Research Article, J Soil Sci Plant Health Vol: 2 Issue: 2
Soil Physical and Chemical Characteristics Influencing Hydrogen Sulfide Toxicity
Julia M Fryer1, Trenton L Roberts1*, Yeshi Wamishe2, Jarrod T Hardke2 and David M Miller1
1Department of Crop, Soil, and Environmental Sciences at the University of Arkansas, Fayetteville, AR, USA
2Rice Research and Extension Center, University of Arkansas Division of Agriculture, Stuttgart, AR, USA
*Corresponding Author : Trenton Roberts
Department of Crop, Soil, and Environmental Sciences, University of Arkansas, 115 Plant Science, Fayetteville, AR, USA
Tel: 479-575-6752
E-mail: tlrobert@uark.edu
Received: June 01, 2018 Accepted: June 18, 2018 Published: June 25, 2018
Citation: Fryer JM, Roberts TL, Wamishe Y, Hardke JT, Miller DM (2018) Soil Physical and Chemical Characteristics Influencing Hydrogen Sulfide Toxicity. J Soil Sci Plant Health 2:2.
Abstract
Hydrogen sulfide (H2S) toxicity is a poorly understood physiological disorder that occurs under anaerobic conditions and can cause substantial yield loss in rice (Oryza sativa L.). Though the reduction of sulfate (SO42-) to H2S is the causes of toxicity, there are many factors that influence the extent to which this occurs. Two greenhouse studies were designed to investigate the chemical and physical characteristics of four soils in Arkansas where this disorder occurs regularly (H and HR-W), sometimes occurs (HR-E), and has never been reported (PTRS). The three soils that have had this disorder (H, HR-W, and HR-E) contained approximately 30% more silt than PTRS. Mehlich 3 extractable SO42- and Fe concentrations were significantly different among the soils. In the first study, the effect of soil sterilization on SO42- concentration was examined. This study showed that SO42- concentrations over time were significantly greater in the sterilized soils from day 7-77 (p=0.0231 to <0.0001) indicating that microbes play a key role in the disappearance of SO42-. Sulfate concentrations were different from day 21-77 (p=0.0310 to <0.0001), however H and PTRS were not statistically different. Redox potential dropped more rapidly in H than PTRS, suggesting that redox potential greatly influences the occurrence of H2S toxicity. When rice was grown, there was again a statistical difference between locations (p=0.0405 to 0.0095), however H contained the most SO42- and PTRS the least. The most rapid decline in SO42- occurred after two weeks of flooding, which coincides with the onset of symptoms in the field. Within four weeks after flooding, H lost 20.7 mg SO42- kg-1 soil solution whereas PTRS lost 13.5 SO42- kg-1 soil solutions. These results indicate that the rate of SO42- reduction, decline in redox potential, and activity of microorganisms all play a role in the occurrence of H2S toxicity.
Keywords: Rice; Hydrogen sulphide; Toxicity; Soil properties
Introduction
With the value of rice sales in Arkansas reaching nearly one billion dollars [1], rice production is a vital component of the economy in Arkansas. For the past 44 years, Arkansas has been the leader in rice production in the United States, responsible for producing over half of the rice in the country [2]. In order to produce this quantity of rice, over 25,000 jobs in Arkansas are associated with rice production [3]. Because rice production provides a major food staple, creates thousands of jobs, and contributes billions to the economy, researchers must continually address challenges and develop solutions and advancements for producing quality rice crops.
In recent years, reports of H2S toxicity have increased in Arkansas. Hydrogen sulfide toxicity appears to be caused by excessive S and Fe in the root zone, though there are likely many contributing factors. Soils prone to H2S toxicity have been termed “akiochi” soils, which is Japanese for “autumn decline”. This disorder was first identified in Japan before the 1950s and was classified as a “serious physiological disease” [4]. Yoshida [5] later referred to this problem as a “nutritional disorder”, and this may, indeed, be a more accurate term. When soils are under anaerobic conditions, SO42- reduces to H2S, a gas toxic to plant roots. However, H2S typically reacts with reducible Fe3+ in the soil and precipitates out as insoluble FeS, preventing the buildup and toxicity of H2S [5]. However, in soils prone to this disorder, H2S does not precipitate out but builds up in the rhizosphere, inhibiting root respiration and nutrient and water uptake due to the lack of energy supplied from respiration [6]. If root exposure to H2S is prolonged, roots will eventually die and rot [7].
Hydrogen sulfide toxicity weakens plants causing them to be more prone to invasion by opportunistic disease organisms. Brown spot (historically referred to as Helminthosporium leaf spot), rice blast, and crown rot have all been found in increased severity on plants affected by H2S toxicity [6]. Under severe conditions, opportunistic fungi causing crown rot can invade the root crown, resulting in plant death.
The H2S toxicity phenomenon is not well understood and has been problematic to overcome. Little conclusive information on soil physical and chemical characteristics has been confirmed regarding when this disorder will occur and to what level of severity [8-10].
Physical characteristics of akiochi soils vary. In Japan and Korea, H2S toxicity is typically reported in sandy soils with low cation exchange capacity (CEC), low active Fe, high organic matter, and high soluble SO42- content [6,11-13] In Arkansas, however, based on field observations in 2004 by the University of Arkansas Cooperative Extension Service, H2S toxicity occurred in fields with a high soil pH and in soil textures from silt loam to clay loam [9].
Additionally, organic matter (OM) is an important physical characteristic for identifying when and where H2S toxicity will occur. The majority of S in soil comes from OM as microbes mineralize organic-S to SO42- [14]. Reddy et al. [15] notes that SO42- reduction to H2S naturally occurs when sufficient easily decomposable OM is present in flooded soil devoid of oxygen (O), NO-3, manganese (Mn) and Fe. Rice straw is considered easily decomposable OM [16] and would thus promote the reduction of SO42- to H2S.
The chemical transformations of S in the soil are dynamic and change with the environment. Immobilization, mineralization, sulfide production, and the production of volatile S compounds are common fates of sulphur in anaerobic soil conditions [17]. With approximately 90% of the total S under soil found as organic-S [14], the fate of S depends principally on the activity of soil microorganisms [18], particularly Desulfovibrio and Desulfotomacuclum [15,19]. Soil microbes catalyze oxidation and reduction reactions as they decompose organic materials.
Redox potential (Eh), the measure of the tendency of chemical species to gain electrons, is a useful measure of what is happening chemically in soils. Microorganisms along with abiotic factors influence the Eh of soil. Typically, Eh ranges from 700 to -300 millivolts (mV) depending on the soil pH, though may be even lower than -300 mV [20]. Though abiotic factors can influence Eh, Eh is mainly influenced by biotic factors [20]. Under flooded conditions, microbes quickly deplete the soil of dissolved O2 then move on to using the oxidized states of N, Mn oxides, Fe, and SO42- as secondary terminal electron acceptors for respiration. During anaerobic conditions, Eh decreases; pH changes; denitrification occurs; and Mn (IV), Fe (III), and SO42- are reduced. In addition, organic acids are produced and carbon dioxide (CO2) accumulates in the soil [21]. Under anaerobic conditions, the S cycle is completely microbial [15] which suggests that SO42- would not be reduced and Eh would not rapidly decline if the soil were to be sterilized prior to flooding.
The goal of this research was to investigate differences in physiochemical properties among soils in Arkansas that exhibit varying degrees of this disorder. Reduction of SO42-, changes in Eh, and the effects of soil sterilization were evaluated in an attempt to link the occurrence of H2S toxicity to specific soil physical and chemical characteristics.
