Research Article, J Clin Exp Oncol Vol: 7 Issue: 1
Serum Leptin Might Be Causally Correlated to Invasive Ductal Breast Carcinoma
Saad M Al-Shibli1, Nasser M Amjad2, Muna K Al-Kubaisi3, Norra Harun4, Emad M Nafie1 and Shaikh Mizan1*
1Department of Basic Medical Sciences, Kulliyyah of Medicine, International Islamic University Malaysia, Pahang, Malaysia
2Department of Surgery, Kulliyyah of Medicine, International Islamic University Malaysia, Pahang, Malaysia
3Department of Obstetrics and Gynaecology, Kulliyyah of Medicine, International Islamic University Malaysia, Pahang, Malaysia
4sDepartment of Pathology, Hospital Tengku Ampuan Afzan, Pahang, Malaysia
*Corresponding Author : Shaikh Mizan
Department of Basic Medical Sciences, Kulliyyah of Medicine, International Islamic University Malaysia, Pahang, Malaysia
Tel: (60) 9-570-4496/(60) 112 639-6670
Fax: (60) 9-571-6770
E-mail: shaikhmizan2015@gmail.com
Received: January 25, 2018 Accepted: February 08, 2018 Published: February 15, 2018
Citation: Al-Shibli SM, Amjad NM, Al-Kubaisi MK, Harun N, Nafie EM , et al. (2018) Serum Leptin Might Be Causally Correlated to Invasive Ductal Breast Carcinoma. J Clin Exp Oncol 7:1. doi: 10.4172/2324-9110.1000211
Abstract
Introduction: Leptin, the hormone secreted from white adipose tissue is suspected of being a causative factor in tumorigenesis in various tissues. Thus it might be a link between obesity and breast cancer.
Objectives: In this project we tried to study causative association of obesity and serum leptin with invasive ductal breast cancer (IDC) in a sample of Malaysian population.
Methods: We measured various obesity parameters, and estimated serum leptin levels in a group of healthy control and IDC patients. After confirming diagnosis by standard histopathological methods, serum leptin levels were estimated using ELISA. Both the pre-and postoperative leptin levels were measured with the Patient group.
Results: The difference between the serum leptin levels of the Control and the Patient group were highly significant with a P value of < 0.001. There was no significant difference in the serum leptin level between the pre and post-operative states in the IDC patients (P=0.414). There was significant positive correlation between the serum leptin level and the Body Mass Index (BMI) with the Control group (rs=0.598, P < 0.01), however, the breast cancer cases showed a very weakly positive correlation (rs = 0.217, P > 0.05).
Conclusion: Since high serum leptin in IDC patients persists unabated in the post-operative state, we infer that the source of leptin in these cases cannot be the breast cancer tissue itself and leptin cannot be a marker for breast cancer. Highly significant association between serum leptin and IDC patients suggests that leptin in serum might play a causative role in the tumorigenesis of breast cancer, and it might be an important connection between obesity and breast cancer. Therefore, leptin signal transduction pathway might be a prospective therapeutic target in the treatment and prevention of IDC.
Keywords: Leptin; Serum leptin; Invasive ductal carcinoma; Breast cancer; Obesity
Abbreviations
IDC: Invasive Ductal Carcinoma of Breast; BMI: Body Mass Index; WHR: Waist Hip Ratio; WC: Waist Circumference
Introduction
Breast cancer is the commonest cancer in women [1,2]. Although it occurs only among women, it is the second commonest cancer after lung cancer, with an estimated 1.7 million new cases diagnosed globally in 2012 [3]. Its incidence in the Western countries has risen by over 30% in the last 25 years. The increase is variously attributed to increased life expectancy, better screening and diagnosis, urbanization, adoption of sedentary lifestyles, and obesity [2,4].
Although there are some controversy about whether obesity increases the risk of breast cancer in pre-menopausal women, the overall suggestion appears to be in favor of higher risk of breast cancer, poorer treatment response and increased mortality in both pre and post-menopausal women [5,6]. Moreover central obesity, particularly among Asian women, has shown plausible association with breast cancer even in pre-menopausal women [7].
