Research Article, J Clin Exp Oncol Vol: 6 Issue: 7
Single Versus Double Ring Structure: Search for Best Anti-Neoplastic Driver in Colon and Pancreatic Cancer Cells-Taurultam or Taurolidine?
Buchholz M1*,#, Berg J1#, Braumann C1, Majchrzak-Stiller B1, Hahn S2, Pfirrmann RW3, UhI W1 and Chromik AM4
1Department of General and Visceral Surgery, St. Josef-Hospital, Ruhr- University Bochum, Germany
2Department of Molecular Gastrointestinal Oncology, Ruhr-University Bochum, Germany
3Geistlich Pharma AG, Wolhusen, Switzerland
4Department of General and Visceral Surgery, Asklepios Klinikum Harburg, Hamburg-Harburg, Germany
*Corresponding Author : Buchholz M
Department of General and Visceral Surgery, Division of Molecular and Clinical Research, St. Josef-Hospital, Ruhr-University Bochum, 44791 Bochum, Germany
Tel: +49 234 509 6236
Fax: +49 234 509 3595
E-mail: marie.buchholz-a7y@rub.de
#These authors contributed equally for this work
Received: September 08, 2017 Accepted: October 11, 2017 Published: October 18, 2017
Citation: Buchholz M, Berg J, Braumann C, Majchrzak-Stiller B, Hahn S, et al. (2017) Single Versus Double Ring Structure: Search for Best Anti-Neoplastic Driver in Colon and Pancreatic Cancer Cells -Taurultam or Taurolidine?. J Clin Exp Oncol 6:6. doi: 10.4172/2324-9110.1000205
Abstract
Background: Since the molecular mechanism of the well-known anti-infective and antineoplastic substance Taurolidine (TRD) is still unknown, we sought to analyze the anti-neoplastic capacity of its main metabolite Taurultam (TAU) in malignant human cell lines derived from pancreatic cancer (AsPC-1, BxPC-3, HPAF II, MiaPaca-2, Panc-1) and colon cancer (SW-480, HT-29 and HCT-116) in vitro.
Methods: Cell lines were incubated with TAU or TRD in increasing concentrations for 24h and 48h. Comprehensive analyses were performed to quantify the anti-neoplastic activity of TAU: Analysis of cytotoxicity via MTT-assay, inhibition of proliferation via BrdU and induction of apoptosis and necrosis via FACS-analysis. Furthermore, cell growth was monitored using a real-time cell analyzer.
Results: TAU revealed a significant cytotoxic and anti-proliferative effect on all pancreatic and colon cancer cell-lines as well in MTT- and BrdU- assays as in the real-time cell analyzer. Furthermore, FACS analyses were characterized by a significant apoptotic and necrotic response upon stimulation with TAU. In contrast to TRD antineoplastic effects were noticeable lower.
Conclusion: It could be demonstrated for the first time, that TAU provides antineoplastic effects operating through mechanisms like its parent compound TRD. However, our results show clearly that TAU is not the only anti-neoplastic active metabolite of TRD. Hence, our data suggest that the efficiency of TRD against cancer cells is rather based on the methylol-containing species released during hydrolysis. These promising results are the first step towards the development of a novel substance combining the high anti-neoplastic capacity of TRD with better molecular properties of TAU, like a higher solubility in aqueous solution.
Keywords: MTaurolidine; Taurultam; Apoptosis; Chemotherapy; Cancer; Methylol-containing species; Gastrointestinal cancer
Abbreviations
Annexin V-FITC: Annexin V-Fluorescein; BrdU: 5-bromo-2-deoxyuridine; CI: Cell index; DMEM: Dulbecco’s modified eagle medium; DMSO: Dimethylsulfoxide; ELISA: Enzyme Linked Immunosorbent Assay; FACS: Fluoreszcence-activated cell scanning; NAC: N-acetlycysteine; PCD: Programmed cell death; PDAC: Pancreatic ductal adenocarcinoma; PI: Propidiumiodide; RPMI 1640: Roswell park memorial institute medium 1640; TRD: Taurolidine; TAU: Taurultam; Z-VAD: carbobenzoxy-valyl-alanylaspartyl-[ O- methyl]- fluoromethylketone
Introduction
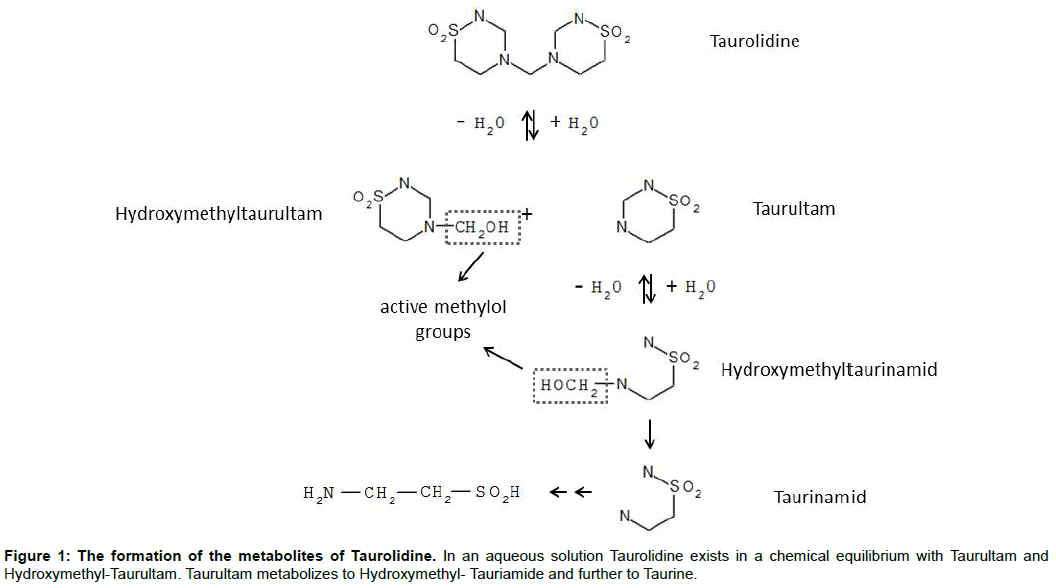
Taurultam is a metabolite of Taurolidine (bis (1,1-dioxoperhydro -1,2,4 - thiadiazinyl - 4) methane) and consists of one annular structure [1]. Two molecules of Taurultam rise by hydrolysis of Taurolidine (double ring structure) and are metabolized first to tauriamide and further to Taurine and CO2 [2]. Originally taurolidine is an antimicrobial substance, which is successfully used in the treatment of bacterial and fungal induced infections, like peritonitis [4,5]. Furthermore Taurolidine is used for the successful treatment and prophylaxis of catheter associated infections [6,7]. This antimicrobial effect has caused by Methylol-containing species, which are created during the hydrolysis of Taurolidine (Figure 1) and interact with bacterial and fungal cell walls [8,9].