Materials and Methods
Soil description
Soil was collected from three locations in Arkansas during 2015. Surface soil was collected from fields in Hunter, AR; Hickory Ridge, AR; and the Pine Tree Research Station in Colt, AR. The field in Hunter, AR (H) was reported to have symptoms of H2S toxicity every year in which rice was planted. Soil was collected separately from the east and west ends of the Hickory Ridge field, where H2S toxicity was reported to occur every year when rice was planted in the west end of the field (HR-W), and H2S toxicity occurred approximately half the time when planted to rice on the east end of the field (HR-E). Hydrogen sulfide toxicity had never been reported in rice growing on the soil collected from the Pine Tree Research Station (PTRS).
A preliminary soil test was conducted to assess pH (1:2 v:v soil:water ratio) [22], soil texture [23], total nitrogen (TN) [24], total carbon (TC) [25], soil OM via loss on ignition (LOI) [26], and Mehlich 3 extractable nutrients, P, K, and S [27]. Detailed soil chemical and physical information is listed in Table 1.
| Location ID | H2S Occurrence | Soil Series | Soil Classification | Soil Texture† | pH‡ | LOI§ | TN¶ | TC¶ | P# | K# | S# | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sand | Silt | Clay | |||||||||||
| -------g/kg-------- | ------------g/kg--------- | ----mg/kg--- | |||||||||||
| HR-W | Always | Henry Silt Loam | Coarse-silty, mixed, active, thermic Typic Fragiaqualfs | 6 | 79 | 15 | 80.1 | 20.69 | 00.1261 | 10.3694 | 24 | 71 | 14 |
| HR-E | Sometimes | Henry Silt Loam | Coarse-silty, mixed, active, thermic Typic Fragiaqualfs | 12 | 74 | 14 | 80.1 | 20.03 | 00.0661 | 00.9426 | 19 | 46 | 10 |
| H | Always | Hillemann Silt Loam | Fine-silty, mixed, active, thermic Albic Glossic Natraqualfs | 14 | 73 | 13 | 70.9 | 20.01 | 00.0677 | 00.9425 | 16 | 53 | 16 |
| PTRS | Never | Calloway Silt Loam | Fine-silty, mixed, active, thermic Aquic Fraglossudalfs | 35 | 48 | 17 | 70.6 | 20.08 | 00.0806 | 00.9128 | 70 | 114 | 9 |
Table 1: Selected soil chemical and physical properties from locations used in greenhouse experiments.
Sterilization
Soil from each location was sterilized using an autoclave. Fifteen liters of soil from each location was brought to approximately field capacity using deionized water and covered with aluminum foil. After allowing soil to sit at room temperature for three days, each location was separated into four polyethylene biohazard autoclave bags, one gallon of soil per bag. Bags were then placed onto cookie sheets and soil was spread as thin as possible to maximize surface area exposure. The soil was then autoclaved for one hour at 122°C and a pressure of 1.1 kg cm-1. Soil was then removed from the autoclave and allowed to sit at room temperature for three days to allow for any dormant microorganisms to become active. The sterilization process was repeated two more times.
Sixteen two-gallon buckets were sterilized using a dilute bleach solution. After soil was sterilized three times, each bag was emptied into a sterilized pot and covered with plastic wrap until the beginning of the experiment.
Greenhouse redox experiment using sterilized and nonsterilized soils
Prior to sterilization, all soil was sieved using a one cm screen to remove clods and large pieces of organic matter. Four gallons of nonsterilized soil from each location that had not been sterilized were divided into four 7.57-liter buckets, 3.79 liters of soil per bucket, yielding 16 buckets. Buckets were used to ensure that water would not drain out of the system. From each location non-sterilized and sterilized treatment were replicated four times, giving a total of 32 buckets. All treatments were randomized and blocked with one replication occurring in each block. The four blocks were placed over two benches of the greenhouse.
Platinum electrode redox sensors (Sensorex® electrochemical ORP sensor, Garden Grove, CA) were inserted approximately 8 cm deep in to soil in two buckets from each treatment, totaling 16 redox sensors [28]. Porous ceramic cup samplers (IRROMETER® Soil Solution Access Tube–Model SSAT, Riverside, CA) were placed in each pot. Each bucket was flooded with deionized water 10 cm above the soil surface and the flood was maintained throughout the duration of the experiment. Redox was continuously monitored by the electrodes and logged into a data logger.
A 60 cc syringe was used to extract and discard all fluid in the porous ceramic cup sampler. To extract soil solution samples, a vacuum was drawn to-60 cbar in each porous ceramic cup sampler using a hand pump vacuum. The vacuum was maintained for 3 h before the solution was collected. A clean 60 cc syringe was double rinsed with deionized water and used to extract soil solution. The extracted solution was then placed in a scintillation vial containing two drops of concentrated HCl (37%) to acidify the solution to prevent precipitation of solution constituents and to reduce microbial activity. Between each sample extraction, used syringes were double rinsed with deionized water. Sample extracts collected were then stored at room temperature until analyzed for P, K, Ca, Mg, Na, S, Fe (I), Fe (II), Mn, Zn, Cu, and B using an inductively coupled argon plasma (ICAP) spectrophotometry. Soil solution samples were extracted and analyzed following the protocol described in Gao et al. [29] at 1, 2, 7, 14, 21, 28, 35, 42, 49, 63, 77, and 91 days after flooding.
Greenhouse experiment using rice plants grown in the soil samples
After completion of the redox experiment above, a new set of soil samples from H, HR-E, HR-W, and PTRS were collected. Nonsterilized soil samples were used in this experiment. Quadruplicate 3.79 liter samples of each soil were placed in 7.57 liter buckets. Fertilizer was incorporated in the top few cm of the soil at rates of 45 kg P2O5 ha-1 and 67 kg K2O ha-1. Soil was wetted with deionized water and left to sit overnight in the greenhouse. Ten pre-germinated seeds of Cultivar ‘CL 151’ seeds were transferred to each bucket at a depth of 1.5 cm. Soil was misted with deionized water and buckets were then covered with plastic wrap to retain moisture. The plastic wrap was temporarily removed each day to mist soil with deionized water and reduce the potential for soil crusting that could interfere with emergence. After rice seedling stands were well established, seedlings were thinned to five uniform plants in each bucket. An equivalent of 692 kg urea ha-1 was added to each bucket one day prior to flooding, which provided the equivalent of 318 kg N ha-1. The rice was flooded at 5. Leaf stage (V5) with deionized water and platinum electrode redox sensors and ceramic cup samplers were inserted approximately 8 cm into the soil in each bucket. Continuous redox measurements were taken for the duration of the experiment. The flood was maintained approximately 10 cm above the soil surface. Soil solution samples from each pot were collected 1, 2, 7, 14, 21, 28, 35, 42, 49, 63, and 77 days after flooding and analyzed as described above. Soil solution samples were analyzed for P, K, Ca, Mg, Na, S, Fe (I), Fe (II), Mn, Zn, Cu, and B. Plants were monitored for signs of H2S toxicity and root blackening throughout the experiment. This experiment was carried out for 77 days. At last, all rice plant roots were washed and examined for blackening of the roots. Above ground biomass was collected, dried, and ground to pass a one mm sieve. Acid digests of plant material in concentrated HNO3 and 30% H2O2 were analyzed for S by ICAP spectrophotometry [30].
Statistical analysis
The first greenhouse experiment consisted of four locations and two treatments, non-sterilized and sterilized soil. Each treatment was replicated four times totaling 32 individual buckets. Buckets were arranged in a randomized complete block design with replications blocked. Redox sensors were placed at random in two replications across all replications. Soil solution samples were collected from each individual pot. Therefore, an analysis of variance (ANOVA) was conducted by day for soil solution data using JMP® Pro 12 (SAS Institute, Inc., Cary, NC). Comparisons were made at the p ≤ 0.05 significance level to evaluate the effects of sterilization on SO42- reduction and also differences of SO42- reduction between locations. Student’s T Test was used to separate significant means. Soil solution data were analyzed by an ANOVA. And the redox data were used to support the findings of the soil solution. The null hypothesis in this portion of the study comprised: 1) SO42- levels would decrease more rapidly in H and HR-W soils, and slower in HR-E soil and slowest in PTRS soil. 2) SO42- level in sterilized soils declines slower than in nonsterilized soils.