The means of the effects of adiposity on other organs are the hormones and cytokines synthesized and secreted by white adipose tissue, e.g. leptin, adiponectin, resist in, apelin, omentin, tumor necrosis factor-a, IL-6, etc. [8]. Among all these hormones and cytokines, generally called adipokines, the most widely researched and most significant in relation to obesity and tumorigenesis is leptin [9].
Leptin is a multifunctional protein hormone, consisting of 167 amino acids with a molecular mass of 16 kDa. [10,11]. It is physiologically responsible for satiety and weight control through its action on hypothalamic appetite center. When well nutritional, that is the body has got enough fat reserve, ample leptin is produced from the adipose tissue to inhibit appetite center. But incapacity to produce enough leptin obviously results in uninhibited food intake and obesity [12,13]. Paradoxically, obese persons are commonly found with high serum leptin level. The obvious explanation is reduced sensitivity of the hypothalamic neurons to leptin, resulting in a requirement for higher leptin level to inhibit food intake-a higher set point for leptin and thus white adipose tissue mass, the producer of leptin [14,15].
However, higher leptin level has got other consequences. Leptin is a pleiotropic hormone and is a physiological growth factor for various tissues including human breast [16-18] pancreatic β cells [18], hepatic cells [19], colonic epithelial cells [20], lung [21], placenta [22], bone tissue [23], and so on. As is often found with growth factors, over activity of leptin might be suspected to induce tumorigenesis in its target tissues. Leptin has been often reported to be a proliferative, angiogenic and anti-apoptotic factor, which suggest its possible role in the development and progression of cancer [24,25]. Thus it might be the link between obesity and cancer. Studies often suggested causal association between leptin and cancer of various organs, including ovary [26], prostate [27], esophagus [28] and particularly breast [29,30]. However, some researchers as Mantzoros et al. [31] and Coskun et al. [32] found no correlation of leptin with breast cancer. And even there are others as Petridou et al. [33] who found a negative correlation with premenopausal breast cancer patients in samples of western (mostly Caucasoid) population . This study is a further attempt to clarify such ambiguities regarding the correlation of leptin with breast cancer; and it is the first report on sample of the Malaysian population.
We report here anthropometric measurements, estimates of serum leptin level and their relationship in confirmed cases of ductal breast carcinoma, and of otherwise normal females from the same population. We also report pre- and post-operative serum leptin levels of the cases, in order to find out possible role of breast cancer tissue in elevating serum leptin level in such patients. And then we discuss the implications of the findings in prevention and treatment of ductal breast carcinoma.
Materials and Methods
Fifty one (51) patients with invasive ductal carcinoma (IDC) of the breast, admitted in the Department of Surgery, Hospital Tengku Ampuan Afzan (HTAA) Kuantan, Malaysia, from January 2012 to March 2014, were included in this study. The diagnosis was confirmed with histopathological examination of the surgically resected breast tissue, as described in our previous report [34]. The exclusion criteria were patients with type 2 diabetes; those already started adjuvant or neo-adjuvant therapy and patients attending the clinic for follow-up.
The Control group was forty (40) women attending the breast center IIUM (International Islamic University Malaysia), who had negative mammogram during the same investigation period. A written consent was obtained from all the patients and the Control group. Our research protocol had the approval from IIUM Research Ethical Committee (IREC).
Personal information was taken from all the participants by a trained nurse through a personal interview, which was consistent with the guidelines for studies including human subjects. These information included age, ethnicity, parity, menopausal status, medical and family history, and particularly breast cancer history. Body weight was taken to nearest 0.1 kg, height to the nearest 0.1 cm. Body mass index (BMI) was calculated as body weight in kilogram/height in meter square. Serum leptin levels were estimated by ELISA technique (as described in the legend of Figure 1). Waist circumference (WC) was taken midway between the lowest rib and iliac crest. Waist to hip ratios (WHR) was calculated following standard procedures. The ratio was used as a measure for central obesity.