In 1997 the antineoplastic effect of Taurolidine was first demonstrated by Jacobi et al., in vitro in colon carcinoma cells and in vivo in a rat model [10]. Additional investigations confirmed this effect in different malignant cells in vitro [11-16] as well as in vivo [14,16-19]. The mechanisms of its anti-neoplastic activity are not completely understood yet, but it is known that in an aqueous solution a chemical equilibrium between Taurolidine and (one of) its metabolite Taurultam exists, where the equilibrium is strongly shifted in the direction of the metabolite and Taurolidine is presented in a low concentration (2-10%) [2]. Therefore, the aim of this investigation was to examine the anti-neoplastic effect of Taurultam the major breakdown product separately for the first time, to find out whether the anti-neoplastic activity of Taurolidine mainly relies on the effects of this metabolite. The antineoplastic effect of the single ring structured Taurultam was never evaluated using human cancer cell lines before. Therefore this study focuses on the anti-proliferative capacity, the induction of cell death and the dose depending formation of apoptosis and necrosis by Taurultam using different established human cancer cell lines from colon carcinoma and pancreatic cancer. These model cell lines were tested for the effects of Taurolidine and showed the desired response in former investigations [20,21].
Material and Methods
Cell culture and cell lines
Six different cancer cell lines: AsPC-1, CLS Cell Lines Service, Eppelheim, Germany, BxPC-3 ATCC - LGC Standards GmbH, Wesel, Germany, HPAF II, HCT116, SW480, HT 29 American Type Culture Collection, and Manassas were used in this study. HPAF II cells were cultured in Eagles’ Minimum Essential Medium (MEM Eagle) (PAN Biotech GmbH, Aidenbach, Deutschland). AsPC-1 cells and BxPC-3 cells were cultured in RPMI 1640 (PAN Biotech GmbH, Aidenbach), the remaining cell lines (HCT116, HT29 and SW480) were cultured in Dulbecco`s Modified Eagle Medium (PAN Biotech GmbH, Aidenbach, Germany). Cell cultures were supplemented with 10% fetal bovine serum (Biowest Ltd., Nuaillé, Frankreich), 1% Penicillin (10000 U/ml) and Streptomycin (10000 U/ml) (Biowest Ltd., Nuaillé, Frankreich) as well as 1% L-Glutamin (Biowest Ltd., Nuaillé, Frankreich). The cells were cultured at 5 % (v/v) CO2, 37.0ºC and 95% humidity.
Reagents
Taurolidine (TRD) and Taurultam (TAU) were synthetized, collected and given by Doctor RW Pfirrmann and therefore kindly provided by Geistlich Pharma (Wohlhusen, Switzerland). Taurultam were dissolved in double distillated water; Taurolidine was dissolved in a 5% Povidon (Geistlich Pharma AG, Wolhusen, Switzerland). To be able to compare both substances accurately Tau was always used in a doubled concentration, because 1 Mol TRD is hydrolyzed within the human body into 2 Mol of TAU.
Viability assay MTT
Cytotoxic effects were measured by colorimetric MTT assay (Sigma-Aldrich, Steinheim, Germany). Cells (45000/well) were seeded in a 96-well-format. After 24 hours, cells were treated with different concentrations of TAU for 24 h and 48 h. In order to determine a dose-response effect of TAU, various concentrations of TAU (100 μM, 200 μM, 500 μM, 1000 μM, 1500 μM and 2000 μM) were used. Two concentrations of TRD (250 μM and 1000 μM) served as positive controls, whereas the solvent of the respective substances (5% Povidon solution and double-distilled water) were used as negative controls.
BrdU proliferation assay
Anti-proliferative effect was measured by BrdU (5-bromo- 2’-deoxyuridine) incorporation (Cell Proliferation ELISA, BrdU colorimetric, Roche Applied Science, Mannheim, Germany). Cells (45000/well) were seeded in a 96-well-plate. After 24 h the medium was removed and the cells were incubated with scheduled concentrations of TAU (100 μM, 200 μM, 500 μM, 1000 μM, 1500 μM and 2000 μM). After an incubation period of 6 h the BrdU reagent was added and left for additional 2 h, before cells were introduced to the BrdU proliferation assay (Roche), following the manufactures instructions. Positive and negative controls were used according to the MTT assay. The incubation period add up to 8 h, which showed to be an adequate incubation time in previous investigations [22].