For the second greenhouse study, our experiment was a one factor complete randomized block design, with only one replication blocked. An ANOVA was conducted by day for soil solution data using JMP® Pro 12 (SAS Institute, Inc., Cary, NC). Comparisons were made at the α=0.05 significance level to evaluate differences in SO42- reduction between locations while rice was grown. Student’s T test was used for mean separation. The null hypothesis included: 1) SO42- would be reduced more rapidly in H2S toxicity prone soils than soils compared to soils with no history of the problem. 2) Rice growth would be affected more in soils where SO42- is reduced rapidly in H2S toxicity prone soils. Redox data were used to support soil solution findings.
Results and Discussion
Physical and chemical soil characteristics
Since the dynamics in nutrient availability in flooded systems, is different from dried soil, data collected from extractable plant nutrients, soil characterization, and soil organic matter from the dried soil may not accurately represent the nutrient status after the soil is flooded [31]. However, the data are indicative of the presence of differences among the soil sources (Table 1).
Mehlich 3 extractable S ranged from 9 to 16, with H containing the highest (16 mg S kg soil-1) and PTRS containing the lowest (9 mg S kg soil-1). Concentrations of extractable SO42-S are considered low when less than or equal to 10 mg kg-1 [32]. Due to the majority of S being contained in the organic-S pool, extractable soil nutrient analyses are not the most accurate representation of available S in the soil [31]. This organic-S pool accounts for nearly 90% of the total soil S [33]. One pathway for the release of plant available SO42- from organic-S is mineralization by microorganisms [34]. Unfortunately, there is currently no direct method to evaluate total mineralizable organic-S [35]. Organic matter in each of the soils was assessed by loss on ignition (LOI) in a muffle furnace at 360°C [36]. Organic matter estimates in these soils were fairly consistent between locations, ranging from 2.01-2.69%. Though this is an important source of SO42- as organic-S is mineralized, decomposition and release of SO42- slows considerably under anaerobic conditions [37].
Iron (Fe) appears to impact whether or not H2S toxicity occurs. Soluble Fe was measured using the Mehlich 3 soil test. Though a clear trend of soluble Fe concentration by location is not apparent, PTRS contained the highest concentration of 464 mg Fe kg soil-1, with HR-E and H containing 383 and 385 mg Fe kg soil-1, respectively. Sulfate, OM and soluble Fe all seem to interact to influence the production and toxicity of H2S. When Fe is in the insoluble form of Fe2+, formation of FeS may not occur as microbes utilize SO42- in respiration. In this situation, H2S is likely to be formed and become problematic in these soils [37]. In addition to concentrations of reducible elements such as N, Fe, and Mn, soil texture may also influence H2S toxicity. In all three locations where H2S toxicity has been known to occur, the percentage of silt ranged from 72.9-79.3% whereas PTRS soil contained 47.7%. These data bring up the question of how soil texture may influence H2S toxicity. In Japanese paddy soils, H2S toxicity generally were reported on sandy soils [6,11,13], whereas the textures of our soils in this study were all silt loams.
Effect of flooding on sulfate concentration and redox potential in 7-14 days
A significant difference in concentrations of solution SO42- between the sterilized and non-sterilized soils was found on days 7 and 14 after flooding (p=0.0231 and 0.0005 respectively). Despite this significant difference, we believe that the sterilization was not completely effective for several reasons. In a soil completely devoid of soil microbes, neither SO42- concentration nor Eh would decline to the same degree as in non-sterilized soils. In order for reducing conditions to develop in soils, it is necessary to have anaerobiosis, mineralizable OM and sufficient numbers of viable anaerobic bacteria. Reduction requires three features: anaerobic conditions, presence of OM, and activity of anaerobic bacteria [38]. Without microbes, SO42- concentrations would not decline. While abiotic forces can drive Eh, the most common driving force behind Eh changes is biotic [20]. Redox potential and SO42- concentration steadily declined from day one in both the sterilized and non-sterilized soils indicating the presence of at least a limited population of soil microbes in both treatments.
Another reason for suspecting incomplete sterilization was the germination of weeds in the sterilized soils. Sterilization may have been compromised by air contamination or the presence of highly resistant spores that withstood the sterilization process. Also, steam sterilization may not be the most effective long term sterilization method. Tanaka et al. [39] found that bacteria counts were relatively unaffected after steam sterilization. Eno and Popenoe [40] were able to obtain near complete sterilization through steam, though some fungi and bacteria were still detected in the muck soil. The incomplete sterilization in our study may be due to the nature of the sterilization technique used.
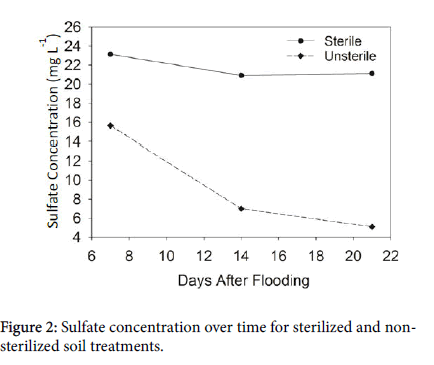
Though the soils were apparently not completely sterilized, the sterilization level we used in this study was effective to some degree. Soil solution from sterilized soil consistently contained significantly higher concentrations of SO42- than soil solution from the nonsterilized soils. This difference was statistically significant on days 7, 14 and 21 (p =0.0231, 0.0005, and <0.0001 respectively). On day 7, the sterilized soils contained 7.49 mg L-1 more sulfate than the nonsterilized soils. Since dead microbial cells contribute to OM residue, this elevated concentration of sulfate in the sterilized soil is likely due to the addition of microorganism detritus as organic-C and S as a result of sterilization [15]. Surviving microbes then had more organic- S to mineralize into SO42-. This may have occurred to some degree, however, mineralization of OM slows greatly once submergence occurs [19].
Initial sterilization occurred 7.5 weeks before the flooding occurred but was sterilized again 12 days before flooding. This time gap allowed for aerobic mineralization to occur by any surviving microbes. The amount of organic-S mineralized under aerobic conditions varies from soil to soil, but according to a study by [34], 4.4-7.2% organic S can be mineralized over a 28 week period. Another theory as to why the sterilized soils contained more sulfate than the non-sterilized soils in the beginning is that autoclaving resulted in a chemical change in the soil. According to Eno and Popenoe [40], steam sterilization changes the soil chemistry by increasing extractable N, P, and S. This could be the case in our soils and thus account for the additional SO42- in solution.
However, Eh for sterilized soils was greater than 300 mV on day 7, indicating that the majority of the soils were still mainly aerobic with O2 as the main terminal electron acceptor (TEA) [35]. Redox potential for the non-sterilized soils was 250 mV indicating that NO3- and Mn4+ were the primary TEAs [15].
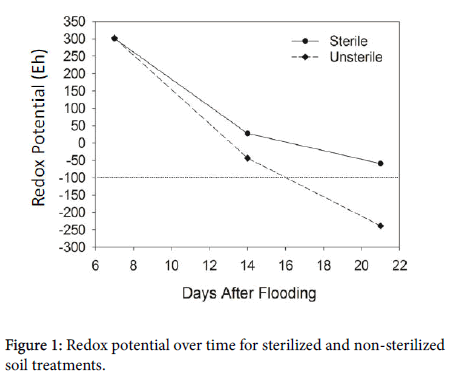
Two weeks after flooding, sterilized soil contained 13.91 mg L-1 more SO42- than the non-sterilized soils. Sterilized soil lost a mere 2.23 mg L-1 over one week whereas non-sterilized soils lost just over half of the SO42- (8.64 mg L-1). The Eh declined for both sterilization treatments, however, Eh for the non-sterilized soils dropped below -20 mV while sterilized soils only declined to 220 mV, a 240 mV difference (Figure 1).
