Research Article, Arch Med Biotechnol Vol: 4 Issue: 1
Study on Guava Leaves Extract: A Folk Medicine
Ratul Deb*
Department of Bio-Sciences, JIS University, Kolkata, India
*Corresponding Author:
Ratul Deb
Department of Bio-Sciences,
JIS University,
Kolkata,
India,
Tel: 8250445733;
E-mail: ratuldeb645@gmail.com
Received date: 21 October, 2021, Manuscript No. AMB-21-45253; Editor assigned date: 26 October, 2021, PreQC No. AMB-21-45253 (PQ); Reviewed date: 09 November, 2021, QC No. AMB-21-45253; Revised date: 31 January, 2023, Manuscript No. AMB-21-45253 (R); Published date: 28 February, 2023, DOI: 10.4172/amb.1000032
Citation: Deb R (2023) Study on Guava Leaves Extract: A Folk Medicine. Arch Med Biotechnol 4:1.
Abstract
Antibiotics are generally used to kill micro-organisms. But nowadays, micro-organisms showed resistance against many antibiotics like benzyl penicillin, methicillin, vancomycin etc. To overcome this problem, we are in need of natural products with antimicrobial activity, as it shows no side effects like the antibiotics. The guava (Psidium guajava) is a phyto-therapeutic plant and it helps in various fields like treatment and manages of various diseases like malaria, gastroenteritis, vomiting, diarrhea, dysentery, wounds, ulcers, and a number of other conditions. As some of these diseases are due to bacterial infection, we in the present study try to evaluate the antibacterial activity of guava leaves extract. Myeloid leukemia’s continue to be difficult to treat at this time too even various medicines is in the relevant field. Novel therapeutic strategies that exclusively target cells as well as various malignant cells. In search for natural product-based anticancer agent I have tried to evaluate the effect of guava leaf extract on Chronic Myeloid Leukemia (CML) cells. The preliminary studies showed potent anti-CML activity.
Keywords: Guava leaves, Anti-bacterial activity, Anti- leukemic activity, Guava leaves
Introduction
Recently natural medicine takes place all in the attention field. Several fruits and fruit extracts mainly tea extract from arrowroot and caffeine, which helped in antimicrobial activity against the E. coli O157:H7 and it is proved in laboratory. Natural medicine manifest relatively high levels of antimicrobial action which can be used in inhibition of the growth of foodborne pathogens. Plant extracts can kill the bacterial cells by the rupture of cell walls and membranes and also extracts may be helpful in solve of the irregular disruption of the intracellular matrix [1].
The plant has a potential activity against diabetes, hypertension, and obesity. In this study, I aim to evaluate the total extracts of P. guajava leaves which is collected from JIS university hostel. Also, I have tried to establish a connection of efficacy using various aqueous and organic solvents against killing or inhibiting the growth of foodborne bacterium Staphylococcus aureus, Escherichia coli, Salmonella enteritidis, and Bacillus cereus which are major cause of illness and spoilage [2].
Belongs to the family Myrtaceae, it is originated in South America tropical region. Guava plants are found and grown in tropical and subtropical areas of the world. The genus Psidium have at about more than 150 species of small trees and shrubs in which only 20 species can produce the edible fruits and the rest are moreover wild and nonedible in nature. The most local cultured species of Psidium is P. guajava L. which is the common guava as our local language. This species is utilized for regulation of vigor, fruit quality improvement management and growth of various pest and disease resistance control [3].
The guava leaves have a height of 2 to 6 inches with 1 to 2 inches in width. When crushed, it has an aromatic flavour and appears dullgreen with stiff at it leaves which are clearly shown [4]. Also, it has various bioactive components which can fight against various pathogens, weight loss process as well as regulation of blood glucose levels. The leaves of guava contain an essential oil rich in various types of substances like cineol, tannins, triterpenes, flavonoids, resin, eugenol and a various number of other substances which are really helpful as a folk medicine [5].