Flow cytometry analysis
Two pancreas carcinoma (AsPc-1, BxPC-3) and two colon carcinoma cell lines (HCT116 and SW480) were incubated with various concentrations of TAU (200 μM, 500 μM and 2000 μM) and TRD (250 μM and 1000 μM) and analyzed by flow cytometry. A 5% povidone solution and double distilled water served as controls. After 24h all cells were collected and resuspended in binding buffer (rhannexin V -FITC kit, Bender Med Systems GmbH, Vienna, Austria). Dilutions of 200 cells/ μl were stained using Annexin V-FITC (BD Biosciences, Heidelberg, Germany) and Propiumiodid (BenderMed Systems GmbH, Vienna, Austria). Dot plots were analyzed using Cell Quest Pro software. Annexin V-FITC negative and PI negative cells were considered viable. Annexin V-FITC positive however PI negative cells were assumed as apoptotic. Annexin V-FITC negative and PI positive cells were labeled necrotic.
xCELLigence system
Cells (6000/well) were seeded in a 16-well-e-plate format and incubated at 37°C with 5% CO2 for 24 h before treated with scheduled concentrations of TAU (200 μM, 500 μM, 2000 μM) or TRD (100 μM, 1000 μM). Cells were monitored by the xCELLigence system every 10 minutes for up to 48 h. Double distilled water and 5% povidone solution served as controls.
Statistical analysis
Depending on the cell line and the assay, four to six passages were incubated with TAU or TRD. Results of the viability Assay MTT, the proliferation assay BrdU and the flow cytometric analysis were statistically evaluated using the data processing program Graphpad Prism software. The results were expressed as mean value and its standard deviation (mean ± SEM).
For comparison one way ANOVA with Tukey’s post-hoc test was performed. P-values ≤ 0.05 were regarded as statistically significant: *=p ≤ 0.05; **=p ≤ 0.01; *** =p ≤ 0.001, n.s.= not significant.
Results
Taurultam and Taurolidine introduce cell death in different cancer cell lines
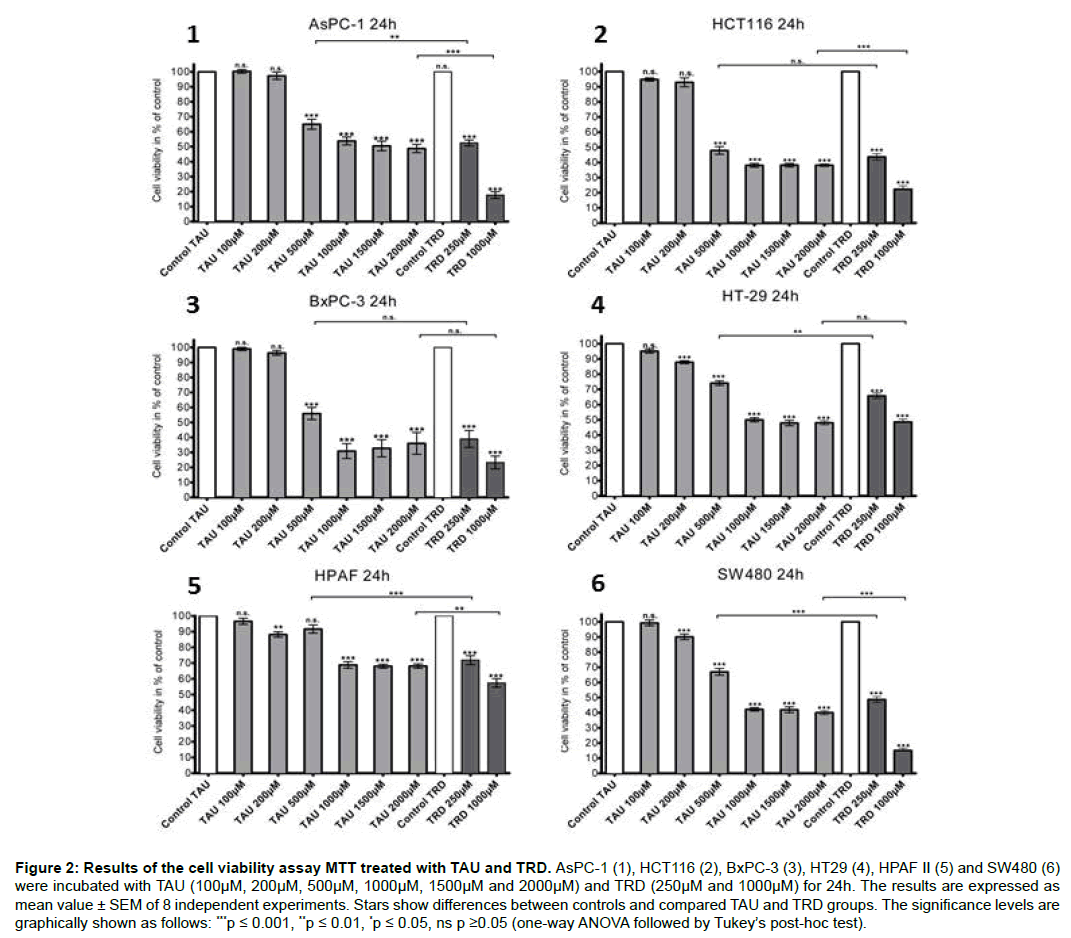
To evaluate the cytotoxic effect of TAU compared to TRD MTT assays were used. The results of the viability assay after treatment with several concentrations of TAU and TRD over 24h and 48h showed a statistically significant decrease of cell viability in all cell lines. After 24h (Figure 2) a response was noticed starting with a concentration of 200 μM TAU in three cell lines (HPAF II, HT-29 and SW480). Values of cell viability rates were measured between 90.1 ± 1.9% (SW480) and 87.9 ± 0.9% (HT-29). In the presence of 1000 μM TAU all cell lines showed a reduction of cell viability, reaching values from 68.9 ± 2.1% (HPAF II) up to 30.9 ± 4.9% (BxPC-3). Cells incubated with 1500 μM and 2000 μM showed also massive cell death. Similar results appeared after 48h (data not shown). The strongest decrease of cell viability was observed from 1000 μM up to 2000 μM, leading to cell viability rates between 50.0 ± 2.8% (HPAF II) and 13.7 ± 5.1% (BxPC-3). However, TRD tends to be more effective in the reduction of viable cells, where both concentrations of TRD (250 μM and 1000 μM) resulted in a stronger significant reduction of viable cells than TAU, except in one cell line (BxPC-3).