With an Eh of 220 mV and a limited microbial presence, the sterilized soils appear to be utilizing other TEAs such as NO3- and Fe3+ [15,41], explaining the very small reduction of SO42-. Sulfate concentration and Eh declined in both sterilization treatments, but the non-sterilized soils experienced a more extreme decline than the sterilized soils. This supports the hypothesis that microbes catalyze in the reduction of SO42- to S2- and thus are important causative agents in the onset of H2S toxicity.
Effect of flooding on sulfate concentration and redox potential in day 21
On day 21, sterilization was again a significant main effect but location also became a significant main effect. For the sterilization main effect, SO42- concentration in the non-sterilized soils only declined by 27% from the previous week whereas 55% of the SO42- was lost between days 7 and 14. The Eh for the non-sterilized soils continued to decline to below -200 mV, well below the Eh where SO42- reduction is expected to occur. Sulfate concentration in the sterilized soils was unchanged from day 14 to day 21, but the Eh declined to approximately -60 mV. In contrast, as the non-sterilized soils approached this Eh, sulfate reduction rapidly took place.
There are several possible explanations for the observed differences in Eh between the sterilized and non-sterilized soils. Several reports in the literature indicate that measuring redox with platinum electrodes may not accurately reflect chemical changes taking place under anoxic conditions [38,42]. Additionally, Eh varies throughout the bulk soil both vertically and horizontally [43]. Taking continuous redox measurements in one spot in our soils could explain the variation in Eh between replications. Another factor influencing Eh measurements are microorganisms. The difference in Eh as well as SO42- concentration between sterilized and non-sterilized soils may indicate differences in microbial populations. The greatest microbial diversity appears in soils with a near neutral pH [44]. The soils used in this study ranged in pH from 7.6-8.1 (Table 1). Once a soil is flooded, pH approaches neutral regardless of whether the pH was previously acidic or alkaline [38]. This research supports the assumption that microbial diversity is likely very high in these four Arkansas soils. However, we do not know what species of microbes are involved. Sterilization limits microbe species diversity as well as density. Hence, the lack of sulfate reduction could indicate that fewer facultative anaerobes are present. Over two weeks (days 7-21), the non-sterilized soils lost approximately 70% of the SO42- in solution while the sterilized soils only lost 10%. While soils did not appear to be completely sterilized, the difference in SO42- concentration between the sterilized and non-sterilized soils indicated that the elimination of microbes greatly impacts SO42- reduction, thus indicating the importance of microorganisms in the H2S toxicity phenomenon.
For the first three weeks after flooding, there were no statistical differences in SO42- concentrations in solution between any of the soil locations. On day 21, SO42- concentrations in solution were significantly different between locations, regardless of sterilization treatment effects. This day is of particular interest since symptoms of H2S toxicity typically appear in the field two to three weeks after flooding [45]. Interestingly, soluble SO42- concentrations in H, PTRS, and HR-W were not significantly different from each other. These three locations contained higher concentrations of soluble SO42- than HR-E, but SO42- concentrations in soils from HR-E and HR-W were also not statistically different (Table 2). These results indicate that the concentration of SO42- in solution on a given day after flooding may not be the best indicator of likelihood of H2S toxicity. Chemical reactions prior to SO42- reduction are likely a better indicator of when and where H2S toxicity will occur. Microbes transform organic-S to H2S under anaerobic conditions [37], but H2S reacts with Fe2+, Mn2+, Cu2+, Cu+, and Zn2+ to form insoluble sulfides [21]. Typically, any H2S formed in the soil would react with Fe2+ present in solution to form insoluble FeS. Without ample amounts of Fe2+ in the soil, H2S can build up and cause toxicity to rice [37].
| Location | H2S Occurrence | SO42-† | Fe | Eh |
|---|---|---|---|---|
| ------mg L-1------ | mV | |||
| H | Always | 21.11 a | 6.8 | -131 |
| PTRS | Never | 19.4 a | 44.77 | 60.7 |
| HR-W | Always | 16.24 ab | 8.69 | -286 |
| HR-E | Sometimes | 13.31 b | 4.71 | -59 |
Table 2: Mean concentrations of soluble SO42-, soluble Fe, and Eh on day 21 for each soil location.
As expected, water-soluble Fe increased over time in each location after soils were flooded (Table 3) [21]. On day 21, PTRS contained nearly 6.5 times higher concentrations of Fe than all other soil locations. According to the Mehlich 3 soil extraction, PTRS did contain the highest concentration of extractable Fe, though it is unclear as to why soluble Fe was so much higher in PTRS than the other soils (Table 3). Based on the elevated soluble Fe concentration and the higher Eh of 60 mV, Fe3+ was likely acting as the primary TEA and SO42- reduction was minimal on this day. With high soluble SO42-, high soluble Fe, and high Eh, the H2S produced once SO42- became the TEA would likely react with the plentiful soluble Fe and precipitate as insoluble FeS, therefore preventing H2S buildup and toxicity (Table 3).
| Treatment | Time in days | |||||||
|---|---|---|---|---|---|---|---|---|
| 1 | 7 | 14 | 21 | 28 | 35 | 42 | 49 | |
| H Sterilized | 0.05 | 1.34 | 4.81 | 7.3 | 8.2 | 11.55 | 13.61 | 12.39 |
| H Unsterilized | 0.03 | 1.86 | 6.16 | 7.57 | 8.53 | 10.71 | 11.57 | 11.01 |
| HR-W Sterilized | 0.05 | 2.12 | 6.17 | 9.33 | 10.14 | 12.61 | 14.14 | 13.98 |
| HR-W Unsterilized | 0.03 | 1.48 | 4.63 | 6.69 | 7.88 | 10.77 | 12.21 | 13.43 |
| HR-E Sterilized | 0.04 | 0.38 | 2.87 | 4.95 | 6.89 | 6.49 | 9.63 | 10.74 |
| HR-E Unsterilized | 0.03 | 0.54 | 3.46 | 4.82 | 5.72 | 7.85 | 8.7 | 9.83 |
| PTRS Sterilized | 0.12 | 7.65 | 31.92 | 50.72 | 56.36 | 69.24 | 68.49 | 56.9 |
| PTRS Unsterilized | 0.02 | 0.68 | 3.31 | 3.91 | 3.53 | 4.51 | 4.98 | 4.99 |
Table 3: Mean soluble Fe concentrations over time for each treatment.
In contrast, on day 21 the H location contained high concentrations of SO42-, low Fe, and a low Eh. Sulfate was likely the TEA on this day with the low Eh (-131 mV). Since soluble Fe concentrations were relatively low (6.8 mg L-1), H2S formed during SO42- reduction would not have enough Fe2+ to react with to precipitate out, meaning that H2S would likely build up in the soil to the point of toxicity.
Effect of flooding on sulfate concentration and redox potential in days 28-77
From days 28 to 77, a significant interaction between sterilization and location occurred for soluble SO42- concentration over time (p values ranged from 0.0105 to <0.0001). Sterilized soils consistently contained higher concentrations of SO42- than the non-sterilized soils, regardless of location. Non-sterilized soils had the lowest concentrations of SO42- but were not statistically different between locations until day 63. However, on days 63 and 77, numerical differences in SO42- concentration were so small that statistical differences were negligible in practical application. On day 77, the difference in SO42- concentration between the highest sterilized soil and lowest non-sterilized soil was only 2.76 mg L-1 which is a minimal difference in a practical sense.
