not showed any remarkable differences among the groups; moreover, both discriminate and indiscriminate groups of broiler chicks were showed lucrative meat quality. Hematological analysis of Total Erythrocyte Count (TEC), Hemoglobin Content (Hb) and Packed Cell Volume (PCV) showed no significant differences among the groups. There were no significant differences of AST and ALT enzymes level both in treated and non-treated birds. TLC analysis demonstrated no Tolfenamic acid residue in liver, kidney, spleen, thigh muscle and breast muscle both in discriminate and indiscriminate birds respectively. Therapeutic efficacy of Tufnil (R) Vet Bolus was 75%, 85%, 92% and 80% in cow, calf, goat and cat respectively.
Conclusions: Tolfenamic acid is highly effective drug for stress and fever treatment and moderately effective drug for pain treatment. Judicious use of the drug has no adverse effects on body physiology and no residual hazards in edible soft tissues.
Keywords: ALT, AST, Efficacy, Poultry, Safety, Tolfenamic acid
Introduction
Tolfenamic acid is a non-steroidal anti-inflammatory drug extensively used in human for the treatment of anti-inflammation, analgesia and antipyretic action. The drug has been developed around 25 years ago for human treatment. More recently this drug has been formulated for veterinary practices. The drug is intended as complementary treatment for pain and fever. This drug can be administered by oral, i/v and i/m routes. Ideal dose of tolfenamic acid is 2 mg/kg body weight/day for 2 days or more as needed for the pattern of diseases. In developed countries, tolfenamic acid is also a popular drug for animal treatment, however, in Bangladesh; it is a new drug for veterinary uses.
Cyclooxygenase (COX) catalyzes the conversion of arachidonic acid into Prosta Glandins (PGs), which play a significant role in health and diseases in the Gastro Intestinal (GI) tract and in the renal, skeletal, and ocular systems [1]. They are generated from arachidonate by the action of cyclooxygenase isoenzymes, and their biosynthesis is blocked by nonsteroidal anti-inflammatory drugs, including those selective for inhibition of cyclooxygenase-2 [2]. Nonsteroidal Anti-Inflammatory Drugs (NSAIDs) are commonly prescribed to decrease the inflammation, fever and pain [3,4]. Some NSAIDs including aspirin, indomethacin and sulindac have antipyretic, antirheumatoid, anti-inflammatory and anticancer activities. In veterinary practices, NSAID is used as an adjunct therapy with antibiotics in the treatment of acute and chronic inflammatory musculoskeletal conditions, bacterial and viral infections, endotoxemia, gastroenteritis, laminitis and mastitis [6]. Besides, it is also useful in pneumonia, dehorning, disbudding and surgical procedures. Tolfenamic acid is a cyclooxygenase inhibitor anti-inflammatory drug. It inhibits prostaglandin release through inhibition of prostaglandin receptors [4]. It also hampers the platelets aggregation. Recently, tolfenamic acid showed its anti-inflammatory effects on HCT116 & HEK 293 human cell lines and RAW 264.7 mouse macrophages through suppression of JNK/NF-?B pathway [5]. Clinician suggested that tolfenamic acid is well absorbed orally and generally reach peak level at 2 to 4 hours of oral administration. Tolfenamic acid (2 mg/kg body weight) in combination with long-acting oxytetracycline (20 mg/kg body weight) demonstrated significant (P<0.04) clinical improvement in a blinded multicenter trial in 313 cattle having respiratory diseases [7]. The treatment regimen of tolfenamic acid once or twice with 48 hours inter dosing interval. Drug interaction is an important characteristic of drug formulation and its uses. Like other drugs, tolfenamic acid should be used with caution with anticoagulants like warfarin, coumarin derivatives and it increases serum digoxin level and should not be used in animals receiving methotrexate, a most potent drug used for inflammatory arthritis. Tolfenamic acid and meloxicam demonstrated similar preoperative and postoperative analgesic effects in cat following ovariohysterectomy [8]. Tolfenamic acid and meloxicam administered preoperatively provided a similar analgesic effect in the postoperative period lasting 24 hours. Tolfenamic acid pharmacokinetic/ pharmacodynamics modelling study exhibited lowering of PGE2, beta-glucuronidase and bradykinin-induced swelling in calves [9]. Intradermal injection of carrageenan, zymosan & dextan and intracaveally carrageenan in ruminant calves model for pharmacodynamics and pharmacokinetics of tolfenamic acid indicated inhibition of PGE2 synthesis and attenuating cardinal signs of inflammation (heat and swelling) suggested that a dosage of 2 mg/kg administered intramuscularly should be effective clinically as an anti-inflammatory agent [10]. Tolfenamic acid inhibited Prosta Glandin (PG) E2 synthesis in vivo in inflammatory exudate by 53%-86% for up to 48h after both TA treatments [11]. Yet no extensive research works has been investigated on tolfenamic acid in Bangladesh. So far we know this is the first research work of tolfenamic acid in poultry in Bangladesh as well as therapeutic efficacy in large and small animals. Our extensive research of tolfenamic acid (Tufnil(R) Vet Bolus) clearly demonstrated that the new journey of this drug in veterinary practices would bring a good impact on livestock and poultry sectors.