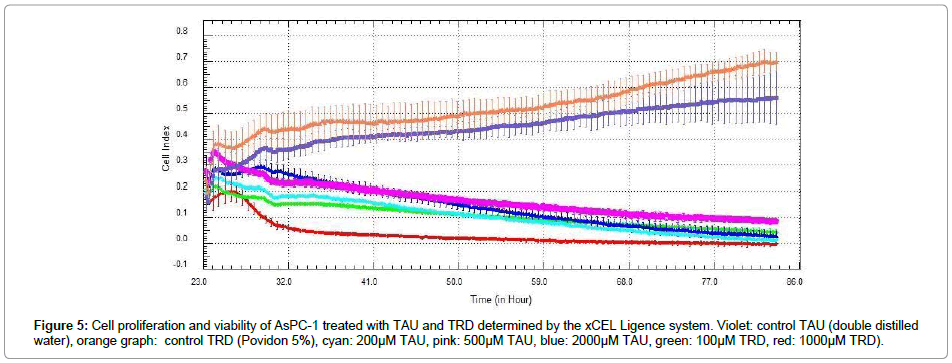
Figure 2: Results of the cell viability assay MTT treated with TAU and TRD. AsPC-1 (1), HCT116 (2), BxPC-3 (3), HT29 (4), HPAF II (5) and SW480 (6) were incubated with TAU (100μM, 200μM, 500μM, 1000μM, 1500μM and 2000μM) and TRD (250μM and 1000μM) for 24h. The results are expressed as mean value ± SEM of 8 independent experiments. Stars show differences between controls and compared TAU and TRD groups. The significance levels are graphically shown as follows: ***p ≤ 0.001, **p ≤ 0.01, *p ≤ 0.05, ns p ≥0.05 (one-way ANOVA followed by Tukey’s post-hoc test).
TAU inhibits proliferation of different cancer cell lines in a dose depended manor
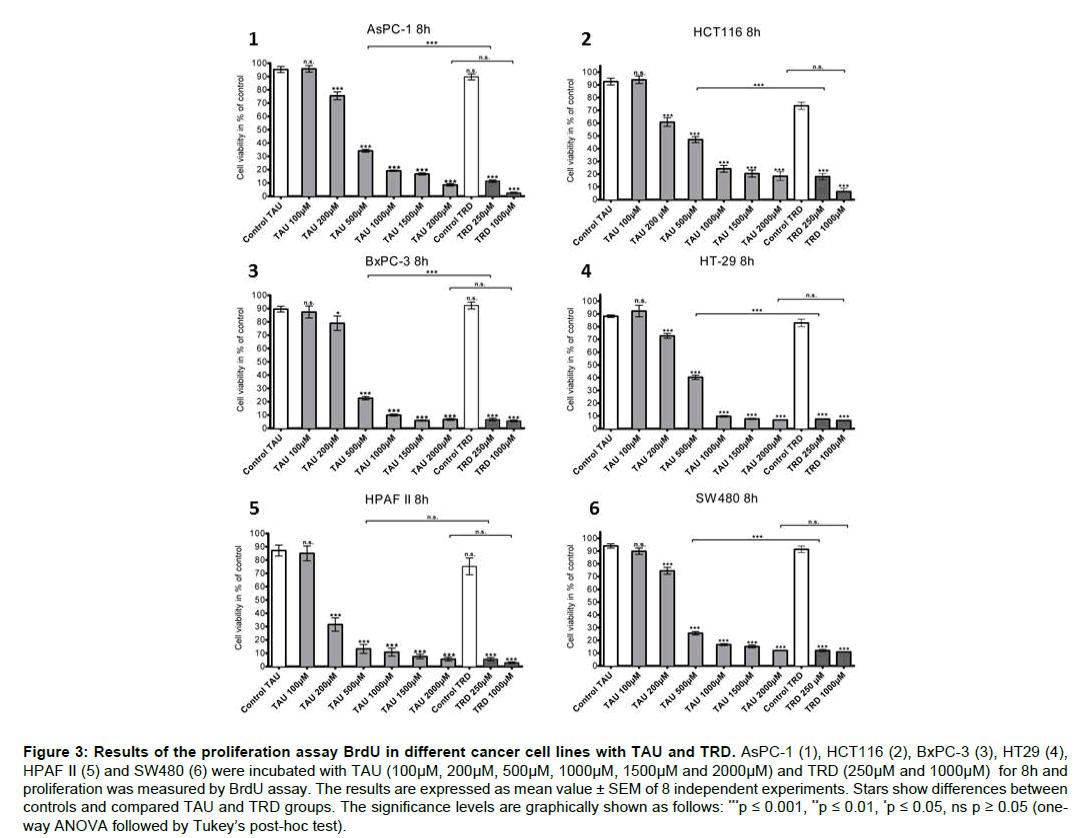
BrdU proliferation test was used to investigate the antiproliferative effect of TAU compared to TRD. The results, shown in Figure 3, indicate that TAU reduces cell proliferation significantly in a dose depended manor, starting with a concentration of 200 μM. Treatment leads to values between 79.0 ± 5.5% (BxPC-3) and 31.6 ± 5.0% (HPAF II). The values of the proliferation rate at the highest concentration of TAU ranked between 18.4 ± 3.4% (HCT116) and 5.5 ± 1.4% (HPAF II). Comparing the results of TAU (500 μM) and TRD (250 μM), TRD caused a significantly higher decrease of cell proliferation in all treated cell lines, except HPAF II. There were no significant differences between the higher concentrations (TAU 2000 μM and TRD 1000 μM) in all tested cancer cell lines. Overall, these results indicate that TAU shows a dose depended effect on cell proliferation.