For all non-sterilized soils from each location, the majority of the SO42- in solution was reduced by day 28 with little numerical difference in concentrations from days 28-77. In contrast, SO42- concentration in sterilized soils continued to be reduced from days 28-42. The majority of the SO42- in the sterilized soils was reduced by day 42, but each location continued to decrease in SO42- concentration for the remaining days with minimal differences in concentration. The difference in SO42- concentration between sterilized and non-sterilized soils was likely due to larger microbial populations in the nonsterilized soils which used more TEAs more rapidly than the limited number of microbes in the sterilized soils. This resulted in more SO42- reduction at a faster rate in the non-sterilized soils for each location.
The magnitude of difference in soluble SO42- concentration between the sterilized and non-sterilized soils of each location is notable (Figure 2). At the beginning of the statistical interaction, SO42- concentration in sterilized soils from PTRS were 6.5 times higher than the SO42- concentrations in the non-sterilized PTRS soils. On this same day, sterilized soils from H contained five times more soluble SO42- than the non-sterilized H soils. Both Hickory Ridge locations had differences in SO42- concentration between the sterilized and non-sterilized soils. However, the 3.5 and 2.5 fold differences were not as extreme as the differences in H and PTRS. The difference between the Hickory Ridge locations and PTRS and H could be due to differences in microorganism population sizes and diversity, amounts of organic substrates in the soils, as well as Eh and pH. Fierer and Jackson [44] found that soil pH affected microbial diversity on a local scale with more diversity and richness occurring around a neutral pH. Several studies throughout the literature show that steam sterilization using an autoclave can alter soil chemical properties including pH and extractable S [40,46,47]. Comparing and contrasting chemical changes in the sterilized and non-sterilized soils by location brought some understanding to these extreme differences in soluble SO42- concentrations between the sterilized and non-sterilized soils.
As previously noted, sterilization likely caused an increase in soluble SO42- by adding more mineralizable S to the system by killing microorganisms. Samples from 24 h after flooding showed that soluble SO42- concentrations in the sterilized soils were higher by 9.45, 11.95, 12.61, and 2.88 mg L-1 for H, PTRS, HR-E, and HR-W respectively compared to the non-sterilized soils of each location. Though this was not of statistical interest (p=0.8879), elevated SO42- concentrations on day one is notable. However, by day 28 this difference became statistically significant as an interaction with location (p=0.0014). This elevated SO42- concentration in the sterilized soils and the interaction with location may be of importance in understanding under what conditions H2S toxicity is likely to occur. By day 28, over half of soluble SO42- was reduced in the sterilized soils of each location. From days 28 to 77, SO42- concentration declined sharply for one week then gradually declined approaching a minimum value asymptotically in the sterilized soils (Figure 2). Redox potential varied by location during this time period.
Both sterilized Hickory Ridge locations experienced rapid SO42- reduction from days 28 to 35. In the sterilized HR-E soils, Eh reached the SO42- reducing potential, -100 mV, shortly before day 28 and declined to -230 mV by day 28. Redox potential continued to decline until day 42, reaching and maintaining a minimum value around -320 mV with small fluctuations over time but never rising above -300 mV However for sterilized HR-W soils, Eh reached the SO42- reducing potential one week before HR-E and was 50 mV lower on day 28. Redox potential gradually declined until day 49 then remained around -300 mV for the remainder of the experiment. Interestingly, on the first day of Eh data (72 h after flooding), HR-W was 150 mV higher than HR-E and declined more rapidly reaching the SO42- reducing potential and the maximum negative value before HR-E. Differences in Eh between locations and sterilization treatments shortly after flooding could be due to the quantity of OM present as was seen in Gao et al. [16]. On day one of sampling, HR-W contained 6.2 mg L-1 less soluble SO42- than HR-E. One possible explanation for the faster decline in Eh and lower starting concentration of SO42- in sterilized HR-W soils is that fewer microbes were killed during sterilization compared to the amount killed in sterilized HR-E soils. The presence of highly resistant spores in one location could account for differences in sterilization effectiveness [46]. A faster decline in Eh would be more likely with more microorganisms present, less organic-S added through dead microbes, and less soluble SO42- in solution. With potentially fewer microbes eliminated during sterilization, this could possibly explain differences in SO42- concentrations between the sterilized and nonsterilized soils in the HR-W soil were greater than the corresponding difference as the other two locations. The differences in effectiveness of sterilization between HR-E and HR-W may indicate differences in microbial populations. With similar concentrations of SO42- but slightly different Eh trends and different microbial populations, this could explain why H2S toxicity occurs regularly in half of the field and only occurs occasionally in the other half.
Sterilized PTRS soils also experienced rapid reduction of SO42- from days 28-35 but reduced the greatest quantity of SO42- out of all sterilized locations. For the duration of the significant interaction, sterilized PTRS soils contained the most SO42- out of all locations, sterilized and non-sterilized, except for the sterilized H soil which was not statistically different. Additionally, sterilized PTRS soil contained numerically the highest concentration of soluble SO42- on day one though this was not statistically significant.
Redox potential was below SO42- reducing potential on day 28 at -163 mV, but was over 100 mV higher than both Hickory Ridge locations and 68 mV higher than H. Redox potential continued to rapidly decline until day 42 at which time Eh settled around -340 mV for the remainder of the experiment. This was the lowest Eh of all sterilized and non-sterilized soil locations, though only by a few mV. This decline in Eh again supports the suspicion that sterilization was not entirely effective. Despite having the lowest Eh, sterilized PTRS soils contained the most SO42- along with sterilized H soils for the duration of the experiment. This research, along with others, does indicate that Eh is difficult to use as an indicator of the progression of reducing conditions in the soil, though does identify oxic and anoxic conditions well [29]. Redox potential alone may not be the best indicator of whether H2S toxicity is likely to occur or not, though it may still be a useful tool in understanding this complex disorder.
Another unique attribute of the sterilized PTRS soils compared to all other locations and sterilization treatments was the abundant production of soluble Fe. While Fe concentration increased logarithmically for all other sterilized soils, Fe increased in the sterilized PTRS soils following a quadratic trend (Table 3). At the peak of soluble Fe in solution, sterilized PTRS soils contained nearly 6.5 times more Fe than the other sterilized soil locations. On day 35, Fe began to decline indicating the possibility of FeS precipitation due to the increase of H2S from the rapid reduction of SO42- the week before. Though sterilization can affect soil chemistry, this drastic change in soluble Fe in sterilized PTRS soils is difficult to explain. No other sterilized soil experienced increased Fe to this extent. However, all other soils, both sterilized and non-sterilized soil, did increase in Fe concentration and plateaued as expected [21]. Non-sterilized PTRS soils contained the lowest concentration of soluble Fe which could indicate that the Fe had been reacting with any H2S produced, therefore preventing H2S toxicity.
As with sterilized PTRS soils, a rapid decline in SO42- concentration occurred between days 28 and 35 for sterilized H soils. Sulfate concentration in sterilized H soils followed the same trend as the sterilized PTRS soils and the two were not statistically different for the duration of the significant interaction. Unlike PTRS, Eh had already declined to and plateaued at a relatively high Eh of approximately -270 mV for the remainder of the experiment. Though not statistically significant, Eh in sterilized H soils was numerically higher than all other locations once each soil reached a minimum Eh value. The nonsterilized H soils followed a similar Eh trend. Eh fell rapidly after flooding and reached a minimum value by day 28. However, nonsterilized H soil was around 80 mV lower than the sterilized soil, and the Eh of the non-sterilized soils reached similar values in all other non-sterilized soil locations.