Material and Methods
Statement of ethical approval
The research work was done according to the ethical and welfare guidelines set by the Animal Welfare & Experimental Ethics Committee of Bangladesh Agricultural University, AWEEC/BAU/2021(07)
Chick collection and experimental design
We followed the experimental guideline of animal welfare & experimental ethics committee of Bangladesh agricultural university. One hundred healthy day-old broiler chicks were collected from the hatchery of AG agro Bangladesh Ltd. Gajipur, Bangladesh. These were not SPF. The birds were reared and vaccinated properly. On the day 14th birds were randomly divided into three groups namely control (group A, n=30), discriminate (group B, n=30) and indiscriminate (group C, n=30) group respectively. It is to be mentioned that there is no hard and fast rules for the sample size in poultry from the experimental ethical committee, however, it is better if the sample size is 10 or more. Intensive cage rearing system was followed with a floor space of average 450-525 square centimeter per birds. The control group was left untreated, whereas, discriminate group was treated with tolfenamic acid in drinking water (200 mg/2 L drinking water) for one week followed by withdrawal period of one week. On the other hand, tolfenamic acid treatment was continued in the indiscriminate group until the day of sacrifice without maintaining any withdrawal period. Poultry feed were supplied by nourish poultry feed Bangladesh Ltd. firstly as pre-starter (nutritional components: ME (kcal/kg), 2950; CP%, 21; Ca% 1; P%, 0.45%; CF%, 5; Lysine%, 1.15; Methionine, 0.4; Vitamins and minerals%, ad libitum, humidity%, 12) followed by grower feed (nutritional components: ME (kcal/kg), 3000; CP%, 20; Ca% .95; P%, 0.45%; CF%, 5; Lysine%, 1.05; Methionine, 0.45; Vitamins and minerals%, ad libitum, humidity%, 12)
Body weight measurement and physical condition analysis
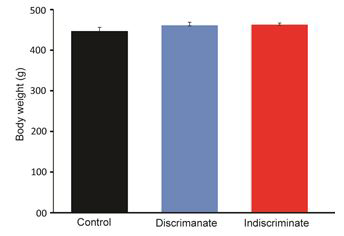
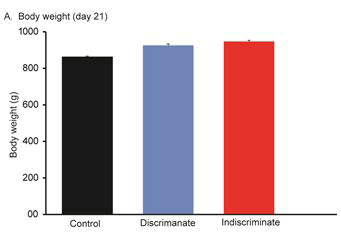
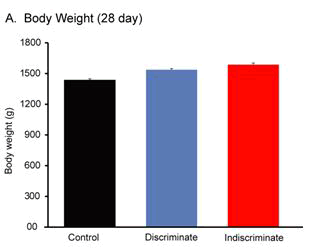
Body weights of all groups of birds were recorded on day 14th, day 21st and 28th respectively through digital balance. Group of bird’s body weight was expressed in Mean ± SE. Physical conditions of birds were observed every day either by open eye examination or close inspections such as movement of birds, feathers appearance, movement of neck etc.
Blood collection
On 28th day, blood samples were collected from all groups (Control, discriminate & indiscriminate) of birds into sterile heparinized and non-heparinized vials and were immediately stored into refrigerator separately to perform hematological tests and blood serum enzymatic test (AST and ALT).