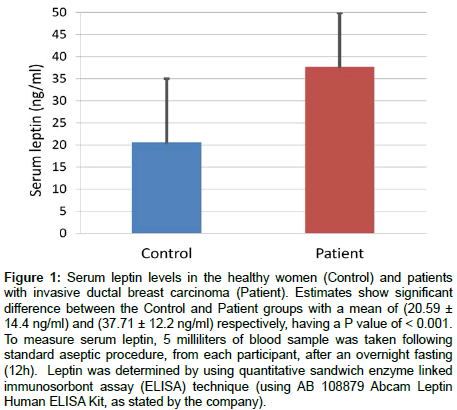
Figure 1: Serum leptin levels in the healthy women (Control) and patients with invasive ductal breast carcinoma (Patient). Estimates show significant difference between the Control and Patient groups with a mean of (20.59 ± 14.4 ng/ml) and (37.71 ± 12.2 ng/ml) respectively, having a P value of < 0.001. To measure serum leptin, 5 milliliters of blood sample was taken following standard aseptic procedure, from each participant, after an overnight fasting (12h). Leptin was determined by using quantitative sandwich enzyme linked immunosorbont assay (ELISA) technique (using AB 108879 Abcam Leptin Human ELISA Kit, as stated by the company).
The breast cancer patients were followed from the preoperative period when anthropometric measurement and blood samples were taken. Then they underwent surgical resection of their tumors in the Department of Surgery, HTAA (Hospital Tengku Ampuan Afzan) Kuantan, from January 2012 to March 2014 by either total mastectomy or wide local excision.
Serum of the individuals from Control and Patient groups were collected and leptin levels were estimated. The method of estimation and results are presented in Figures 1 and 2.
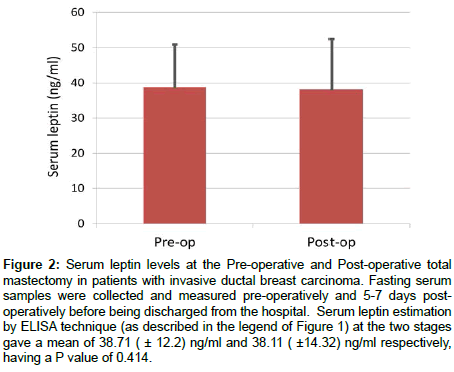
Figure 2: Serum leptin levels at the Pre-operative and Post-operative total mastectomy in patients with invasive ductal breast carcinoma. Fasting serum samples were collected and measured pre-operatively and 5-7 days postoperatively before being discharged from the hospital. Serum leptin estimation by ELISA technique (as described in the legend of Figure 1) at the two stages gave a mean of 38.71 ( ± 12.2) ng/ml and 38.11 ( ±14.32) ng/ml respectively, having a P value of 0.414.
Statistical Analysis
All data were checked for normality and homogeneity of variance before analysis. The relationship between circulating serum leptin with demographic features (ethnicity, age, BMI, W/H ratio and menopausal status) were examined by independent sample t-test, Pearson bivariate correlation test and Chi square (χ2) test. Values with P < 0.05 were considered statistically significant. The statistical software SPSS (version 22.0) was used for all statistical analyses.
Ethical Approvals
The research protocol was approved by the International Islamic University Malaysia (IIUM) Research Ethical Committee (IREC). Approval reference number is IIUM/305/14/11/2/IREC 516 and was registered with the National Malaysian Research Registration under reference number NMRR-16-1271-31307.
Results
The demographic characteristics of the Control group showed a mean age of 49.7( ± 4.85) years, mean BMI of 25.74( ± 5.2) kg/m2 and mean WHR of 0.89 ( ± 0.06). About 65% of the individuals in the Control groups were premenopausal. The Patient group had a mean age of 52.35( ± 8.95) years, mean BMI of 26.07( ± 5.51) kg/m2, and 61.5% of them were premenopausal.
Our results showed a highly significant difference in the serum leptin level between the Control group and the Patient group as shown in Figure 1.
No significant difference was found between the preoperative and postoperative serum leptin levels in the Patient group, as shown in Figure 2.