While some interesting differences were observed between locations for the sterilized soils, the importance of this data becomes apparent when compared to their non-sterilized soil counterparts. Two major general differences were observed between the sterilized and nonsterilized soils for all locations. One, SO42- was reduced to the reached value for all non-sterilized soils by day 35, while SO42- concentrations were much higher and were still being reduced in all the sterilized soils. There are likely more SO42- reducing bacteria present in the nonsterilized soils which explain why sulfate was reduced to the minimum value more rapidly. Other measurements could have been useful to explain this such as pH and temperature [34]. Secondly, the minimum Eh eventually reached by all soils had a larger spread in the sterilized soils while the non-sterilized soils all reached similar values. This is interesting because H2S toxicity occurs naturally in some of these field soils (non-sterilized), but we do not see much difference in Eh between locations. Since sulfate concentrations in non-sterilized PTRS and H were not statistically different from each other and Eh for both were very similar, we can conclude that sulfate reduction and Eh are not strong indicators of whether or not H2S toxicity will occur. However, this conclusion may not hold in the presence of growing rice.
Greenhouse experiment using rice plants grown in the soil samples
Due to equipment malfunction, nearly half of redox data were not good enough to be used. The two successfully recorded replications were not consistent enough to call the data reliable. Therefore, a portion of the data was used as supplemental to the soil solution data wherever appropriate. The sources of errors were possibly from the redox sensors, data logger or oxygenation of the system from deionized water used t to maintain flood until the rice plants matured.. In the watering process, the deionized water was poured into each bucket using another bucket that possibly stirred the system allowing more oxygen. Another possibility for differences between replications is that Eh varies greatly throughout soil [43]. Oxygen could potentially be trapped in different micro and macropores at the tip of the electrode causing higher Eh readings than we would see in other areas within that soil profile. Besides, mosaics of high and low Eh throughout a soil are likely [43]. However, we did not have evidence of which factors were for the disparity between replications to take actions in follow up experiments.
Rice plants grown in the soil samples days 1-28
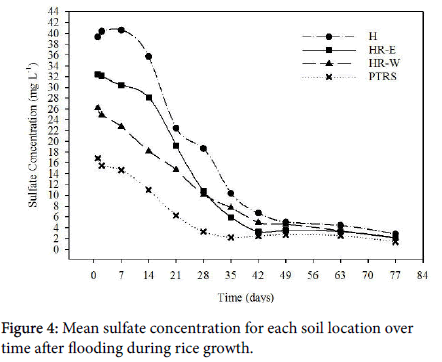
During rice growth, sulfate concentrations in soil solution were significantly different between locations from days 1-28 and 63-77 (p values ranged from 0.0405 to 0.0095) (Figure 3). However, on days 63 and 77, differences in SO42- were 1.4-2 mg L-1 different between the location with the highest concentration and the location with the lowest concentration and are therefore not of practical interest.
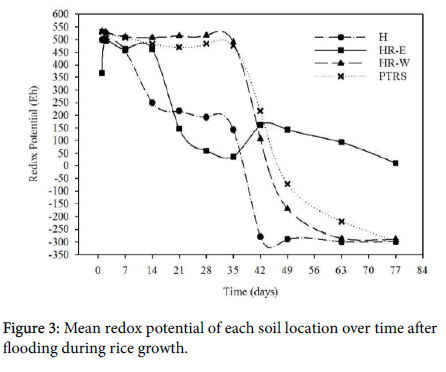
The overall trend in SO42- loss over time greatly differed from the first experiment. Rather than SO42- concentration immediately declining, SO42- concentrations decreased gradually for approximately one week after flooding before rapidly declining then after. Sulfate concentration was high during the first week post flooding in the H soils. Though SO42- increased just over one mg L-1, H is the only location that had a slight increase rather than a slight decrease over the first week. The delayed decline in SO42- concentration was likely due to the rice growing in the soil. Rice roots released O2 into the rhizosphere through the aerenchyma, oxidizing many compounds near the root zone to allow plant uptake [48,49]. Small aerobic zones were likely created in the rhizosphere allowing microbes to continue utilizing O2 as the terminal electron acceptor and delayed the reduction of SO42- due to the diffusion of O2 into the soil from rice roots [5]. Though O2 is depleted within a few hours of flooding [37], O2 release from the roots may have been enough to delay SO42- reduction for a week. The first week of redox data supports the delayed reduction with Eh values all remaining near 500 mV for each location except HR-E which was near 350 mV 24 h after flooding then increased to nearly 500 mV the next day (Figure 4). Since the majority of the redox data for HR-E was very inconsistent between the two replications, this could likely be due to equipment error.
Sulfate rapidly declined two weeks after flooding (Figure 3) which again is consistent with when symptoms typically start appearing in the field. However, above ground symptoms of H2S toxicity did not appear in any treatment. At the termination of the experiment, roots were removed, washed, and examined for accumulation of black iron sulfides, and H2S toxicity. However, no signs of H2S toxicity were detected. This was likely due to the big volume of roots in each bucket. Root respiration in a small, closed system may account for lack of H2S toxicity.
By day 49, all locations began to asymptotically approach a minimum value of SO42-, but no statistical difference was found between locations as the decline of SO42- concentration slowed. By day 77, SO42- concentrations of all locations were less than 3 mg L-1. Unlike the first experiment, H and PTRS were significantly different with H containing substantially more SO42- than PTRS. Concentrations of SO42- in solution 24 h after flooding differed greatly between these two locations, with soluble SO42- concentrations of 39.3 mg L-1 and 16.8 mg L-1 for H and PTRS, respectively. These value were much higher than the Mehlich 3 extractable S from the initial bulk soil samples and the SO42- concentration of the first experiment 24 h after flooding. One contributing factor was likely microbial activity mineralizing organic-S to SO42- for six months between the initial bulk soil test and the beginning of the second experiment. Storage in the warm greenhouse environment along with slowly air drying likely promoted mineralization [50]. However, the Mehlich 3 soil test results represent the nutrient index of soils before flooding. Once soil has been flooded, the chemistry changes substantially which likely caused the differences between the Mehlich 3 results and the SO42- in solution 24 h after flooding.
Between 24 h and 28 days after flooding, approximately 7 mg L-1 more SO42- was reduced in H than PTRS (Figure 3). However, H still contained 15.4 mg L-1 more soluble SO42- as PTRS which had reduced to 3.26 mg L-1. By the termination of the experiment, H reduced twice as much SO42- than PTRS with a total of 36.5 mg L-1 reduced, whereas PTRS reduced 15.4 mg L-1. Unfortunately, Eh data was not consistent between replications for H after one week of flooding. However, Eh data for PTRS was consistent between replications for five weeks after flooding and can be used to support solution data (Figure 4). For the first 28 days of flooding, Eh of PTRS remained fairly steady, around 500 mV. In comparison, during the first week of flooding, Eh of H declined from 500 mV to 450 mV. Redox potential for H continued to decline whereas Eh of PTRS remained fairly steady for 4 weeks (Figure 4). If this was true, the decline in Eh in H soils would have reached the SO42- reducing potential weeks before PTRS which would have promoted more SO42- reduction, accounting for the rapid reduction of SO42- .
Though H and PTRS locations were statistically different during the first four weeks after flooding and were the highest and lowest concentrations in this experiment, soluble SO42- concentrations in the Hickory Ridge locations fell in between those two extremes. While HR-E and HR-W were not statistically different from each other, SO42- concentration in HR-E was also not statistically different from H during the first four weeks after flooding. A sharp decline in soluble SO42- occurred in both of these locations from days 14 to 28, and both likely experienced similar rapid declines in Eh. Replications of Eh data for HR-E were consistent from the beginning of the experiment until day 21 and showed a sharp decline between days 14 and 21 with Eh dropping from nearly 500 mV to 150 mV. If this trend continued, SO42- reducing potential would be obtained weeks before PTRS and HR-W (Figure 3).
While soluble SO42- concentrations in HR-E and H were not statistically different during the first four weeks after flooding, the same was true of soluble SO42- concentration in HR-W and PTRS. Though there was nearly a 10 mg SO42- L-1 difference in these two locations, reduction occurred at the same rate (Figure 3). In both locations, SO42- reduced steadily for five weeks before approaching the minimum content asymptotically. From what was able to be interpreted from Eh data, PTRS and HR-W followed similar Eh patterns of maintaining a high, aerobic Eh for the first 5 weeks after flooding then ending with a low Eh near -300 mV by the termination of the experiment.