Gross meat quality
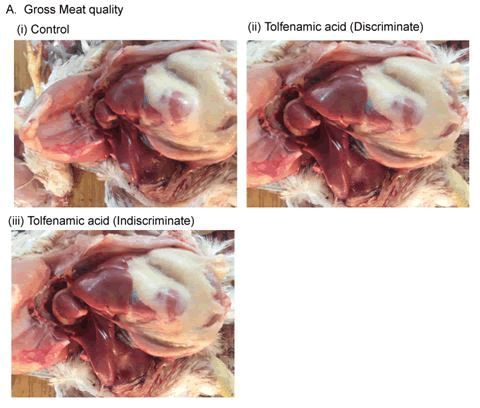
Gross meat quality, whole GIT tract were observed carefully during the sacrifice of each birds. Color of meat, smell of meat, presence of any unwanted spots, blood color etc. were considered the criteria for determination of gross meat quality.
Sample collection
Samples were collected on the day of 29th. After sacrificing the birds; liver, kidney, spleen, thigh & breast muscles were collected for Thin Layer Chromatography analysis.
Chemicals and standard drugs
All chemicals and reagents were standard grade. HPLC grade methanol (Merck-Germany), Trichloracetic Acid (TCA), diethyl ether and acetone were used. Tolfenamic acid pure chemical was obtained from ESKAYEF Pharmaceuticals Ltd, Bangladesh. The standard for the TLC analysis was prepared by dissolving 0.1 gm of tolfenamic powder in 4 mL solution of methanol. Standard solution was stored in -4 °C.
Statement of ethical approval
Tolfenamic acid (Tufnil(R) Vet Bolus) from ESKAYEF pharmaceutical company, Bangladesh Ltd. has been approved for research on poultry and pet, small & large animals for safety and efficacy study (approval number: ahd/mkt/21/297).
Sample preparation for TLC analysis
Thin layer chromatography samples of liver, kidney, spleen, thigh muscle and breast muscle were prepared and kept under -20 °C until analysis. 5 g of each sample was measured and homogenized at 10 mL phosphate buffer (pH 7.2). Then 2 mL trichloroacetic acid (30%) supplemented carefully & mixed and then centrifuged at 7000 rpm for 20 minutes. Supernatant was separated, collected and same volume of diethyl ether mixed and kept for 20 minutes and then upper oily portion was discarded. This extraction of supernatant was repeated twice with diethyl ether. The extract was evaporated until dryness and reconstituted with mobile phase solution {Methanol-acetone (1:1), Butanol-DW-AA (6:2:2), n-Hexane, Butanol-AA-methanol (6:2:2), Acetone-AA (1:1)}. Then, extracts were collected into screw capped vial with proper care and kept into refrigerator for further analysis. Total procedure was done according to the method described by [4].
Thin layer chromatography analysis
TLC was analyzed with some modification as described by [12]. TLC plate (MN-Germany), TLC tank, UV detection box (256 nm) and other necessary instruments were used. In this experiment, standard tolfenamic acid was used to identify the residues of Tolfenamic acid. Spots were visualized in UV detection box at 256 nm and the outlines of the spots were marked with a series of dots using sharp pencil for calculation of Retention Factor (RF).
Hematology and enzyme
Total Erythrocyte Count (TEC), Erythrocyte Sedimentation Rate (ESR) Hemoglobin content (Hb%) and Packed Cell Volume (PCV) were studied for hematological investigation [13]. For determination of hematological parameter, blood samples were collected at the end of experiment (28th day) from all groups and examined. Aspartate Aminotransferase (AST) and Alanine Aminotransferase (ALT) were examination and analyzed by Mispa CCXL (Switzerland GmbH).
Therapeutic efficacy
This study was conducted from August to December, 2021. Therapeutic efficacy was investigated in Veterinary Teaching Hospital, Bangladesh Agricultural University, Mymensingh, Bangladesh on large, small and pet animals etc. Treatment strategy was decided after the observation of sign, symptoms, history and tentative diagnosis as well final diagnosis. The patients were suffering from mastitis, respiratory diseases, musculoskeletal pain, broken bone, fever, pain, lameness etc. Specific treatment was provided along with the tolfenamic acid (2 mg/kg body weight). Clinical efficacies were recorded by direct observation as well as from owner’s information. Data was collected from patient’s cards and prescription papers in Veterinary Teaching hospital. Rational use of drugs was made according to drug’s information and literature.