Linear regression plot between the BMI and leptin level in the Control and Patient group were analyzed and the findings are presented in Figure 3.
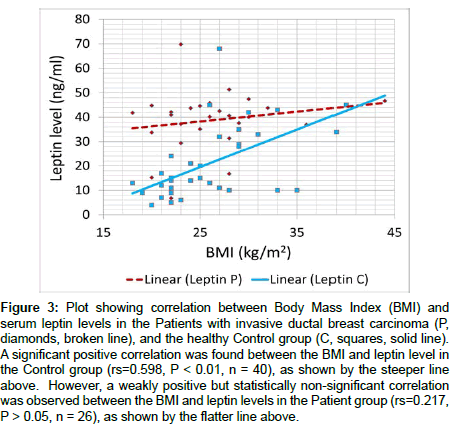
Figure 3: Plot showing correlation between Body Mass Index (BMI) and serum leptin levels in the Patients with invasive ductal breast carcinoma (P, diamonds, broken line), and the healthy Control group (C, squares, solid line). A significant positive correlation was found between the BMI and leptin level in the Control group (rs=0.598, P < 0.01, n = 40), as shown by the steeper line above. However, a weakly positive but statistically non-significant correlation was observed between the BMI and leptin levels in the Patient group (rs=0.217, P > 0.05, n = 26), as shown by the flatter line above.
Discussion
The results show a very significant difference in the mean value of serum leptin level between the Control and the Patient group (Figure 1). This is the first report of its kind about Malaysian population. This finding is consistent with other researchers who reported about other population groups from Taiwan [35], Iran [36] and by Niu et al. in their meta-analysis of published literature from PubMed and the Chinese National Knowledge Infrastructure (CNKI) [30]. All these studies, including the present one, is also supported by direct in vitro studies that leptin enhances proliferation of various normal and cancerous breast cell lines [16,37]. Leptin has also been shown to up regulate specifically vascular endothelial growth factor VEGF and induce angiogenesis in cell culture studies [9,24]. Thus significantly high serum leptin in the Patient group might be suspected as a causal or aggravating factor for development of ductal breast carcinoma.
However, there is a possibility that the cancerous tissue can produce leptin and release it into the blood, thus higher serum leptin could be an effect of the cancer. Indeed, there are reports of tumors that secrete leptin either in tissue fluid [38,39] or in blood [40]. The first one reports that leptin concentrations in saliva samples are significantly increased in patients with parotid tumors [38], however, the patients in these cases show normal serum leptin level. According to the second report, leptin is secreted in the tears of eyes affected by adenoid cystic carcinoma (ACC), and chronic sclerosing dacryoadenitis (CSD). Its concentration markedly reduces postoperatively. However, this report does not say anything about contribution of such leptin-secreting cancer to serum leptin level. In the third report, 30 cases of well-differentiated thyroid cancer show a fall of serum leptin level from a mean value of 19.25 ng/ml to 0.90 ng/ ml (P<0.001), after surgical resection of the cancer.
To study such possibility we estimated serum leptin values before and 5-7 days after total mastectomies and found no significant difference between the means (Figure 2). We know of another such report on breast carcinoma where serum leptin levels are estimated before and 30 days after mastectomy and no significant difference is found [35]. Given the short half-life of only several hours for leptin in blood [41,42] and poor follow up opportunity, we considered a postoperative measurement 5-7 days after, during discharge from the hospital would be acceptable. These findings indicate that breast carcinoma cells in our patients did not contribute significantly to the serum leptin, even if they produced and secreted any. These findings suggest more towards a causal role of high serum leptin in developing ductal breast carcinoma, and that serum leptin level cannot be used as a marker for breast cancer.