Though the frequency of H2S toxicity occurring in the field is different between HR-E and HR-W, these two soils were not statically different from each other for the duration of the experiment. Despite having the same pH, according to the Mehlich 3 soil report HR-W contained higher concentrations of nearly every nutrient as well as higher LOI, %N and %C (Table 1). Additionally, the Eh reacted differently between these soil locations. Redox potential in the HR-W soil maintained near 500 mV for the first 6 weeks after flooding before rapidly declining to below -100 mV by the end of the experiment. Redox potential for HR-E, however, increased during the first week after flooding, maintained near 500 mV for two weeks, then rapidly declined. Unfortunately, data between replications of HR-E was inconsistent after three weeks so Eh for the rest of the experiment is unknown. However, based on the previous experiment and data from the literature, a reasonable conclusion is that Eh declined to anaerobic levels at least by six weeks after flooding [16,21,51,52].
Differences in Eh between locations are particularly interesting when compared to the results of the first experiment. With an immediate decline in Eh during the first experiment, the most likely explanation for the delay in Eh decline was the diffusion of O2 into the rhizosphere from the rice roots [5,48]. While there is no definitive answer as to why Eh declined more rapidly in H and HR-E than PTRS and HR-W, there are several possibilities. First, microbial populations may indeed be different, especially between H and PTRS. Reduction is driven by anaerobic respiration [38], so the differences in these two flooded soils may be due to the presence of different species of anaerobic microbes. Bacterial community structure is believed to be strongly correlated with soil pH [44], and soil pH differed between H, PTRS, and the Hickory Ridge field. However, since HR-E and HR-W have the same soil pH yet different Eh trends, microbial populations alone may not be the driving factor. Another possible factor influencing Eh was nutrient concentration differences, particularly Fe content. Decline in Eh was likely resisted by PTRS soil due to the elevated reducible Fe content which can help prevent a decline in Eh [5]. This would also explain why Eh in HR-W declined two weeks after HR-E since Fe concentrations were higher in HR-W.
Though root blackening symptoms of H2S toxicity did not appear during this experiment, leaf tissue was analyzed for nutrient content since H2S toxicity damages roots and impedes nutrient and water uptake. Visual symptoms of potassium (K) deficiencies appeared in several plants and, according to the leaf tissue report; K levels were below optimum in each location [9]. H, HR-W, and HR-E were all below the critical level for deficiency for K [53]. Sulfur concentration was also below the critical level for deficiency in HR-W and PTRS [53]. Though there were nutrient deficiency problems throughout all the soil locations, H2S was not likely the cause. In cases of H2S toxicity, P is the greatest and most common deficiency followed by K [5]. However, P concentrations in leaf tissue were above optimum in all locations [53]. The K and S deficiencies were likely due to low levels in the soil and an insufficient K2O fertilizer application rate.
With rice growing in these soils, soluble SO42- concentrations and Eh reacted differently than when soil alone was submerged. While symptoms of H2S toxicity did not clearly shown during the second experiment, some differences between soil locations were identified as potentially influencing factors to this nutritional disorder. Based on our study, still more aspects including the physical, chemical biotic and abiotic factors and their interactions need to be investigated to definitively know H2S toxicity phenomenon in rice or other plants grown in submerged conditions.
Summary
The primary objectives of these two studies were to investigate the chemical and physical characteristics of a variety of soils that have experienced H2S toxicity to varying degrees compared to a soil where H2S toxicity has never been reported. Soluble SO42- concentrations in solution, Eh, and sterilization were all examined in each of these soils both with and without rice growing. When comparing soil test results of the four locations, Mehlich 3 extractable SO42- and percent silt were greater in all three soils that had experience H2S toxicity. The amount of these substrates presents in the soil likely influence the chemical reactions that took place once the soils were submerged. However, Mehlich 3 extractable SO42- is not an exact representation of nutrient availability once the soil is anaerobic. Additionally, plant available S is difficult to accurately assess since the majority of S is located in the organic-S pool which cannot be quantified accurately. In these Arkansas soils, silt was the dominant texture whereas sand predominates in Japanese soils prone to H2S toxicity. Though texture may not be a critical factor in H2S toxicity, it may influence the occurrence to some degree. However, this information does indicate differences that likely alter the environment and favor the production of H2S to the point of toxicity.
By sterilizing the soils, we were able to determine that the reduction of sulfate and decline in redox potential is primarily driven by microorganisms. With reduced populations, SO42- concentrations remained greater in all location compared to the all non-sterilized soils (p=0.0231 to <0.0001). Redox potential declined over time in both treatments though at a slower rate and with more variation between locations in the sterilized soils. Fourteen to 42 days passed before Eh dropped below -100 mV in the sterilized soils but only between 14 to 28 days passed for all non-sterilized soils to reach this redox potential.
Soluble SO42- concentrations immediately began to decline in all soil locations regardless of sterilization treatment in the first experiment. Unexpectedly, there was not a significant difference between the nonsterilized H and PTRS soils for the first 28 days after flooding. However, from days 28-42 after flooding, there was a significant interaction between location and sterilization treatment. Again, sterilized soils all contained greater concentrations of soluble SO42- than non-sterilized soil from each location. The magnitudes of the differences between the sterilized and non-sterilized soil treatments for each individual soil location were significantly different. In PTRS soil, sterilized soil contained 6.5 times more soluble SO42- than the nonsterilized and sterilized H soil contained 5 times more soluble SO42- than the non-sterilized H soil. However, sterilized HR-W and HR-E soils only contained 2.5 and 3.5 times more than their non-sterilized counterparts, respectively. As time progressed, differences between soluble SO42- concentrations in the sterilized and non-sterilized soils reduced but were still 1.5-3 times different by day 77 depending on location. These differences were likely due to microbial population density and diversity differences between soil locations as well as between the sterilization treatments. Other influential factors which may cause such different soluble SO42- concentrations were total initial amounts of SO42- , Fe, and OM in the soil, Eh, and soil pH.
The goal of the second experiment was to evaluate the rate and degree of SO42- and Eh reduction after flooding during rice growth. Soil from the same locations as the first experiment was used. In the presence of rice, soluble SO42- and Eh reacted differently. Instead of soluble SO42- immediately declining, SO42- concentrations remained fairly steady in each location for the first week after flooding before beginning to decline. From the data that was able to be interpreted from, decline in Eh also delayed in this study compared to the first study. These differences from the first study were attributed to the diffusion of oxygen from the rice roots into the rhizosphere. Though symptoms of H2S toxicity did not appear in any of the plants, we were able to determine from information from both studies that H2S toxicity is a multifaceted nutritional disorder. Further examination of soil chemistry, soil physical characteristics, biotic and abiotic influences are necessary to understand the causes of H2S toxicity.
References
- USDA-NASS (2017) State agriculture overview. Arkansas, USA.
- Hardke JT, Wilson CE (2012) Trends in Arkansas rice production. BR Wells’s Rice Res Stud 1: 38-47.
- Richardson JW, Outlaw JL (2010) Economic contributions of the US rice industry to the US economy. Texas Agri Life Research, Texas A&M University, USA.
- Baba I, Inada K, Tajima K (1964) Mineral nutrition of the occurrence of physiological diseases. Proceedings of The mineral nutrition of the rice plant. John Hopkins Press, Baltimore, USA.
- Yoshida S (1981) Fundamentals of rice crop science. Int Rice Res Inst, Los Banos, Laguna, Philippines.
- Tanaka A, Yoshida S (1966) Nutritional disorders of the rice plant. Technical Bulletin 7. IRRI, Los Banos, Philippines.