Maceration, infusion aqueous-alcoholic extraction by fermentation, percolation, digestion, decoction, soxhlet extraction, counter-current extraction, supercritical fluid extraction, and microwave-assisted extraction, and phytanic extraction, ultrasound extraction is the basic extraction procedure. Maceration extraction is basically a crude extraction; in where solvents diffuse into solid plant and solubilize the various compounds which has similar polarity [6]. Effect of the plant material is fully dependable on its origin as well as variations, the time, solvent concentration and polarity, temperature of extraction, various metabolite composition present in following extract. Variations in extraction methods are found in areas of extraction period, the solvent used pH, particle size, enlisted temperature and the solvent-to-sample effective ratio.
The antimicrobial effect of essential oils, methanol, hexane and ethyl acetate extracts was observed by Gonc, Alves et al. Gottlieb and Magalhaes extracted essential oil from fresh leaves of guava using a clevenger type doser. Plant produces non-nutritive phytochemicals for their own protection, but it has an extra-ordinary against various diseases of human. Isolation of two triterpenoids named as guavanoic acid and guava coumaric acid was isolated by Begum et al. Arima and Danno also identified four types of flavonoids which can inhibit the growth of Salmonella enteritidis and Bacillus cereus in partial way [7].
Materials and Methods
Preparation of plant extract
The leaf samples were collected from the guava trees. Random leaf samples were collected into plastic zip lock bags with appropriate labelling and stored in an ice cooler until being transported to the laboratory for extraction [8].
Extraction methods
10 gm of leaf samples were washed in tap water, dried, and crushed in the ice-chilled mortar-pestle, which was placed on an ice-bag, with 20 ml of 70% ethanol. The mixtures were transferred to 50 mL falcon tubes and centrifuged at 6,000 rpm for 40 mins at 4°C. The supernatant was collected by filtering with filter paper and stored at 4°C until use. Some amount of supernatant was lyophilized at 45 psi. Then 5 mg of lyophilised material was mixed with 1 ml of DMSO [9].
Phytochemical analysis
Screening and identification of bioactive chemical constituents in the guava leaves were carried out with the extracts using the standard procedure and chemical test was done by regular method [10].
Test for protein: The protein content of guava leaves extract was determined by Folin-Ciocalteu (lowry) assay. Dilutions of BSA with concentrations of 200 μg/ml, 400 μg/ml, 600 μg/ml, 800 μg/ml and 1000 μg/ml was done by transferring respective amount from the BSA solution (1 mg/ml) and adjusting it to a total volume of 1 ml by distilled water. Reagent A (2% sodium carbonate in 0.1 N sodium hydroxide) and reagent B (0.5% copper sulphate in 1% potassium sodium tartrate) were added in the ratio of 49:1 to form reagent C. Then 5 ml of reagent C was added in all the tubes. The tubes were then incubated at dark for 10 mins. After that, 0.5 ml of 2X Folin- Ciocalteu reagent was added in all the tubes. The tubes were again incubated at dark for 30 mins. Then the absorbance was recorded at 660 nm in the spectrophotometer. The same process was done in case of guava leaves extract with concentrations of 12.5 μg/ml, 25 μg/ml, 50 μg/ml and 75 μg/ml [11].
Test for phenols: The phenol content of guava leaves extract was determined by Folin-Ciocalteu (gallic acid) assay. At first a stock solution of 6 ml of gallic acid was made of concentration 1 mg/ml. Serial dilution was taken with known volume (usually 3 ml) of stock and it was replaced into a known volume of distilled water (mainly 3 ml). This produced 6 ml of the dilute solution and it was marked as T1. The concentration of T1 was 0.5 mg/ml. Again, from T1, 3 ml of solution was taken and was placed into a 3 ml of distilled water and it was marked as T2. The concentration of T2 was 0.25 mg/ml. In the same way, the process was continued for T3, T4 and T5, having concentration 0.125 mg/ml, 0.0625 mg/ml and 0.03125 mg/ml respectively. Now, from the stock solution and all the dilutions (T1, T2, T3, T4 and T5), 0.1 ml was taken out individually and was mixed with 1.9 ml of Folin-Ciocalteu reagent. The tubes were incubated at dark for 10 mins. Then the absorbance was recorded at 720 nm in the spectrophotometer. The same process was done in case of guava leaves extract with concentrations of 12.5 μg/ml, 25 μg/ml, 50 μg/ml, 75 μg/ml and 100 μg/ml [12].
