Review Article, J Vet Sci Med Diagn Vol: 11 Issue: 7
STUDY ON THE PREVALENCE OF BOVINE FASCIOLOSIS IN CHOLE DISTRICT ARSI ZONE, OROMIA REGIONAL STATE, CENTRAL ETHIOPIA
Hussein Mohammed Roba*
Department of Veterinary Medicine, College of Veterinary Medicine, Haramaya University, Haramaya, Ethiopia
*Corresponding Author: Hussein Mohammed Roba
Department of Veterinary
Medicine,
College of Veterinary Medicine,
Haramaya University,
Haramaya,
Ethiopia,
Tel: +913081117;
E-mail: dechashussein2021@gmail.com
Received date: 01 July, 2022, Manuscript No. JVSMD-22-68261; Editor assigned date: 04 July, 2022, PreQC No JVSMD-22-68261 (PQ); Reviewed date: 18 July, 2022, QC No. JVSMD-22-68261; Revised date: 30 August, 2022, Manuscript No. JVSMD-22-68261 (R); Published date: 06 September, 2022, DOI: 10.4172/2325-9590.1000035.
Citation: Roba HM (2022) Study on the Prevalence of Bovine Fasciolosis in Chole District Arsi Zone, Oromia Regional State, Central Ethiopia. J Vet Sci Med Diagn 11:7.
Abstract
A cross sectional study was conducted to determine the prevalence of bovine fasciolosis and assess the risk factors associated with fasciolosis in and around Chole district in East Arsi zone of Oromia Regional State of Ethiopia from November 2017 to April 2018. Parasitological examination was conducted using faecal sedimentation technique to recover parasite egg. A total of 384 cattle faecal samples were subjected to coprological examination. Based on the coprological examination the overall prevalence of fasciolosis was 144 (37.5%). Statistically significant variation (P0.05) in the prevalence of fasciolosis based on sexes and health status of the animal. The present study revealed that infection of cattle by fasciolosis was attributed to the presence of favorable environment for the abundance of intermediate host and the parasite. Based on the manifestation of overt clinical signs, animals were categorised into two parts, as apparently normal and showing overt clinical signs based on weight loss, pallor of the mucous membranes, sub mandibular oedema, dullness, weakness, lack of appetite, animals showing one or more of these signs were considered as manifesting of fascioliasis. The result mentioned here indicated that the disease has a significant position in the veterinary pathology scenario of the area. The studies confirm the endemicity of fascioliasis at the study grazing lands. Appropriate strategies for the control of fascioliasis based on seasonal de worming approach are suggested.
Keywords: Bovine, Coproscopy, Fasciolosis; Prevalence, Snails, Intermediate host
Introduction
Livestock are extremely important in Ethiopia to economic development and to poverty reduction. The latest animal population census by CSA, 2009, shows that Ethiopia has 52 million heads of cattle, 33 million heads of sheep, 30 million goats, 5.8 million equine species, 2.5 million camels and over 42 million poultry and it is the largest in Africa [1]. Livestock are the main stays of the livelihood of the majority of the human population by giving draft power, income to farming communities, means of investment and important source of foreign exchange earning to the nation. Moreover, livestock are important cultural resources, social safety nets and means of saving and are also supply for crop production and transport, as source of meat, milk and egg and source of income.
However the economic benefit derived from the livestock sub sector does not commensurate with the potential and the sub sector remained untapped. The challenge facing livestock development in Ethiopia is daunting. The potential for Ethiopia to improve the productivity of the livestock sub sector is clear, however, a number of constraints need to be addressed. Areas in need of attention include animal health and nutrition, availability of quality support services such as extension service, upgrading and dissemination of technology, package to improve animal breeding, marketing and processing and the collection and analysis of baseline data on which to plan development [2].
The widely prevalent livestock diseases are major constraints to Ethiopian livestock development. The vulnerable of livestock production and trade to disease epidemics is undermining investment in a potentially valuable economic activity which would increase employment in rural areas, raise rural incomes and assist in alleviating poverty [3].
Among the many parasitic problems of the domestic animals, fasciolosis is the major disease which imposes direct and indirect economic impact on livestock production in ruminants which are the natural host for Fasciola infestation particularly in cattle and sheep and occasionally in man. The disease is caused by digenean trematodes of the genus Fasciola commonly referred to as liver flukes. The two species most commonly implicated as the etiological agents of fasciolosis are Fasciola hepatica and Fasciola gigantica in Ethiopia [4]. Fasciola hepatica and Fasciola gigantica are coexist in Ethiopia; mixed infection with both species is encountered in areas between 1200-1800 m.a.s.l [5]. Both F. hepatica and F. gigantica are transmitted by the snails of the family Lymnaesidae. Infestation with fasciolosis is usually associated with grazing wet land and drinking from the snail infesting watering places [6].