Data analysis
Results are expressed as the mean ± SEM. Graph Pad Prism 3 (GraphPad Software, La Jolla, CA, USA) was used to perform statistical analysis of the data. When comparing two groups, the Student's unpaired t-test was employed, whereas when comparing more than two groups, a one-way ANOVA with a Bonferroni post-hoc test was utilized. P<0.05 and P<0.01 were considered statistically significant.
Results
Effects of tolfenamic acid on body weight and meat quality in broilers
Body weight gain is an important parameter of poultry industry. The body weight between discriminate and indiscriminate groups of birds was found slightly increased than the control one, however, these were statistically insignificant among the groups and between the discriminate and indiscriminate groups respectively (Figures 1-3). Gross meat quality was assessed on the appearance, color, smell, presence of unwanted spots if any, blood color etc. Meat quality of discriminate and indiscriminate birds was observed grossly and found very lucrative in appearance (Figure 4). The whole GIT tract was also investigated during sacrifices of birds and found very healthy both control and treated birds (data not shown). These observations indicated that Tufnil(R) Vet relieved the stresses during poultry rearing and improve the weight and meat quality.
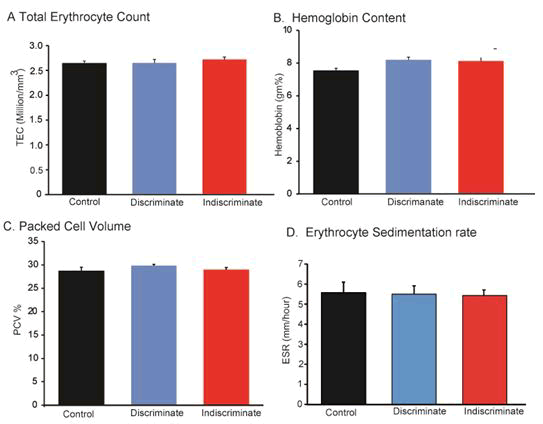
Effects of tolfenamic acid on hematology in broilers
Hematological analysis is an important phenomenon for physiological as well as pathological investigation of body conditions. Hematological parameters such as hemoglobin (Hb) and Total Erythrocyte Count (TEC), Erythrocyte Sedimentation (ESR) and Packed Cell Volume (PCV) were measured in broilers. There were little differences among the control, discriminate and indiscriminate groups of birds (Figure 5) but it was insignificant. This blood profile examination indicated that Tufnil(R) Vet no adverse effects on hematopoietic system in poultry physiology.
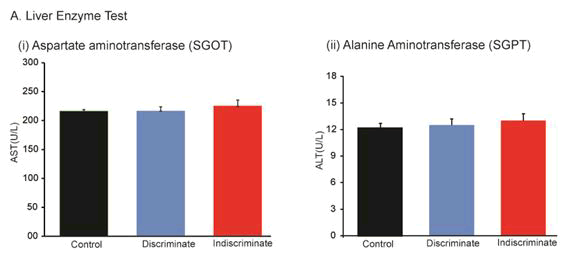
Effects of tolfenamic acid on Aspartate Amino Transferase (AST) and Alanine Aminotransferase (ALT)
Safety is first then the therapy of a drug. AST and ALT enzymatic assessment of liver function was investigated. Under physiological conditions, these enzymes reside within the liver cells to normal cellular functions. However, liver injury causes spill these enzymes into blood raising the AST and ALT blood enzyme level. Mispa CCXL auto analyzers demonstrated that AST level in control, discriminate and indiscriminate were 216 ± 2.8, 225 ± 6.45 and 242 ± 10.12 U/L respectively (Figure 6). Similarly, ALT level in control, discriminate and indiscriminate was 12.5 ± 0.5, 13.08 ± 0.69 and 14.4 ± 0.8 U/L respectively (Figure 6). Both AST and ALT were found insignificant among the groups indicating that Tufnil(R) Vet is not toxic even for indiscriminate use in poultry within the therapeutic index.