Our study showed that serum leptin level was significantly correlated with the body mass index (BMI) in the Control group. It is an well-established fact that serum leptin concentration is correlated with body fat [14,43] and expectedly to BMI [35,44], which is a very useful index of obesity. Figure 3 also shows a very striking feature that while leptin is significantly correlated with breast cancer, in contrast to the Control group the Patient group do not show any significant correlation of leptin with BMI. The correlation curve is almost flat. However, it shows that, except few individuals others have a serum leptin value higher than 30 ng/ml, which is quantitatively indicated by the mode of the two groups being 10 to <15 ng/ml and 40 to <45 ng/ml respectively. It suggests a threshold like effect and a stronger correlation of serum leptin compared to BMI on breast cancer development.
Although WHR (waist to hip ratio) is also considered as an index of adiposity, we found no significant correlations of WHR with the serum leptin level (data not shown). This makes sense as waist circumference is mostly given by abdominal/visceral fat. And it has been often reported that serum leptin has no association with visceral fat; rather subcutaneous fat is the most important determinant of serum leptin [45,46].
We found a weak negative correlation of serum leptin level with age, however, it was statistically insignificant; and we think larger sample size is needed to agree or disagree with other reports as Ostlund et al. [43], who found significantly negative correlation with age in both the sexes. We did not find any significant difference in the serum leptin level between the pre-and post-menopausal women in the Control group. However, to draw more definitive conclusion in these regards larger sample size is needed.
We found significantly negative correlation with age in both the sexes. We did not find any significant difference in the serum leptin level between the pre- and post-menopausal women in the Control group. However, to draw more definitive conclusion in these regards larger sample size is needed.
In conclusion, this study shows a positive correlation of serum leptin level to ductal carcinoma of the breast, and serum leptin is likely to be a causal or aggravating factor for development of ductal breast carcinoma. Leptin might be a bridge between obesity and breast cancer. Since source of high leptin in serum is not breast cancerous tissue, leptin cannot be used as a marker for breast cancer. Along with corroboration from our previous studies on sub-cellular localization of leptin and its receptor in cancer breast tissue [34], we further hypothesize that reducing serum leptin levels or blocking leptin signal transduction pathway might be a prospective therapeutic target in the treatment and prevention of IDC.
Acknowledgement
This research was funded by the International Islamic University Malaysia under Endowment B fund. This work in not indebted to any commercial organization, neither any of the authors has any conflict of interest regarding the work. We thank all the faculties, friends and staffs in Basic Medical Sciences Department for their cordial and invaluable assistance.
References
- Siegel RL, Miller KD, Jemal A (2016) Cancer statistics, 2016. CA Cancer J Clin 66: 7-30. doi:10.3322/caac.21332.
- WHO (2016) Breast cancer: prevention and control, Cancer.
- Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, et al. (2015) Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer 136: E359-86.
- Siegel RL, Miller KD, Jemal A (2015) Cancer statistics, 2015. CA Cancer J Clin 65: 5-29.
- Lennon H, Sperrin M, Badrick E, Renehan AG (2016) The Obesity Paradox in Cancer: a Review. Curr Oncol Rep 18: 56.
- Chan DSM, Vieira AR, Aune D, Bandera EV, Greenwood DC, et al. (2014) Body mass index and survival in women with breast cancer-systematic literature review and meta-analysis of 82 follow-up studies. Ann Oncol 25: 1901-1914.
- Wang X-L, Jia C-X, Liu L-Y, Zhang Q, Li Y-Y, et al. (2013) Obesity, diabetes mellitus, and the risk of female breast cancer in Eastern China. World J Surg Oncol 11: 71.
- Booth A, Magnuson A, Fouts J, Foster MT (2016) Adipose tissue: an endocrine organ playing a role in metabolic regulation. Horm Mol Biol Clin Investig 26: 25-42.
- Lipsey CC, Harbuzariu A, Daley-Brown D, Gonzalez-Perez RR (2016) Oncogenic role of leptin and Notch interleukin-1 leptin crosstalk outcome in cancer. World J Methodol 6: 43-55.
- Friedman JM (1997) Leptin, leptin receptors and the control of body weight. Eur J Med Res 2: 7-13.
- Zhang F, Chen Y, Heiman M, Dimarchi R (2005) Leptin: structure, function and biology. Vitam Horm 71: 345-372.