Fasciolosis or liver fluke is worldwide distributed. Animal health and economic impact of fasciolosis may vary greatly from year to year, depending on the climate, management, level of infection, host immune status and age of the animal. In the endemic areas, several clinical outbreaks are frequent. The clinical manifestation of fasciolosis in infected animal has three forms: Acute, sub-acute and chronic forms [7].
Bovine fasciolosis is an economically important parasitic disease of cattle in tropical and subtropical countries responsible for considerable economic losses in the cattle industry, mainly through mortality, morbidity, reduced growth rate, condemnation of fluke infected liver, increased susceptibility to secondary infections and expense due to control measures. Production loss in livestock industry is estimates at more than 90 million USD annually [8,9].
In spite of the aforementioned prevailing situation and the presence of a number of problems due to fasciolosis, there is scarcity of well-documented information on the occurrence of fasciolosis among cattle in Debremarkos, Ethiopia. So this study was designed with the aims to determine the prevalence of bovine fasciolosis and major risk factors associated with the disease in and around Debre Markos.
Literature Review
Description of the parasite
Fasciolosis is the disease of cattle, sheep, goat, and other ruminants caused by the two most digenetic trematode of F. hepatica and F. gigantica. The members of this genus are commonly known as liver flukes and which infests the livers of various animals specially cattle and sheep. The disease occasionally affects humans, thus considered as zoonotic infection.
Taxonomy and classification
• Kingdom: Animalia
• Phylum: Platyhelminthes
• Class: Trematoda
• Order: Digenea
• Family: Fasciolidae
• Genus: Fasciola
• Species: Fasciola hepatica and Fasciola gigantica
Morphology
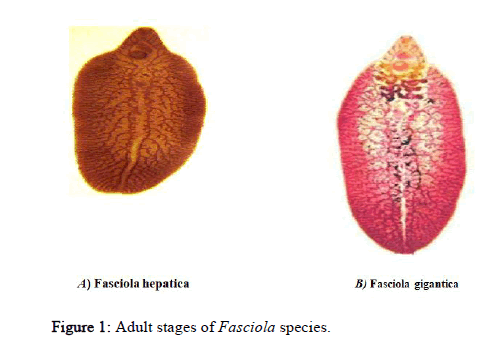
The adult parasite Fasciola hepatica is a flat leaf like body (Figure 1), typical of flukes measures up to 5 cm long and 1.5 cm wide. It has an anterior elongation (cephalic cone) on which the oral and ventral suckers, which are approximately of equal size are located. The intestine of the adult parasite is highly branched, with numerous diverticula extending from the anterior to the posterior end of the body. The pair of testes, also highly branched, is located in the posterior half of the body. The relative compact ovary is located just above the testes and is linked to a short convoluted uterus opening to a genital pore above the ventral sucker. The vitellaria are highly diffuse and branched in the lateral and posterior region of the body [10].
F. gigantica is very similar to F. hepatica but larger in size (2.5 cm - 7.5 cm by 1.2 cm) (Figure 1). It differs from F. hepatica in being more evenly leaf shaped, with scarcely perceptible shoulders in the parallel side of the body, the short anterior cone and the more profusely branched gut caeca.
The eggs of F. hepatica are oval, operculated and large measuring up to 150 μm by 90 μm in size and also very similar in shape to that of F. gigantica. The eggs of F. gigantica are also similar to that of F. hepatica, but it is larger up to 197 μm by 104 μm. Fasciola eggs are distinguished from the eggs of other flukes, especially from the large eggs of paramphistomum. Fasciola eggs have yellowish brown shell with an indistinct operculum and embryonic cells, whereas paramphistomum eggs has transparent shell, distinct operculum with embryonic clear cells and possess a small knob at their posterior end (Figure 2).
Host range
Intermediate host: Fresh water snails of the genus Lymnaea are the intermediate hosts for genus Fasciola. The epidemiology of fasciolosis is dependent on the ecology of the snail intermediate hosts. The habitat requirement of the intermediate hosts of the two most important liver flukes differs slightly. L. natalensis in case of F. gigantica is truly aquatic whereas L. truncatula for F.hepatica is amphibious snails. Most important Lymnaea species in the transmission of F. hepatica includes: Lymnaea truncatula, widespread in Europe, Asia, Africa and North America; L. bulimoides in South America and Caribbean; L. tomentosa in Australia and New Zealand. Other species which have been incriminated in the transmission of F. hepatica include L. viator and L. diaphena (South America, L. columnella (USA, Australia, Central America and New Zealand and L. humilis (North America).