Test for scavenging activity: The scavenging activity of guava leaves extract was determined by hydrogen peroxide scavenging assay. At first, 100 ml of Potassium Buffer Saline (PBS) was prepared with 100 ml double distilled water, 0.8 gm sodium chloride, 0.02 gm potassium chloride, 0.15 gm sodium hydrogen phosphate and 0.024 gm potassium hydrogen phosphate. The OD of PBS is taken. From 100 ml of PBS, 45.3 μl of PBS was taken out and replaced with same volume of 4 mM hydrogen peroxide in the dark and OD was taken. For PBS, dilutions of samples with concentrations of 12.5 μg/ml, 25 μg/ml, 50 μg/ml, 75 μg/ml and 100 μg/ml were done by transferring respective amount from the guava leaves extract and adjusting it to a total volume of 2.6 ml by PBS. For PBS+Hâ??O2, dilutions of samples with concentrations of 12.5 μg/ml, 25 μg/ml, 50 μg/ml, 75 μg/ml and 100 μg/ml was done by transferring respective amount from the guava leaves extract and adjusting it to a total volume of 2.6 ml by PBS +Hâ??O2. In both cases, absorbance was taken at 230 nm in the spectrophotometer [13].
Test for flavonoids: The flavonoid content of guava leaves extract was determined by colorimetric assay. Dilutions of catechin with concentrations of 12.5 μg/ml, 25 μg/ml, 50 μg/ml, 75 μg/ml and 100 μg/ml was done by transferring respective amount from the catechin solution (0.5 mg/ml) and adjusting it to a total volume of 1.5 ml by distilled water. Then 0.1 ml of 10% aluminium carbonate was added in all the tubes. After that, 0.1 ml of IM potassium acetate was added in all the tubes. Then, 2.8 ml of distilled water was added in all the tubes. The tubes were incubated at dark for 30 mins. Then the absorbance was recorded at 415 nm in the spectrophotometer. The same process was done in case of guava leaves extract with concentrations of 12.5 μg/ml, 25 μg/ml, 50 μg/ml and 75 μg/ml [14].
Test for tannin: The flavonoid content of guava leaves extract was determined by tannic acid assay. Dilutions of tannic acid with concentrations of 12.5 μg/ml, 25 μg/ml, 50 μg/ml, 75 μg/ml and 100 μg/ml was done by transferring respective amount from the tannic acid stock solution (1 mg/ml) and adjusting it to a total volume of 1.5 ml by adding ethanol. Then 0.5 ml of 25% sodium carbonate was added in all the tubes. After that, 0.25 ml of Folin-Ciocalteu reagent was added in all the tubes. Then, 2.75 ml of distilled water was added in all the tubes. The tubes were incubated at dark for 30 mins. Then the absorbance was recorded at 725 nm in the spectrophotometer. The same process was done in case of guava leaves extract with concentrations of 12.5 μg/ml, 25 μg/ml, 50 μg/ml and 75 μg/ml [15].
Antibacterial activity
The antibacterial activity of guava leaves extract was determined by agar well diffusion method. At first nutrient agar solution was prepared by mixing nutrient agar powder and agarose in distilled water. The solution was then autoclaved at 121°C with 15 psi for 1 hour. The solution was then poured into 4 agar plates and let them sit still for solidification. After solidification, 10 μl of overnight inoculated nutrient broth solution of Escherichia coli K12 culture was poured respectively in 2 plates and 10 μl of overnight inoculated nutrient broth solution of Pseudomonas geniculata SMKP6 culture was poured respectively in 2 plates. The solutions were spread with a glass spreader and the plates were left in the laminar air flow for 15 min-30 min for drying. After drying, 3 wells were made in each of the 4 plates. In case of E. coli plates, one plate consists of wells for control, 25 μl and 50 μl of guava leaves extract, and the other plate consists of wells for control, 100 μl and 200 μl of guava leaves extract. In case of Pseudomonas plates, one plate consists of wells for control, 25 μl and 50 μl of guava leaves extract, and the other plate consists of wells for control, 100 μl and 200 μl of guava leaves extract. Now the control was poured in the wells for control, and samples are poured in their wells according to their concentration in individual plates. The Escherichia coli K12 plates were kept in incubator at 37°C and Pseudomonas geniculata SMKP6 plates were kept in the dark at room temp for overnight to achieve the result [16].