- Hardke J, Lorenz G, Wamishe Y, Stiles S (2015) Arkansas rice update 6-13-15. Arkansas Corn and Grain Sorghum Weekly Update, Arkansas Row Crops, University of Agriculture, USA.
- Hardke J, Lorenz G, Scott B, Wamishe Y (2013) Arkansas rice update 6-21-13. Arkansas Row Crops, University of Agriculture, USA.
- Wamishe Y (2015) Weather and Akiochi disease of rice – is there a link? Arkansas Row Crops, University of Agriculture, USA.
- Wamishe Y, Cartwright R, Lee F (2013) Management of rice disease. Arkansas Rice Production Handbook, Little Rock, Arkansas, USA.
- Fairhurst T, Witt C, Buresh R, Dobbermann A (2007) Rice: A practical guide to nutrient management. Int Rice Res Inst, Metro Manila, Philippines.
- Groth D, Lee F (2003) Rice: origin, history, technology, and production, John Wiley & Sons, New Jersey, USA.
- Ponnamperuma FN (1965) Dynamic aspects of flooded soils and the nutrition of the rice plant. In The Mineral Nutrition of the Rice Plant. John Hopkins Press, Baltimore, Maryland, USA.
- Germida JJ (1999) Transformations of sulfur. In: Sylvia DM (2nd edtn) Principles and applications of soil microbiology. Prentice hall. USA.
- Reddy KR, Feijtel TC, Patrick Jr WH (1986) Effect of soil redox conditions on microbial oxidation of organic matter. The Role of Organic Matter in Modern Agriculture, Dordrecht, Netherlands.
- Gao S, Tanji KK, Scardaci SC (2004) Impact of rice straw incorporation on soil redox status and sulfide toxicity. Agron J 96: 70-76.
- Lefroy RDB, Mamril CP, Blair GJ, Gonzales PJ (1992) Sulphur cycling in rice wetlands, Sulphur cycling on the continents: Wetlands, terrestrial ecosystems, and associated water bodies. John Wiley & Sons, New York, USA.
- Starkey RL (1950) Relations of microorganisms to transformations of sulfur in soil. Soil Sci 70: 55-66.
- Ponnamperuma FN (1984) Effects of flooding on soils. Flooding and plant growth. Academic Press, Orland, USA.
- Strawn DG, Bohn HL, O’Connor GA (2015) Redox reactions in soil. Soil chemistry, John Wiley and Sons, USA.
- Ponnamperuma FN (1981) Some aspects of the physical chemistry of paddy soils. Academia Sinica, Proceedings of symposium on paddy soils. Science Press, Beijing, China.
- Thomas GW (1996) Soil pH and soil acidity. Methods of soil analysis. SSSA Book Ser SSSA, Madison, USA.
- Gavlak R, Horneck D, Miller RO, Kotuby-Amacher J (2003) Soil, plant and water reference methods for the western region. WCC-103 Publication, Fort Collins, Colorado.
- Bremner JM (1996) Nitrogen-total. Methods of Soil Analysis 3:1085-1121
- Nelson DW, Sommers LE (1996) Total carbon, organic carbon, and organic matter. Methods of Soil Analysis 3: 961-1010.
- Schulte EE, Hopkins BG (1996) Estimation of soil organic matter by weight-loss-on-ignition. Soil organic matter: analysis and interpretation. SSSA Spec Publ, Madison, USA.
- Helmke PA, Sparks DL (1996) Lithium, sodium, potassium, rubidium, and cesium. Methods of soil analysis SSSA, Madison, USA.
- Patrick WH, Gambrell RP, Faulkner SP (1996) Redox measurements of soil. Methods of soil analysis. SSSA Book Ser SSSA, Madison, USA.
- Gao S, Tanji KK, Scardaci SC, Chow AT (2002) Comparison of redox indicators in a paddy soil during rice-growing season. Soil Sci Soc Am J 66: 805-817.
- Jones JB, Case VW (1990) Sampling, handling, and analyzing plant tissue samples. Soil testing and plant analysis. SSSA Book Ser SSSA, Madison, USA.
- Dobermann K, Cassman KG, Mamaril CP, Sheehy JE (1998) Management of phosphorus, potassium, and sulfur in intensive, irrigated lowland rice. Field Crops Res 56: 113-138.
- Espinoza L, Slaton N, Mozaffari M (2007) Understanding the numbers on your soil test report. University of Arkansas, USA.
- Strawn DG, Bohn HL, O’Connor GA (2015) Production and chemistry of soil organic matter. Soil chemistry. John Wiley and Sons, USA.
- Zhou W, Li ST, Wang H, Lin B (1999) Mineralization of organic sulfur and its importance as a reservoir of plant-available sulfur in upland soils of north China. Bio Fert Soils 30: 245-250.
- Freney JR, Jacq VA, Baldensperger JF (1982) The significance of the biological sulfur cycle in rice production. Microbiology of tropical soils and plant productivity. Microbiol Tropic soils plant product 271-317.
- Combs SM, Nathan MV (1998) Soil organic matter. Recommended chemical soil test procedures. Columbia, USA.
- De Datta SK (1981) Principles and practices of rice production. John Wiley and Sons, Toronto, Canada.
- Ponnamperuma FN (1972) The chemistry of submerged soils. Adv Agron 24: 29-96.
- Tanaka S, Kobayashi T, Iwasaki K, Yamane S, Maeda K, et al. (2003) Properties and metabolic diversity of microbial communites in soils treated with steam sterilization compared with methyl bromide and chloropicrin fumigations. Soil Sci Plant Nutr 49: 603-610.
- Eno C, Popenoe H (1964) Gamma radiation compared with steam and methyl bromide as a soil sterilizing agent. Soil Sci Soc Am Proc 28: 533-535.
- Harter RD, McLean EO (1965) Effect of moisture level and incubation time on the chemical equilibria of a Toledo clay loam soil. Agron J 57: 583-588.
- Bohn H (1971) Redox potentials. Soil Sci 112: 39-45.
- Aomine S (1962) A review of research on redox potentials of paddy soils in Japan. Soil Sci 94: 6-13.
- Feirer N, Jackson R (2006) The diversity and biogeography of soil bacterial communities. Proceedings of the National Academy of Sciences, USA.
- Hardke JT, Wamishe Y (2015) Arkansas rice update 2-7-15. Arkansas Row Crops. University of Arkansas Division of Agriculture Research and Extension, USA.
- Skipper HD, Westermann DT (1973) Comparative effects of propylene oxide, sodium azide, and autoclaving on selected soil properties. Soil Bio Biochem 5: 409-414.
- Wolf DC, Dao TH, Scott HD, Lavy TL (1989) Influence of sterilization methods on selected soil microbiological, physical, and chemical properties. J Environ Qual 18: 39-44.
- Ando T, Yoshida S, Nishiyama I (1983) Nature of oxidizing power of rice roots. Plant Soil 72: 57-71
- Joshi MM, Ibrahim IKA, Hollis JP (1975) Hydrogen sulfide: effects on the physiology of rice plants and relation to straighthead disease. Phytopathology 65: 1165-1170.
- Williams CH (1967) Some factors affecting the mineralization of organic sulphur in soils. Plant Soil 26: 205-223.
- Rogers CW, Brye KR, Roberts TL, Norman RJ, Fulford AM (2011) Assessing redox potentials as related to greenhouse gases in flooded paddy soils. BR Wells rice research studies, USA.
- Takai Y, Kamura T (1966) The mechanism of reduction in waterlogged paddy soil. Folia Microbiol 11: 304-313.
- Dobermann K, Fairhurst T (2000) Mineral Deficiencies. Rice: nutrient disorders and nutrient management, Singapore.
 Spanish
Spanish  Chinese
Chinese  Russian
Russian  German
German  French
French  Japanese
Japanese  Portuguese
Portuguese  Hindi
Hindi