Figure 3: Results of the proliferation assay BrdU in different cancer cell lines with TAU and TRD. AsPC-1 (1), HCT116 (2), BxPC-3 (3), HT29 (4), HPAF II (5) and SW480 (6) were incubated with TAU (100μM, 200μM, 500μM, 1000μM, 1500μM and 2000μM) and TRD (250μM and 1000μM) for 8h and proliferation was measured by BrdU assay. The results are expressed as mean value ± SEM of 8 independent experiments. Stars show differences between controls and compared TAU and TRD groups. The significance levels are graphically shown as follows: ***p ≤ 0.001, **p ≤ 0.01, *p ≤ 0.05, ns p ≥ 0.05 (oneway ANOVA followed by Tukey’s post-hoc test).
Taurultam induces apoptotic cell death in human cancer cells
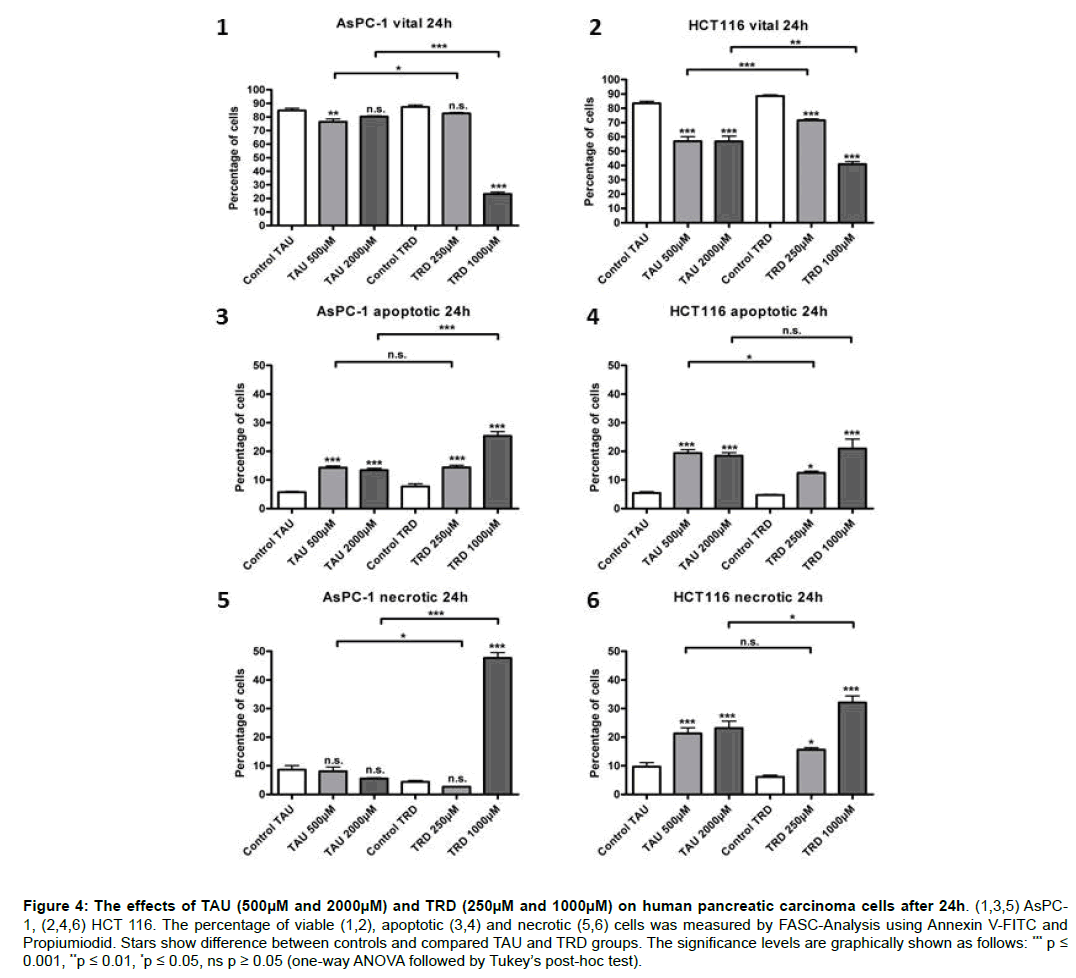
FACS analyses were performed to compare the apoptotic and necrotic effects of both substances. All cell lines (AsPc-1, BxPC-3, HCT116 and SW480) were incubated with different concentrations of TAU and TRD for 24h. The results show a significant decrease of viable cells and an increase of apoptotic and necrotic cells in comparison to the untreated controls. The poorest response to TAU was observed in the cell line AsPC-1. The lower concentration of TAU (500 μM) caused a significant decrease of viable cells 76.5 ± 2.1% and an increase of apoptotic cells (14.3 ± 0.6%) after 24h. A significant increase of apoptotic cells 13.4 ± 0.7% was also caused by the highest concentration (2000 μM), however without a reduction of viable cells (Figure 4). These results suggest a V-shape dose response effect among the cells. Likewise, the colon carcinoma cell line SW480 presented a V-shape dose effect (data not shown), where the lower tested concentration of TAU (500 μM) leaded to a stronger response of the cells (47.2 ± 3.7%) than the higher (2000 μM) concentration (57.6 ± 3.3%). Both concentrations achieved a significant increase of apoptotic cells without any increase of necrosis, values ranging between 49.5 ± 3.7% (500 μM) and 36.1 ± 3.7% (2000 μM). Interestingly, in BxPC-3 (data not shown) and HCT116 (Figure 4) an increase of necrotic cells in addition to apoptotic cells under treatment with TAU was observed, reaching maximum values of 11.3 ± 0.3% (500 μM) necrotic cells in BxPC-3 and 23.1 ± 2.5% (2000 μM) in HCT116.