Anti-leukemic activity
The effects of guava leaves extract on leukemic cells were determined by conventional trypan blue exclusion assay. For this assay, 5 mg of the lyophilized sample was mixed with 1 ml of DMSO to have a concentration of 5 mg/ml which was used for this assay. Chronic Myelogenous Leukemic (CML) cell line K-562 was used in this assay. Cells were seeded in triplicate at a density of 0.1 × 106 in 24 well plate. After that cell were treated with different concentrations ranging from 0 μl/ml, 2.5 μl/ml, 5 μl/ml, 10 μl/ml and 20 μl/ml. After 48 hours of incubation, cells were centrifuged at 2500 rpm for 7 mins. The media was discarded and the pellet was dissolved in 1 ml RPMI (Roswell Park Memorial Institute) 1640 media. Now from this, 10 μl was taken in an eppendorf and 40 μl of trypan blue strain was added to make it 5X dilution. The total stained cells were taken and it was placed on haemocytometer. The cells were counted under inverted phase contrast microscope [17].
Results and Discussion
Protein estimation
The absorbance and graph for standard protein (BSA) concentrations are given below (Table 1 and Figure 1).
Table 1: Absorbance and graph for standard protein (BSA) concentrations.
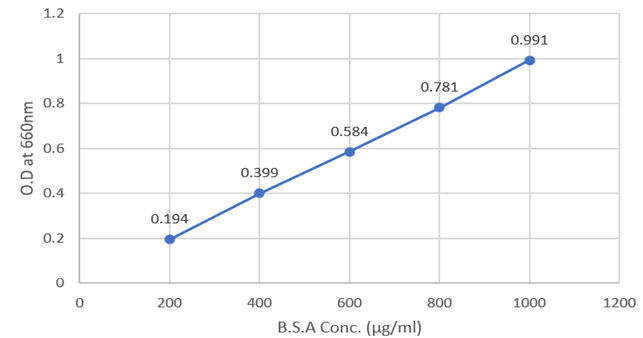
Figure 1: Absorbance for BSA concentration at 660 nm.
Now, the absorbance for guava leaf samples of different concentrations is given below (Table 2 and Figure 2).
Table 2: Absorbance for guava leaf samples of different concentrations.
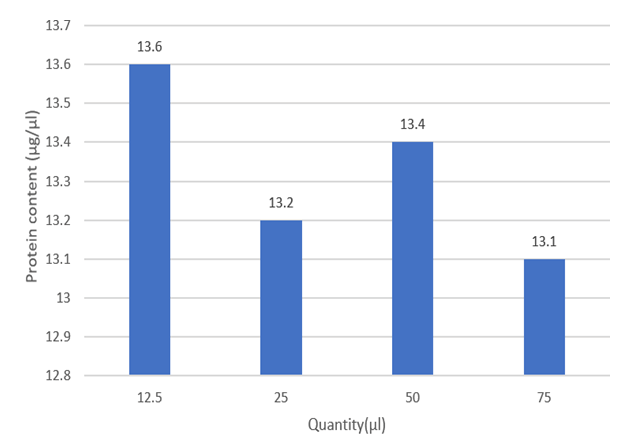
Figure 2: Total protein content of different conc. of guava leaves extract.
Discussion: It was observed that the guava leaf has high protein content which acts as nutritional value when it is used or consumed.
Phenol estimation
The absorbance and graph for gallic acid concentrations are given below (Table 3 and Figure 3).
Table 3: Absorbance and graph for gallic acid concentrations.
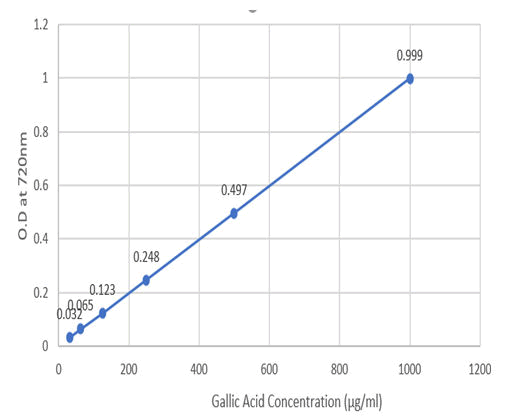
Figure 3: Absorbance for gallic acid conc. at 720 nm.