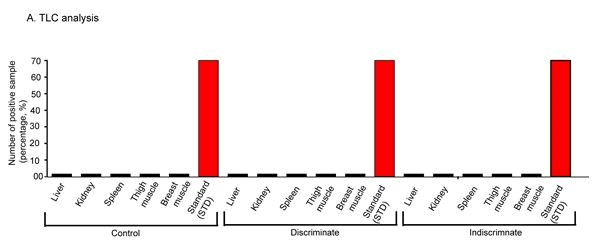
Residual presence of tolfenamic acid in edible poultry tissues in broilers
Drug residue is hazardous to both human and animal health. These drug residues come from non-judicious use of meat and egg producing animal to human health. However, judicious use of drugs can overcome the problem. In this study, tolfenamic acid residue was investigated in broiler poultry after judicious and non-judicious administration. Liver, kidney, spleen, thigh muscle and breast muscle were examined in control, discriminate and indiscriminate groups of birds. No tolfenamic acid residue was detected in liver, kidney, spleen, thigh muscle and breast muscle of control, discriminate and indiscriminate groups of birds (Figure 7). This research findings suggest that Tufnil(R) Vet is safe drug if use judiciously.
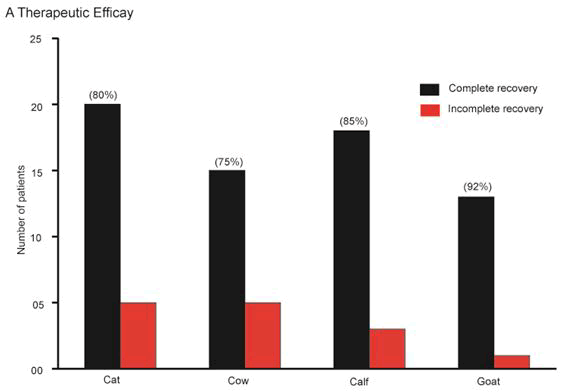
Therapeutic efficacy of tolfenamic acid in large, small and pet animals
Therapeutic efficacy of tolfenamic acid (Tufnil(R) Vet Bolus, 200 mg) (2 mg/kg body weight) was evaluated in in cattle (n=4), calf (n=7), goat (n=14) and cat (n=25) in the veterinary hospitals in Bangladesh Agricultural University, Mymensingh-2202, Bangladesh. Animals were suffering with mastitis, respiratory diseases, pain, fever etc. and tofenamic acid was provided as a supportive therapy in the prescription. Cattle suffering from respiratory diseases well responded with Tufnil(R) Vet bolus treatment. Over all complete recovery rate was 75% in cattle, 85% in calf, 92% in goat and 80% in cat (Figure 8). Diseases pattern, body conditions, fever, feed appetite were found very well after treatment. Goats affected with respiratory problem and fever was treated with Tufnil(R) Vet bolus and almost 90% disease recovery was recorded. Pet animal cat with fever, less appetite as well respiratory problems treated with Tufnil(R) Vet bolus responded with excellent recovery rate. Moreover, a goat with pain & fever treated with Tufnil(R) Vet bolus as a supportive therapy found complete recovery. Therefore, the investigation provides the information that the therapeutic efficacy of Tufnil(R) Vet bolus in mastitis, respiratory diseases, fever, pains etc. as supportive therapy was very satisfactory. There were no adverse effects recorded.
Discussion
There is no genotoxicity report of tolfenamic acid in human health. Moreover, no carcinogenicity was recorded in repeated dose toxicity studies in mice and rats. Sometimes over use and continuous use of tolfenamic acid accelerated gastrointestinal tract erosions and ulcers. Tolfenamic acid markedly reduces the carrageenan, zymosan and dextran-induced intradermal inflammation in calves. The anti-inflammatory effects were pharmacologically through inhibition of serum Thromboxane (TX) B2 and Prosta Glandin (PG) E2 production suggest that tolfenamic acid@dose of 2 mg should be effective clinically as an anti-inflammatory drugs [10]. Endogenous prostaglandins may protect the gastric mucosa as well as prostaglandin deficiency is a strong predisposing factor for gastric ulceration [14]. Cyclo-oxygenase is a widely distributed enzyme which converts arachidonic acid to the cyclic endoperoxide precursors of prostaglandins, thromboxanes and prostacyclin [15]. The main mode of action of non-steroidal anti-inflammatory drugs is the inhibition of prostanoid synthesis. Body weight is an important parameter of poultry industry. The body weight between discriminate and indiscriminate groups of birds was found slightly increased than the control birds. Tolfenamic acid treated broiler’s meat was found lucrative in appearance. Effects of tolfenamic acid on GIT tract was investigated and found very healthy GIT like the control one. These observations indicated that tolfenamic acid is not harmful for poultry rearing system rather it is helpful to remove the stress and improve the weight gain and meat quality. Although inhibition of prostanoid synthesis is considered the key mode of pharmacodynamics of tolfenamic acid, however, inhibition of LTB4–induced chemotaxis is a non-ligand specific additional mechanism of action of tolfenamic [16]. Hematological analysis is a simple phenomenon to understand the physiology and pathology of a biological subject. The immune system of poultry is diagnosed by investigating the blood profile of poultry. Blood profile examinations is not only the diagnostic tool, it is also a major tool