The intermediate hosts for F. hepatica are amphibious snails that live close to the edges slow moving no stagnant water they periodically emerge on to surrounding mud. They are capable of withstanding summer drought or winter freezing for several months by hibernating deep in the mud. Optimal conditions include a slightly acidic pH environment and slowly moving water medium and they feed mostly on algae. The optimal temperature ranges for the development of snail is 15-26°C [11].
The most important intermediate hosts of F. gigantica are L. natalensis and L. auricularia [12]. Other species serving as secondarily hosts to this species are L. rufescens and L. acuminate (Indo-Pakistan and L. rubiginosa (Malaysia. L. natalensis is a strictly aquatic snail often found in Africa. It requires well oxygenated non polluted water bodies and can aestivate during dry periods. Optimal temperature requirement for the completion of parasite developmental stages within the snails is 22-26°C.
Final host: Hosts of F. hepatica are most mammals including man, sheep and cattle being most important. F. gigantica affects a wide range of domestic animals and is found in lowland areas replacing F. hepatica. Final host is responsible for the maturation and laying a huge number of eggs. In unusual host, such as man the fluke may be found in the lungs, under the skin or in other location [13].
Epidemiology
The epidemiology of fasciolosis is depends on the ecology of the snail intermediate hosts. The most important intermediate hosts of Fasciola are L. trucatula and L. natalensis. In developed countries, the incidence of F. hepatica ranges up to 77% [14]. Unlike Fasciola gigantica that occurs mainly in tropical areas such as Africa, Middle East, Eastern Europe and South and Eastern Asia, Fasciola hepatica is a temperate species and it is found Southern America, Europe, Australia and Africa, but also found in highlands of Ethiopia and Kenya. Due to the colonizing ability of the intermediate hosts and the parasites ability to infect a large range of primary hosts, F. hepatica has succeeded in expanding from the European original geographical area and nowadays it is considered very cosmopolitan in its distribution and can be found in almost all temperate regions. Fasciola hepatica is present in a very wide diversity of environments.
Snails of the genus Lymnaea are the intermediate hosts for genus Fasciola. L. trucatula snail is an amphibious with the wide spread in Europe, Asia, Africa and North America; L. tomentosa in Australia, New Zealand; L. columella in North America, Australia and New Zealand; and L. humilis in North America. L. truncatula is the most common intermediate host for F. hepatica in different parts of the world. The most important intermediate host of F. gigantica is L. natalensis and is a true aquatic snail often found in Africa.
Factors affecting the distribution of fasciolosis: Availability of suitable snail habitats; snail of the genus Lymnea is mainly involved as intermediate host in the life cycle. The important Lymnea species of snails involved in the transmission of fasciolosis vary in their geographic distribution. The habitat requirements of the two important liver flukes differ slightly. F. hepatica is found in an area above 1800 m.a.s.l while its tropical counterpart, F. gigantica, occur below 1200 m.a.s.l. In between these altitude limits, both species coexists where the ecology is conducive for both snail hosts L. truncatula and L. natalensis respectively, and mixed infections prevailed.
L. truncatula prefers wet mud to free water, and permanent habitats include the banks of ditches or streams and the edges of small ponds. Following heavy rainfall or flooding, temporary habitats may be provided by hoof marks. Though a slightly acidic pH environment is optimal for L. truncatula, excessively acid pH levels are detrimental.
Temperature: A mean day/night temperature of 10°C or above is necessary for both snails to breed and for the development of F. hepatica within the snails, and all activity cease at 5°C. This is also the minimum range for development and hatching of F. hepatica eggs. However, it is only when temperature rise to 15°C and are maintained above that level, that a significant multiplication of snail and the fluke larval stages ensues.
Moisture: The ideal moisture condition for snail breeding and the development of F. hepatica with in snails are provided when rain fall exceeds transpiration and filed saturation is attained. Such conditions are also essential for the development of fluke eggs, for miracidia searching for snails and for the dispersal of cercaria being shed from the snail.