Now, the absorbance for guava leaf samples of different concentrations is given below (Table 4 and Figure 4).
Table 4: Absorbance for guava leaf samples of different concentrations.
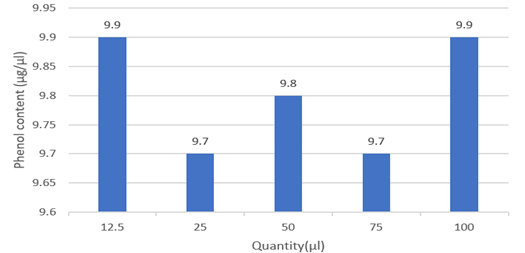
Figure 4: Total phenol content of different conc. of guava leaves extract.
Discussion: It was observed that the guava leaf has high phenol content. High phenol generally contributes to the leaf's antioxidant potential.
Scavenging activity
Now, the absorbance of different items is given below (Table 5).
Table 5: Image showing scavenging activity.
Now, the absorbance for guava leaf samples of different concentrations with blank (PBS) are given below (Table 6).
Table 6: Absorbance for guava leaf samples of different concentrations.
Now, the absorbance for guava leaf samples of different concentrations with control (PBS+Hâ??Oâ??) are given below (Tables 7 and 8) (Figure 5).
Table 7: Absorbance for guava leaf samples of different concentrations.
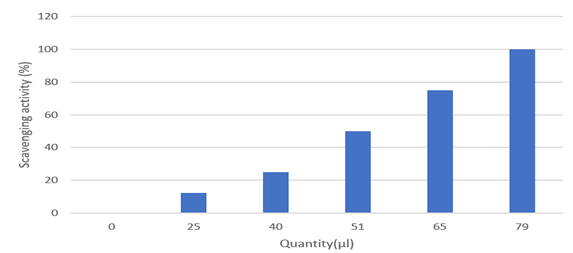
Figure 5: Percentage of scavengers of different conc. of guava leaf extract.
Table 8: Percentage of scavengers of different conc. of guava leaf extract.
Discussion: The guava leaf has a high scavenging activity. Hydrogen peroxide persist in natural condition at low concentration levels in the surrounding parameters like air, water, human body, plants, microorganisms, food and beverages. The hydrogen peroxide scavenging activity in guava leaves can be correlated to the presence of total phenols in the extract.
Flavonoid estimation
The absorbance and graph for catechin concentrations are given below (Table 9 and Figure 6).
Table 9: Absorbance and graph for catechin concentrations.
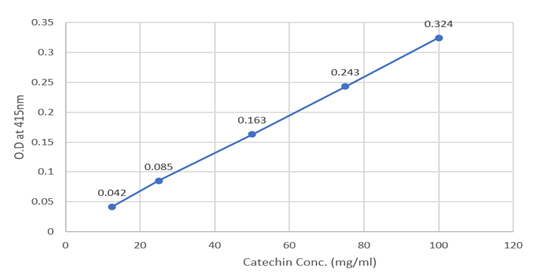
Figure 6: Absorption for catechin conc. at 415 nm.
Now, the absorbance for guava leaf samples of different concentrations is given below (Table 10 and Figure 7).
Table 10: Absorbance for guava leaf samples of different concentrations.
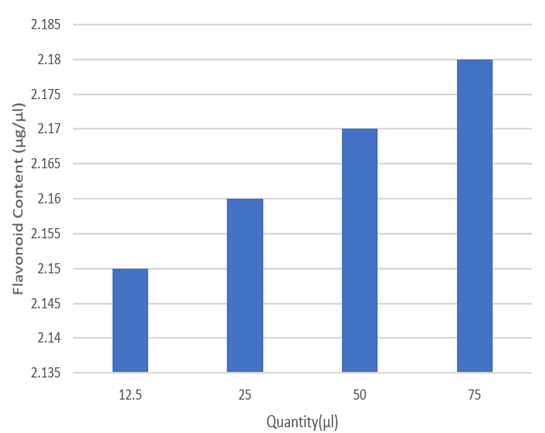
Figure 7: Total flavonoid content of different conc. of guava leaves extract.