Figure 4: The effects of TAU (500μM and 2000μM) and TRD (250μM and 1000μM) on human pancreatic carcinoma cells after 24h. (1,3,5) AsPC-1, (2,4,6) HCT 116. The percentage of viable (1,2), apoptotic (3,4) and necrotic (5,6) cells was measured by FASC-Analysis using Annexin V-FITC and Propiumiodid. Stars show difference between controls and compared TAU and TRD groups. The significance levels are graphically shown as follows: *** p ≤ 0.001, **p ≤ 0.01, *p ≤ 0.05, ns p ≥ 0.05 (one-way ANOVA followed by Tukey’s post-hoc test).
Considering the results of the cell line BxPC-3 compared with the untreated control, TAU achieved a statistically significant reduction of viable cells in a dose depending manor, resulting in 45.1 ± 0.4% (2000 μM). Furthermore, TAU implied a statistically significant increase of apoptotic cells, leading to results of 29.4 ± 0.4% (500 μM) and 29.9 ± 0.6% (2000 μM).
A significant effect on cell viability of HCT116 cells was caused by treatment with both concentrations of TAU (500 μM and 2000 μM) leading to an increase of apoptotic cells. A Maximum of 56.9 ± 3.2% (500 μM) viable and 19.4 ± 1.2% (500 μM) apoptotic cells was reached. Treatment with 2000 μM TAU could not increase the pro-apoptotic effect, thus the dose response effect did not appear proportional. Comparative, two concentrations of TRD (250 μM and 1000 μM) were used because previous investigations showed that 250 μM was the lowest concentration with a significant effect on human cancer cells and 1000 μM TRD caused massive rise of apoptotic and necrotic cells [21].
Comparing the two lowest concentrations of TAU (500 μM) and TRD (250 μM), TAU was able to reduce the number of viable cells similar effective than TRD. This effect was especially determined by an increase of apoptotic cells. However, the concentration of 1000 μM TRD was able to reduce the number of viable cells more effectively (p<0.001) than TAU (2000 μM) in all examined cell lines. Interestingly the amount of reduction of viable cells by TRD was especially caused by necrosis and less apoptosis (Figure 4) in contrast to treatment with TAU, which caused rather an increase of apoptosis than necrosis.
Analysis by the xCELLigence system
To evaluate the effects on cell proliferation and viability the real time, label free cell proliferation assay xCELLigence was performed. Figure 5 illustrates the results of the proliferative viability measurement by the system of the cell line AsPC-1 treated with various concentrations of TAU and TRD. A reduction of the CI of all tested concentrations was observed. Especially the concentration of 1000 μM TRD showed an early massive drop of the cell index, not achieved by the concentration of 2000 μM TAU. The concentrations of 200 μM TAU and 100μM TRD caused a comparative drop of the CI. The concentrations 500 μM and 2000 μM TAU resulted in the poorest drop in the growth curve. All other tested cell lines showed a similar response to the treatment with TAU and TRD (data not shown).
Discussion
The anti-neoplastic effect of TRD was already shown in prior studies by cell viability assays like MTT [20,23] as well as by cell proliferation assays like BrdU [12,16,22] and FACS analysis [12,16,22,23]. However, there was no data in regards to the antineoplastic effect by its major breakdown product TAU on human cancer cells in the literature by now. This study was conducted to determine the anti-neoplastic activity of TAU on human pancreatic and colon cancer cell lines for the first time to elucidate whether the anti-neoplastic effect of TRD is mainly caused by its metabolite TAU. We were able to show, that TAU, as TRD, caused a significant reduction of viable cells in all analyzed cell lines (AsPC-1, BxPC-3, HCT116 and SW480) in a dose depending manor, determined by MTT assay. However, the results showed that TRD appeared to be more effective to reduce cell viability in pancreatic and colon cancer cell lines.