of research in poultry birds. It is an investigation of analysis and guideline of stress factor caused by environmental and other factors. We have analyzed hemoglobin (Hb) level of blood in all groups of birds and found there are differences but that is very negligible. Like Hb, Total Erythrocyte Count (TEC), Erythrocyte Sedimentation (ESR) and Packed Cell Volume (PCV) were found non-significant among the groups of birds. Blood analysis indicated that tolfenamic acid has no toxic effects on hematopoietic system in broilers birds rather it increased the quality of life of birds. We then examined the liver physiology of broilers by investing the AST and ALT level. In physiological conditions, these two enzymes maintain liver homeostasis and body metabolic function. Liver damage causes spill out these enzymes from hepatocytes and reach to blood. There were no significant differences in AST or ALT among discriminate, indiscriminate and control groups of broilers respectively. We found tolfenamic acid in therapeutic dose@2 mg/kg body weight did not hamper liver enzyme AST or ALT function and was non-toxic to broiler birds. It is to be mentioned that we did not investigate dose dependent toxicity and safety level.
Good health is associated with safe food which is free from drug residues, poisons and toxins. Food is a basic need for human health. Nowadays food safety issue is a burning question in the present world because there is a good relationship between human health and safe food [17]. Drug residue is harmful to human health as well as animal health. These drug residues come from non-judicious use of meat and egg producing animal to human health. However, judicious use of drugs can overcome the problem. Livestock is a potential source of food of animal origin. The use of veterinary drugs in food-producing animals leads to drug residues in animal derived products (meat, milk and eggs) and poses serious public health hazard. Among drug residues antibiotic residue is most important because there are various major areas of concern over the presence of residues of antibiotics in animal-derived foodstuffs with regard to human health like hypersensitivity reaction, carcinogenicity, mutagenicity, teratogenicity and the development of antimicrobial drug resistance and the possible transfer of resistant pathogens from animal food products to human. Recently, tolfenamic acid is prescribed to treat inflammation, fever, pain, stress and so on [3, 4] in poultry and livestock farming system in Bangladesh. Therefore, poultry and livestock treated with tolfenamic acid required for specific withdrawal period until all residues are depleted to safe levels before their tissues can be used for human consumption. In fact, there is no extensive research work on the tolfenamic acid residue detection and hazard analysis in Bangladesh. Tolfenamic acid residue was investigated in broiler poultry after judicious and non-judicious administration. Liver, kidney, spleen, thigh muscle and breast muscle were examined in control, discriminate and indiscriminate groups of birds respectively. No residues of tolfenamic acid was detected both in discriminate and indiscriminate groups of birds. Drug potency is very important characteristics of a drug. Each and every drug has its own inherent qualities to treat the diseases and prophylaxis. Tolfenamic acid as a supportive therapy for mastitis, respiratory diseases, pain, fever etc. was found very effective [4-8]. The mode of anti-inflammatory effects of tolfenamic acid is inhibition of prostaglandin production in the body and inflammatory sites [4].
Conclusion
This research findings suggest that tolfenamic acid (Tufnil(R) Vet Bolus) is safe drug if use judiciously. Potency of drug is very important for a drug to treat the diseases. Therapeutic efficacy of tolfenamic acid (2 mg/kg body weight) was very successful as supportive therapy for mastitis, respiratory diseases, and diseases associated with pain & fever; moreover, no adverse effects were recorded. Therefore, it could be concluded a good start in Bangladesh. Farther research study is needed for more information of safety and efficacy of the tolfenamic acid.
Acknowledgement
The Authors are thankful to Professor Dr. Md. Taohidul Islam, Professor, Department of Medicine, Bangladesh Agricultural University, Mymensingh-2202, Bangladesh for providing technical support and therapeutic facilities.