Discussion: It was observed that the guava leaf has moderate flavonoid content. Flavonoids are types of hydroxylated polyphenolic compounds contained by plants which give a certain range of response to microbial infections. Compound’s ability has been used to form complexes with extracellular and soluble proteins and various bacterial cell walls. Depending on their structure, flavonoids are able to scavenge practically all known ROS.
Tannin estimation
The absorbance and graph for tannin concentrations are given below (Table 11 and Figure 8).
Table 11: Absorbance and graph for tannin concentrations.
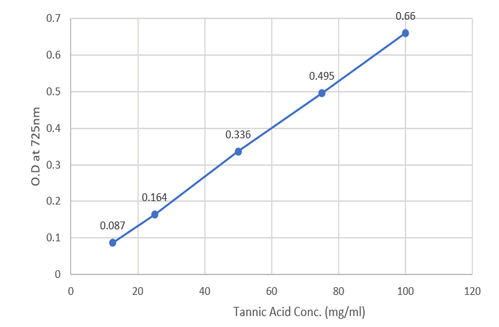
Figure 8: Absorbance for tannic acid conc. at 725 nm.
Now, the absorbance for guava leaf samples of different concentrations is given below (Table 12 and Figure 9).
Table 12: Absorbance for guava leaf samples of different concentrations.
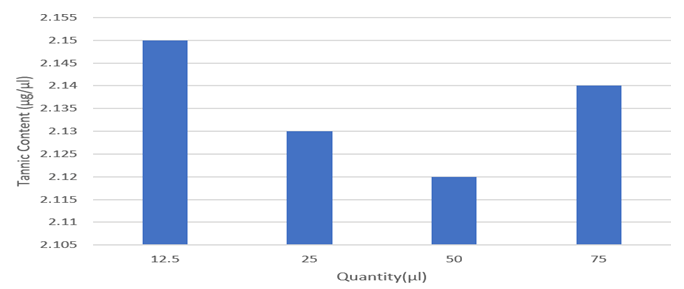
Figure 9: Total tannin content of different conc. of guava leaves extract.
Discussion: It was observed that the guava leaf has moderate tannin content. Tannin is a polyphenolic compound that are useful and an it binds and precipitates proteins and various other organic compounds including various types of amino acids and alkaloids. Tannin-protein complex can provide more persistent antioxidant activity than regular. Also, it acts as nutritional value when it is used or consumed.
Antibacterial activity
Fig. A and B represent the plates that were spreaded with Pseudomonas geniculata SMKP6. In Fig. A, the 3 wells represent control, 25 μl and 50 μl of extract. Similarly, in Fig. B, the 3 wells represent control, 100 μl and 200 μl of extract (Table 13 and Figure 10.
Table 13: The plates that were spreaded with Pseudomonas geniculata SMKP6.
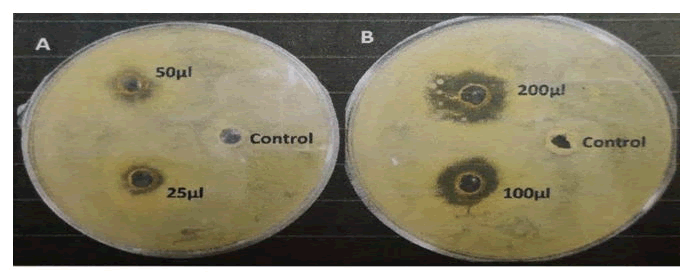
Figure 10: Zone of inhibition by control and different amounts of guava leaves extracts in Pseudomonas geniculata SMKP6 spreaded NA plate.
Fig. C and D represent the plates that were spreaded with Escherichia coli K12. In Fig. C, the 3 wells represent control, 25 μl and 50 μl of extract. Similarly, in Fig. D, the 3 wells represent control, 100 μl and 200 μl of extract (Table 14 and Figure 11).
Table 14: The plates that were spreaded with Escherichia coli K12.
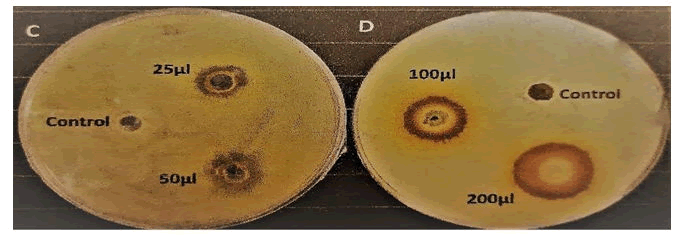
Figure 11: Zone of the inhibition by control and different amounts of gauva leaves extract in Escherichia coli K12 spreaded NA plates.