Similar findings were observed by cell proliferation assay with BrdU. Here, both substances showed a proportional dose response reduction of the cell proliferation (Figure 3). In comparison with the cytotoxic effect measured by MTT an anti-proliferative effect could be observed at a lower concentration of TAU in all analyzed cell lines. These findings suggest that the anti-proliferative effect of TAU might be slightly stronger than the cytotoxic effect. Furthermore, these results match those observed in earlier studies, where a proportional dose response of cell proliferation was observed under treatment with TRD [22]. For a concentration of 250 μM TRD, a statistically stronger decrease of cell proliferation was observed compared to a concentration of 500 μM TAU. Even though, both substances showed an anti-proliferative effect, the findings clearly suggest that TRD seemed to have a stronger effect on the analyzed colon and pancreatic cancer cells.
Therefore, we conclude that the antineoplastic activity of TAU is less effective than the capacity of TRD, even when used in an adjusted quantity of 2 Mol. Hence, there is some evidence, that the metabolite and major breakdown product TAU might not be the only effective component of TRD.
A plausible explanation for these findings is that the antineoplastic effect of TRD is caused by methylol-containing species, which are already known to interact with bacterial and fungal cell walls thereby mediating the bactericidal effect of the substance [8,9]. Based on our results, we assume that these species could also generate the antineoplastic effect of TRD. On this context, the different efficiency of both substances could be explained by the different amount of methylol-containing species released during hydrolysis (Figure 1), because during hydrolysis of 1 Mol TAU less methylol-containing species are released than during hydrolysis of 1 Mol TRD.
FACS analysis supported the results of both viability assays within this study. The lowest effective concentration of TAU at about 500 μM was observed among all analyzed cell. Surprisingly, differences were found between the dose response effects of TAU among the four treated cell lines analyzed by FACS analysis. In AsPC-1 and SW480 no direct dose response was observed. Here a concentration of 500 μM TAU implied a slightly bigger decline of cell viability whereas the higher (2000 μM) concentration was less effective (Figure 4). HCT116 cells showed a similar response within all tested concentrations of TAU. BxPC-3 was the only cell line, which was characterized by a proportional dose effect, observed after treatment with TAU. Our results are in a line with the findings of other studies, which also observed these different dose-response patterns between different cancer cell lines after treatment with TRD [13,21].
Contrary to our expectations, the results of this study show that the treatment with TRD resulted in a statistically significant (p<0.001) bigger rise of necrotic cells than the treatment with TAU. Treatment with TAU caused rather an increase of apoptosis than necrosis. We can only speculate about the reason of these findings; however the design of our analysis could reveal a possible explanation. Apoptosis leads to caspases-depending cell destruction and further to phagocytosis of the apoptotic cells. In a cell culture setting, which lacks phagocytosis a secondary necrosis follows, which is characterized by the same appearance of primary necrosis [24]. In our in vitro studies there is no possibility of phagocytosis by inflammatory cells, which are not present in cell cultures. The marker used for necrosis (PI) could ultimately not differentiate between a substance-induced primary necrosis and secondary necrosis due to the lack of phagocytosis. Further work is required to clarify whether TRD or TAU lead to the induction of primary necrosis within tumor cells.
The xCELLigence system continuously monitors cell adhesion and cell proliferation for real time and label-free. It was used successfully in different studies to quantify the response of human cancer cells to anti-neoplastic substances [25,26]. The growth curves of the untreated controls showed characteristic profile of cell growth. In contrast cells treated with TAU and TRD showed the expected response among all cell lines, resulting in an obvious slope of the growth curves. These findings are in agreement with those observed in the MTT and BrdU assays as well as with the FACS analysis of our study. Again, the results advise that the treatment with TRD led to a stronger growth reduction compared to the treatment with TAU among all cancer cell lines.
Conclusion
TAU has an anti-proliferative and cytotoxic effect on different colon and pancreatic cancer cell lines. However, the results advise clearly, that TRD has a stronger effect on cancer cells than its metabolite TAU. These findings suggest that TAU does not cause the anti-neoplastic effect of TRD alone. Future trials should access the impact of the methylol-containing species on malignant cancer cells, which could be, based on the results of the current study, the possible mediators of the anti-neoplastic activity of TRD. The results of our study are also an important step towards extending our knowledge of the effectivity of TAU as an anti-neoplastic single ring structured molecule. In contrast to TRD, TAU shows a higher solubility in water, which would lead to higher concentrations and lower volume during therapeutic approaches. The analysis of TAU undertaken here provides the basis for further chemical modifications of TAU to increase the anti-neoplastic potential as a clinical relevant agent.
Authors’ Contributions
MB and JB made substantial contributions to conception and design, acquisition of data as well as analysis and interpretation of data. AC made substantial contributions to conception and design. SH guided the xCelligence experiments. RP synthesized the analyzed agent. BMS, CB and WU revised the manuscript. All authors have read and approved the manuscript and take public responsibility for it.
References
- Jacobi CA, Menenakos C, Braumann C (2005) Taurolidine--a new drug with anti-tumor and anti-angiogenic effects. Anticancer Drugs 16: 917-921.
- Stendel R, Scheurer L, Schlatterer K, Stalder U, Pfirrmann RW, et al. (2007) Pharmacokinetics of taurolidine following repeated intravenous infusions measured by HPLC-ESI-MS/MS of the derivatives taurultame and taurinamide in glioblastoma patients. Clin Pharmacokinet 46: 513-524.
- Gong L, Greenberg HE, Liu LL, Perhach JL, Waldman SA, et al. (2007) The pharmacokinetics of taurolidine metabolites in healthy volunteers. J Clin Pharmacol 47: 697-703.