Moisture is the critical factor, determining the presence and the extent of snail habitat, which serves as transmission foci for liver flukes. The interaction between moisture and temperature determines the survival and reproduction rate of the snail and the parasites. The liver flukes have a versatile survival strategy, certain stages of the parasite and their intermediate hosts have relatively well developed ability to persist through adverse weather conditions such as drought and freezing. Thus, persistence of infections form one season for the next may occur by several mechanisms; as adult fluke in mammalian host, as eggs on pasture, as larvae developing in snails and as metacercaria encysted on herbage.
Source of infection: Mammals become infected with Fasciola hepatica when grazing in pastures with contaminated vegetation. Infection occurs through the ingestion of metacercarial cysts located on the plants surface. Water has also been cited as a source of infection through direct drinking of cysts on water surface [15].
Life cycle
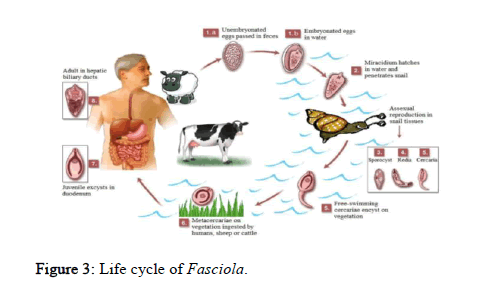
The life cycle is heterogenic and complex involving several phases and two hosts; a mammalian definitive host and an amphibious snail acting as the intermediate host. There may be some variations in the Fasciola life cycle, particularly within different definitive hosts, but the main factors affecting the life cycle tend to be the requirement of suitable temperature and sufficient moisture. The oval shaped eggs are laid by the adult parasite in the bile duct system of the definitive host and pass into the host alimentary tract, specifically the duodenum, with the bile. The eggs then leave the host through the feces [16].
Eggs have a thin shell and at this stage they are still not embryonated. Despite their thin shell, eggs are structurally strong. Embryonation occurs only after the eggs are liberated from the fecal matter and is dependent on a number of physico-chemical factors, although temperature and the availability of moisture are considered most important. The eggs hatch and release free-swimming miracidia that find and penetrate the tissues of their molluscan intermediate hosts: F. hepatica typically infects the fresh water snail Galba truncatula (formerly known as Lymnaea truncatula) in Europe and parts of Asia, whereas F. gigantica can infect a greater range of snail species including Lymnaea natalensis in Africa and Lymnaea rubiginosa in Asia [17].
The miracidium is the first free-living larval stage of F. hepatica and is a non-feeding organism; therefore its life span is determinate by the amount of energy stored. It is a fragile ciliated larva which swims vigorously in order to locate the intermediate host, and then it actively penetrates the snail using a retractile papilliform process possibly facilitated by the secretion of proteolytic enzymes. The need to find a suitable host to penetrate is an urgent one [18].
Miracidia that fail to do so generally die within 24 hours. After penetrating the snail, specifically inside the muscle tissue, the miracidium loses its cilia and metamorphoses into a rounded sporocyst, which migrates into the snail’s digestive gland. Usually every sporocyst gives rise to 5 to 8 motile rediae. Under adverse conditions, the rediae delay their development and give rise to a second generation of rediae, also by mitotic divisions. However, in adequate conditions, the germ cells in the brood chamber of the rediae develop into cercariae.
The mobile cercaria generally leaves the snail by migrating through the snail’s tissues and become free swimming. The cercariae release happens during moist conditions when a critical temperature of 10°C is exceeded. The whole process, from snail infection by the miracidium to cercariae emergence usually lasts 40 to 80 days and is temperature dependent; warmer temperatures reduce the number of days needed. After emerging from the snail, the cercaria attaches to various objects such as submerged blades of grass or other aquatic vegetation like water cress. The tail falls away and the cercarial body secretes a four layered cyst covering from cystogenous glands located on the lateral regions of the body. The formation of the cyst wall may take up to two days.
The metacercaria (the encysted and resistant form of the cercariae) is the infective form to the definitive host. One miracidium hatching from an egg can produce up to 4,000 infective cysts (metacercariae) due to the vegetative multiplication (mitotic division) at the sporocyst and redia stages. Metacercariae are able to survive for up to a year in appropriate conditions, like high humidity and cool temperatures, but show reduced survivability at increased temperature and in dry conditions [19].
Upon being swallowed, along with the contaminated vegetation, by the definitive host, the metacercarial cysts enter the small intestine and are stimulated to excyst, releasing the juvenile parasite. The excitement, which occurs in the duodenum, is stimulated by high carbon dioxide concentrations, reducing conditions and temperature at 39°C. Newly excysted juveniles penetrate the intestinal wall and enter the peritoneal cavity within 24 hours. From there, they migrate directly to the liver over a period of approximately five days. The immature flukes (also referred to as adeloscaria) then penetrate the liver tissues and migrate through the liver parenchyma consuming liver cells and blood for about six weeks until they find the bile ducts [20].