Discussion: It was observed that the zone of inhibition of different amounts of guava leaves extract was higher than that of control (99% ethanol). Also, the zone of inhibitions of guava leaves extract was greater in case of Escherichia coli K12 than that of Pseudomonas geniculata SMKP6. So, it proves that guava leaf extract has higher antibacterial activity on Escherichia coli K12 than that of Pseudomonas geniculata SMKP6. Basically, guava leaf has lower antibacterial activity in case of gram-negative bacteria than that of gram-positive bacteria.
Anti-leukemic activity
Discussion: Guava leaf extract has promising anticancer effect. We have examined the effect of the guava leaf extract on chronic myelogenous cell line K562. The trypan blue exclusion assay showed that the extract has anti-proliferative effect from minimal concentration like 2.5 μg/ml at 48 h. The 50% Inhibitory Concentration (IC 50) of guava leaf extract is 3.5 μg/ml+2 μg/ml (Figure 12).
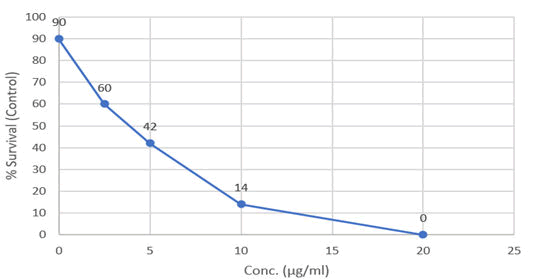
Figure 12: Anti-proliferative activity of gauva leaves extract.
Conclusion
Psidium guajava is a common tree in tropical and subtropical region. Its leaves are not commonly taken as D food but yet it has a high nutritional profile. The macronutrients and micronutrients were analysed by various types of standard methods. The biochemical studies revels that the concentration of carbohydrate, protein, starch and amino acid present in the sample is very high than their RDA value. It is also determined that guava leaves are the good source of vitamin C and vitamin B, calcium, magnesium, phosphorus and iron and various substances. Comparison between the guava leaf and guava fruit based on concentration of micronutrients showed result that, the leaves have more concentration in vitamin B, iron, calcium, magnesium, phosphorus and various substances. So, I can conclude the leaves are rich in nutrients than fruit. Raw guava can help to decrease blood pressure and blood lipid in body as it has high vitamin C and soluble fibre content. Guava leaves play an important role in improving body immunity and helps in keeping small blood vessels healthy as vitamin C is present in high range. Guava leaves contain vitamin B complex that is helpful in improving blood circulation areas leads to the brain, also effect the stimulating cognitive function and relaxing the nerve cells. Guava leaves can help against diseases such as osteoporosis, hypo calcemia, hypophosphatemia etc because of the enrichment of high concentration of calcium and phosphorus. It is also used in hair regrowth as it is rich in vitamin B and C which really nourish the follicles and help in hair growth. The vitamin C helps to boost the collagen activity which helps hair grow out faster and healthier. Guava leaf helps in relieving cough and cold as it helps get rid of mucus due to high presence of iron compound as well as it also disinfects the respiratory tract, throat and lungs. Though it is cheaply and easily available right in our surroundings, we do not make any use out of it. Beside all of these, the research shows that guava leaves could be used to prepare organic tea that lower cholesterol levels and diabetics and it is ongoing in different shadow. We can bring back this folk medicine and reuse them in broader and specific manner which is much better than those chemical drugs. The present study showed that guava leaf is a reservoir of potent secondary metabolites. Those metabolites make the extract a potential antibacterial and probable anti-cancerous agent. I need to go further specific studies on this ground to evaluate more accurate and specific molecular pathway involved with all of the processes.
References
- Kim S, Fung DYC (2004) Antibacterial effect of crude waterâ?soluble arrowroot (Puerariae radix) tea extracts on foodâ?borne pathogens in liquid medium. Letters Appl Microbiol 39: 319-325.