- Linder MM, Ott W, Wesch G, Wicki O, Marti MC, et al. (1981) Therapy of purulent peritonitis. Documentation of 78 cases and experience with taurolin. Langenbecks Arch Chir 353: 241-250.
- Reith HB (1997) Therapy of peritonitis today. Surgical management and adjuvant therapy strategies. Langenbecks Arch Chir 382: S14-S17.
- Koldehoff M, Zakrzewski JL (2004) Taurolidine is effective in the treatment of central venous catheter-related bloodstream infections in cancer patients. Int J Antimicrob Agents 24: 491-495.
- Weber M, Meyer F, Halloul Z (2009) Spectrum of indications and perioperative management in i. v. port-a-cath explantation--alternative administration of taurolin in case of i. v. port-a-cath infection. Zentralbl Chir 134: 350-356.
- Staubach KH (1997) Adjuvant therapy of peritonitis with taurolidine. Modulation of mediator liberation. Langenbecks Arch Chir 382: S26-S30.
- Caruso F, Darnowski JW, Opazo C, Goldberg A, Kishore N, et al. (2010) Taurolidine antiadhesive properties on interaction with E. coli; its transformation in biological environment and interaction with bacteria cell wall. PLoS One 5: e8927.
- Jacobi CA, Sabat R, Ordemann J, Wenger F, Volk HD, et al. (1997) Peritoneal instillation of taurolidine and heparin for preventing intraperitoneal tumor growth and trocar metastases in laparoscopic operations in the rat model. Langenbecks Arch Chir 382: S31-S36.
- Aceto N, Bertino P, Barbone D, Tassi G, Manzo L, et al. (2009) Taurolidine and oxidative stress: a rationale for local treatment of mesothelioma. Eur Respir J 34: 1399-1407.
- Daigeler A, Brenzel C, Bulut D, Geisler A, Hilgert C, et al. (2008) TRAIL and Taurolidine induce apoptosis and decrease proliferation in human fibrosarcoma. J Exp Clin Cancer Res 27: 82.
- Daigeler A, Chromik AM, Geisler A, Bulut D, Hilgert C, et al. (2008) Synergistic apoptotic effects of taurolidine and TRAIL on squamous carcinoma cells of the esophagus. Int J Oncol 32:1205-1220.
- Darnowski JW, Goulette FA, Cousens LP, Chatterjee D, Calabresi P (2004) Mechanistic and antineoplastic evaluation of taurolidine in the DU145 model of human prostate cancer. Cancer Chemother Pharmacol 54: 249-258.
- Karlisch C, Harati K, Chromik AM, Bulut D, Klein-Hitpass L, et al. (2013) Effects of TRAIL and taurolidine on apoptosis and proliferation in human rhabdomyosarcoma, leiomyosarcoma and epithelioid cell sarcoma. Int J Oncol 42: 945-956.
- McCourt M, Wang JH, Sookhai S, Redmond HP (2000) Taurolidine inhibits tumor cell growth in vitro and in vivo. Ann Surg Oncol 7: 685-691.
- Braumann C, Jacobi CA, Rogalla S, Menenakos C, Fuehrer K, et al. (2007) The tumor suppressive reagent taurolidine inhibits growth of malignant melanoma--a mouse model. J Surg Res 143: 372-378.
- Braumann C, Ordemann J, Wildbrett P, Jacobi CA (2000) Influence of intraperitoneal and systemic application of taurolidine and taurolidine/heparin during laparoscopy on intraperitoneal and subcutaneous tumour growth in rats. Clin Exp Metastasis 18: 547-552.
- Hoksch B, Rufer B, Gazdhar, Bilici M, Beshay M, et al. (2009) Taurolidine in the prevention and therapy of lung metastases. Eur J Cardiothorac Surg 36: 1058-1063.
- Braumann C, Henke W, Jacobi CA, Dubiel W (2004) The tumor-suppressive reagent taurolidine is an inhibitor of protein biosynthesis. Int J Cancer 112: 225-230.
- Chromik AM, Daigeler A, Bulut D, Flier A, May C, et al. (2010) Comparative analysis of cell death induction by Taurolidine in different malignant human cancer cell lines. J Exp Clin Cancer Res 29: 21.
- Chromik AM, Hahn SA, Daigeler A, Flier A, Bulut D, et al. (2010) Gene expression analysis of cell death induction by taurolidine in different malignant cell lines. BMC Cancer 10: 595.
- Opitz I, Sigrist B, Hillinger S, Lardinois D, Stahel R, et al. (2007) Taurolidine and povidone-iodine induce different types of cell death in malignant pleural mesothelioma. Lung Cancer 56: 327-336.
- Vanden BT, Vanlangenakker N, Parthoens E, Deckers W, Devos M, et al. (2010) Necroptosis, necrosis and secondary necrosis converge on similar cellular disintegration features. Cell Death Differ 17: 922-930.
- Tan YF, Mundargi RC, Chen MH, Lessig J, Neu B, et al. (2014) Layer-by-layer nanoparticles as an efficient siRNA delivery vehicle for SPARC silencing. Small 10: 1790-1798.
- Darbre PD, Mannello F, Exley C (2013) Aluminium and breast cancer: Sources of exposure, tissue measurements and mechanisms of toxicological actions on breast biology. J Inorg Biochem 128: 257-261.
 Spanish
Spanish  Chinese
Chinese  Russian
Russian  German
German  French
French  Japanese
Japanese  Portuguese
Portuguese  Hindi
Hindi