- Tartaglia LA, Dembski M, Weng X, Deng N, Culpepper J, et al. (1995) Identification and expression cloning of a leptin receptor, OB-R. Cell 83: 1263-1271.
- Lee GH, Proenca R, Montez JM, Carroll KM, Darvishzadeh JG, et al. (1996) Abnormal splicing of the leptin receptor in diabetic mice. Nature 379: 632-635.
- Considine RV, Sinha MK, Heiman ML, Kriauciunas A, Stephens TW, et al. (1996) Serum immunoreactive-leptin concentrations in normal-weight and obese humans. N Engl J Med 334: 292-295.
- Sari A (2013) The relationship between leptin and fatty acid. J Mol Biomark Diagn 4: 2.
- Hu X, Juneja SC, Maihle NJ, Cleary MP (2002) Leptin--a growth factor in normal and malignant breast cells and for normal mammary gland development. J Natl Cancer Inst 94: 1704-1711.
- Esper RM, Dame M, McClintock S, Holt PR, Dannenberg AJ, et al. (2015) Leptin and Adiponectin Modulate the Self-renewal of Normal Human Breast Epithelial Stem Cells. Cancer Prev Res (Phila) 8: 1174-1183.
- Morioka T, Asilmaz E, Hu J, Dishinger JF, Kurpad AJ, et al. (2007) Disruption of leptin receptor expression in the pancreas directly affects beta cell growth and function in mice. J Clin Invest 117: 2860-2868.
- Chen C, Chang YC, Liu CL, Liu TP, Chang KJ, et al. (2007) Leptin induces proliferation and anti-apoptosis in human hepatocarcinoma cells by up-regulating cyclin D1 and down-regulating Bax via a Janus kinase 2-linked pathway. Endocr Relat Cancer 14: 513-529.
- Hardwick JC, Van Den Brink GR, Offerhaus GJ, Van Deventer SJ, Peppelenbosch MP (2001) Leptin is a growth factor for colonic epithelial cells. Gastroenterology 121: 79-90.
- Tsuchiya T, Shimizu H, Horie T, Mori M (1999) Expression of leptin receptor in lung: leptin as a growth factor. Eur J Pharmacol 365: 273-279.
- Masuzaki H, Ogawa Y, Sagawa N, Hosoda K, Matsumoto T, et al. (1997) Nonadipose tissue production of leptin: leptin as a novel placenta-derived hormone in humans. Nat Med 3: 1029-1033.
- Steppan CM, Crawford DT, Chidsey-Frink KL, Ke H, Swick AG (2000) Leptin is a potent stimulator of bone growth in ob/ob mice. Regul Pept 92: 73-78.
- Gonzalez RR, Cherfils S, Escobar M, Yoo JH, Carino C, et al. (2006) Leptin signaling promotes the growth of mammary tumors and increases the expression of vascular endothelial growth factor (VEGF) and its receptor type two (VEGF-R2). J Biol Chem 281: 26320-26328.
- Surmacz E (2013) Leptin and adiponectin: emerging therapeutic targets in breast cancer. J Mammary Gland Biol Neoplasia 18: 321-332.
- Uddin S, Bu R, Ahmed M, Abubaker J, Al-Dayel F, et al. (2009) Overexpression of leptin receptor predicts an unfavorable outcome in Middle Eastern ovarian cancer. Mol Cancer 8: 74.
- Hoon Kim J, Lee SY, Myung SC, Kim YS, Kim T-H, et al., (2008) Clinical significance of the leptin and leptin receptor expressions in prostate tissues. Asian J Androl 10: 923-928
- Howard JM, Beddy P, Ennis D, Keogan M, Pidgeon GP, et al. (2010) Associations between leptin and adiponectin receptor upregulation, visceral obesity and tumour stage in oesophageal and junctional adenocarcinoma. Br J Surg 97: 1020-1027.
- Ishikawa M, Kitayama J, Nagawa H (2004) Enhanced expression of leptin and leptin receptor (OB-R) in human breast cancer. Clin Cancer Res 10: 4325-4331.