References
- Radi ZA (2009) Pathophysiology of cyclooxygenase inhibition in animal models. Toxicol Pathol 37: 34-46. [Crossref][Google Scholar][Indexed]
- Ricciotti E, FitzGerald GA (2011) Prostaglandins and inflammation. Arterioscler Thromb Vasc Biol 31: 986-1000. [Crossref][Google Scholar][Indexed]
- Abdulla A, Adams N, Bone M, Elliot AM, Gaffin J, et al. (2013) Guidance on the management of pain in older people. Age Ageing 42: i1-57. [Crossref][Google Scholar][Indexed]
- Tokola RA, Kangasniemi P, Neuvonen PJ, Tokola O (1984) Tolfenamic acid, metoclopramide, caffeine and their combinations in the treatment of migraine attacks. 4: 253-63. [Crossref][Google Scholar][Indexed]
- Shao HJ, Lou Z, Jeong JB, Kim KJ, Lee J, et al. (2015) Tolfenamic Acid Suppresses Inflammatory Stimuli-Mediated Activation of NF-kappaB Signaling. Biomol Ther (Seoul) 23: 39-44. [Crossref][Google scholar][Indexed]
- Dinakaran V, Dumka VK (2013) Pharmacokinetics of tolfenamic acid following two oral dose levels in buffalo calves. J Vet Pharmacol Ther 36: 306-308. [Crossref][Google Scholar][Indexed]
- Deleforge J, Thomas E, Davot JL, Boisrame et al. (1994) A field evaluation of the efficacy of tolfenamic acid and oxytetracycline in the treatment of bovine respiratory disease. J Vet Pharmacol Ther. 17: 43-7. [Crossref][Google Scholar][Indexed]
- De-La-Vibora BJ, Lascelles BDX, Fernández PG, Freire M, Segura IAGD (2008) Efficacy of tolfenamic acid and meloxicam in the control of postoperative pain following ovariohysterectomy in the cat. Vet Anaesth Analg 35: 501-10. [Crossref][Google Scholar][Indexed]
- Landoni MF, Cunningham FM, Lees P (1996) Pharmacokinetics and pharmacodynamics of tolfenamic acid in calves. Res Vet Sci 61: 26-32. [Crossref][Google Scholar][Indexed]
- Lees P, Mckeller QA, Foot R, Gettinby G (1998) Pharmacodynamics and pharmacokinetics of tolfenamic acid in ruminating calves: evaluation in models of acute inflammation. Vet J 155: 275-88. [Crossref][Google Scholar][Indexed]
- Sidhu PK, Landoni MF, Lees P (2006) Pharmacokinetic and pharmacodynamic interactions of tolfenamic acid and marbofloxacin in goats. Res Vet Sci 80: 79-90. [Crossref][Google Scholar][Indexed]
- Fitzhugh AL, Anadu NO, Waterhouse DJ, Hrabie JA, Saavedra JE, et al. (2002) Qualitative thin-layer and high-performance liquid chromatographic analysis of 1-substituted diazen-1-ium-1,2-diolates on aminopropyl-bonded silica gel. Anal Biochem 301: 97-102. [Crossref][Google Scholar][Indexed]
- Lamberg S, Rothstein R (1977) Laboratory manual of hematology and urine analysis. 1st ed. AG publishing Company Inc. Conencticut, USA.
- Hawkey CJ (1986) Synthesis of prostaglandin E2, thromboxane B2 and prostaglandin catabolism in gastritis and gastric ulcer. Gut 27: 1484-92. [Crossref][Google Scholar][Indexed]
- Higgs GA, Moncada S, Salmon JA, Seager K (1983) The source of thromboxane and prostaglandins in experimental inflammation. Br J Pharmacol 79: 863-8. [Crossref][GoogleScholar][Indexed]
- Kankaanranta, H., E. Moilanen, and H. Vapaatalo, Tolfenamic acid inhibits leukotriene B4-induced chemotaxis of polymorphonuclear leukocytes in vitro. Inflammation, 1991. 15(2): p. 137-43. [Crossref][Google Scholar][Indexed]
- Rather IA, Koh WY, Paek WK, Lim J (2017) The sources of chemical contaminants in food and their health implications. Front Pharmacol 8: 830. [Cross Ref][Google Scholar][Indexed]
 Spanish
Spanish  Chinese
Chinese  Russian
Russian  German
German  French
French  Japanese
Japanese  Portuguese
Portuguese  Hindi
Hindi