[Crossref] [Google Scholar] [PubMed]
- Ibrahim SA, Salameh MM, Phetsomphou S, Yang H, Seo CW (2006) Application of caffeine, 1, 3, 7-trimethylxanthine, to control Escherichia coli 157: H7. Food Chem 99: 645-650.
- Abdelrahim SI, Almagboul AZ, Omer MEA, Elegami A (2002) Antimicrobial activity of Psidium guajava L. Fitoterapia 73: 713-715.
[Crossref] [Google Scholar] [PubMed]
- Jaiarj P, Khoohaswan P, Wongkrajang Y, Peungvicha P, Suriyawong P, et al. (1999) Anticough and antimicrobial activities of Psidium guajava Linn. leaf extract. J Ethnopharmacol 67: 203-212.
[Crossref] [Google Scholar] [PubMed]
- Lutterodt GD (1992) Inhibition of microlax-induced experimental diarrhoea with narcotic-like extracts of Psidium guajava leaf in rats. J Ethnopharmacol 37: 151-157.
[Crossref] [Google Scholar] [PubMed]
- Begum S, Hassan SI, Ali SN, Siddiqui BS (2004) Chemical constituents from the leaves of Psidium guajava. Nat Prod Res 18: 135-140.
[Crossref] [Google Scholar] [PubMed]
- Morales MA, Tortoriello J, Meckes M, Paz D, Lozoya X (1994) Calcium-antagonist effect of quercetin and its relation with the spasmolytic properties of Psidium guajava L. Archiv Med Res 25: 17-21.
[Google Scholar] [PubMed]
- Sunagawa M, Shimada S, Zhang Z, Oonishi A, Nakamura M, et al. (2004) Plasma insulin concentration was increased by long-term ingestion of guava juice in spontaneous non-insulin-dependent diabetes mellitus (NIDDM) rats. J Health Sci 50: 674-678.
- Ncube NS, Afolayan J, Okoh AI (2008) Assessment techniques of antimicrobial properties of natural compounds of plant origin: Current methods and future trends. African J Biotechnol 7.
- Goncalves FA, Andrade NM, Bezerra JN, Macrae A, Sousa OVD, et al. (2008) Antibacterial activity of Guava, Psidium guajava Linnaeus, leaf extracts on diarrhea-causing enteric bacteria isolated from Seabob shrimp, Xiphopenaeus kroyeri (Heller). Revista do Instituto de Medicina Tropical de São Paulo 50: 11-15.
- Gottlieb OR, Magalhaes MT (1960) Essential oil of the bark and wood of Aniba canellila. Perfum Essential Oil Record 51: 69-70.
- Ibrahim SA, Yang G, Song D, Tse TS (2011) Antimicrobial effect of guava on Escherichia coli 157: H7 and Salmonella typhimurium in liquid medium. Int J Food Prop 14: 102-109.
- Mahfuzul Hoque MD, Bari ML, Inatsu Y, Juneja VK, Kawamoto S (2007) Antibacterial activity of guava (Psidium guajava L.) and neem (Azadirachta indica A. Juss.) extracts against foodborne pathogens and spoilage bacteria. Foodborne Pathogens Dis 4: 481-488.
[Crossref] [Google Scholar] [PubMed]
- Burt S (2004) Essential oils: Their antibacterial properties and potential applications in foods a review. Int J Food Microbiol 94: 223-253.
- Juven BJ, Kanner J, Schved, F, Weisslowicz H (1994) Factors that interact with the antibacterial action of thyme essential oil and its active constituents. J Applied Bacteriol 76: 626-631.
[Crossref] [Google Scholar] [PubMed]
- Sanches NR, Garcia Cortez DA, Schiavini MS, Nakamura CV, Dias Filho BP (2005) An evaluation of antibacterial activities of Psidium guajava (L.). Brazilian Archiv Biol Technol 48: 429-436.
- Sacchetti G, Maietti S, Muzzoli M, Scaglianti M, Manfredini S, et al. (2005) Comparative evaluation of 11 essential oils of different origin as functional antioxidants, antiradicals and antimicrobials in foods. Food Chem 91: 621-632.
 Spanish
Spanish  Chinese
Chinese  Russian
Russian  German
German  French
French  Japanese
Japanese  Portuguese
Portuguese  Hindi
Hindi