- Niu J, Jiang L, Guo W, Shao L, Liu Y, et al. (2013) The Association between Leptin Level and Breast Cancer: A Meta-Analysis. PLoS One 8: e67349.
- Mantzoros CS, Bolhke K, Moschos S, Cramer DW (1999) Leptin in relation to carcinoma in situ of the breast: a study of pre-menopausal cases and controls. Int J Cancer 80: 523-526.
- Coskun U, Günel N, Toruner FB, Sancak B, Onuk E, et al. (2003) Serum leptin, prolactin and vascular endothelial growth factor (VEGF) levels in patients with breast cancer. Neoplasma 50: 41-46.
- Petridou E, Papadiamantis Y, Markopoulos C, Spanos E, Dessypris N, et al. (2000) Leptin and insulin growth factor I in relation to breast cancer (Greece). Cancer Causes Control 11: 383-388.
- Al-Shibli SM, Amjad NM, Al-Kubaisi MK, Mizan S (2017) Subcellular localization of leptin and leptin receptor in breast cancer detected in an electron microscopic study. Biochem Biophys Res Commun 482: 1102-1106.
- Chen DC, Chung YF, Yeh YT, Chaung HC, Kuo FC, et al. (2006) Serum adiponectin and leptin levels in Taiwanese breast cancer patients. Cancer Lett 237: 109-14.
- Mohammadzadeh G, Ghaffari MA, Bafandeh A, Hosseini SM (2014) Association of serum soluble leptin receptor and leptin levels with breast cancer. J Res Med Sci 19: 433-438.
- Chen C, Chang YC, Liu CL, Chang KJ, Guo IC (2006) Leptin-induced growth of human ZR-75-1 breast cancer cells is associated with up-regulation of cyclin D1 and c-Myc and down-regulation of tumor suppressor p53 and p21WAF1/CIP1. Breast Cancer Res Treat 98: 121-132.
- Schapher M, Wendler O, Gröschl M, Schäfer R, Iro H, et al. (2009) Salivary leptin as a candidate diagnostic marker in salivary gland tumors. Clin Chem 55: 914-922.
- Kim YJ, Kim YS, Chin S, Yoon JS, Lee SY, et al. (2015) Cytoplasmic and nuclear leptin expression in lacrimal gland tumours: a pilot study. Br J Ophthalmol 99: 1306-1310.
- Rehem RA, Elwafa WA, Elwafa RA, Abdel-Aziz TE (2014) Study of serum leptin in well-differentiated thyroid carcinoma: correlation with patient and tumor characteristics. World J Surg 38: 2621-2617.
- Hill RA, Margetic S, Pegg GG, Gazzola C (1998) Leptin: its pharmacokinetics and tissue distribution. Int J Obes Relat Metab Disord 22: 765-770.
- Wong SL, DePaoli AM, Lee JH, Mantzoros CS (2004) Leptin hormonal kinetics in the fed state: effects of adiposity, age, and gender on endogenous leptin production and clearance rates. J Clin Endocrinol Metab 89: 2672-2677.
- Ostlund RE, Yang JW, Klein S, Gingerich R (1996) Relation between plasma leptin concentration and body fat, gender, diet, age, and metabolic covariates. J Clin Endocrinol Metab 81: 3909-3913.
- Maffei M, Halaas J, Ravussin E, Pratley RE, Lee GH, et al. (1995) Leptin levels in human and rodent: measurement of plasma leptin and ob RNA in obese and weight-reduced subjects. Nat Med 1: 1155-1161.
- Takahashi M, Funahashi T, Shimomura I, Miyaoka K, Matsuzawa Y (1996) Plasma leptin levels and body fat distribution. Horm Metab Res 28: 751-752.
- Wauters M, Mertens I, Considine R, De Leeuw I, Van Gaal L (1998) Are leptin levels dependent on body fat distribution in obese men and women? Eat Weight Disord 3: 124-130.
 Spanish
Spanish  Chinese
Chinese  Russian
Russian  German
German  French
French  Japanese
Japanese  Portuguese
Portuguese  Hindi
Hindi