After about four weeks in the bile ducts, the flukes reach sexual maturity, generally within 3 months of the initial infection. Flukes attach to the bile duct wall using their suckers, with their spines securing them in place. This abrades the host epithelia and ruptures blood vessels, providing the parasite with additional nutrition. Adult F. hepatica can survive for many years in the livers of infected hosts; one to two years in cattle or as long as 20 years in sheep (Figure 3).
Pathogenesis
Pathogenesis of fasciolosis varies according to the number of metacercariae ingested, phases of parasitic development in the liver and species of host involved. Essentially the pathogenesis is two phases. The first phase occurs during migration in the liver parenchyma (migratory phase) and is associated with liver damage and hemorrhage. The parenchymal phase begins when excysted juvenile flukes penetrate the intestinal wall. After the penetration of the intestine, flukes migrate within the abdominal cavity and penetrate the liver or other organs [21,22].
During migration of flukes, tissues are mechanically destroyed and inflammation appears around migratory tracks of flukes. The second phase occurs when the parasite enter the biliary ducts of the liver (biliary phase) and results from the hematophagic activity of the adult flukes and from damage to the biliary mucosa by their circular spines. In biliary ducts, flukes mature, feed on blood and produce eggs. Hypertrophy of biliary ducts associated with obstruction of the lumen occurs as a result of tissue damage.
Pathogenesis as a direct result of the fluke’s activity may be either chronic or acute. Acute fasciolosis occurs during the pre adult migration of the flukes in parenchyma of the liver. The lesions of the acute form are those of simple traumatic hepatitis or traumatic hepatitis linked to an infectious hepatitis. Immature flukes are tissue feeders but may accidentally ingest some blood and the minor degree of anemia that develops in the first four to five weeks of infection probably reflects the loss of blood in to the migratory tracks of the young fluke. Quiescent spores of Clostridium novyi may become activated by the anaerobic necrotic conditions created in the liver parenchyma by migrating young F.hepatica causing the development of infectious necrotic hepatitis (Black disease) in sheep and cattle. This migration has also been thought to stimulate the development of occasional cases of bacillary hemoglobinuria in cattle.
In chronic fasciolosis, the damage is primarily to the liver develops slowly and is due to the activity of the adult flukes in the bile ducts. This causes cholangitis, biliary obstruction of hepatic tissue and fibrosis and anemia. The cause of anemia seen in chronic fasciolosis can be explained by the blood sucking activity of the flukes and the continuous drain in iron reserves. Hypoalbuminemia is more marked in the chronic disease and is due mainly to the increase protein plasma leakage in the gut [23]. Their by reinforcing immune responses both pigs and horses are generally highly resistant to infection with F. hepatica but differ in their mode of resistance. Horses overcome the migrating flukes at an early stage so that only a few flukes reach the liver, while in the pig the resistance mechanism operates in the liver parenchyma [24].
Necropsy findings
Acute hepatic fasciolosis: Acute hepatic fasciolosis is characterized by badly damage and swollen liver. The peritoneal cavity may contain an excess of blood stained serum. The liver capsule has many small perforations and sub scapular hemorrhages. The parenchyma shows tracts of damaged tissue and is more friable than normal. The immature flukes are often so small that they are not readily discernible. They are most easily demonstrated by slicing a piece of liver thinly and shaking in water, permeating the flukes to settle to the bottom. The size of the flukes may allow estimation of the duration of the infection and these to help to determine which pasture are hazardous [25].
Chronic hepatic fasciolosis: Chronic hepatic fasciolosis is characterized by the presence of grossly enlarged and thickened bile ducts, particularly in the ventral lobe of the liver. The bile ducts may protrude above the surface of the liver and cysts may present due to blockage of ducts with flukes and desquamating epithelial cells. Calcification of the bile duct wall is the common finding in cattle but not in sheep. The hepatic parenchyma is extensively fibrosed and the hepatic lymph nodes are dark brown in color, anemia, edema and emaciation are attendant abnormalities.
Clinical signs
Several clinical syndromes may be associated with liver fluke infection, may vary depending on the forms of the disease (acute, sub acute and chronic). Acute fasciolosis corresponds to the migratory stages of the life cycle, occurs as disease outbreak following a massive (>5000), but relatively short term intake of metacercaria. Most symptoms of acute fasciolosis (including fever, abdominal pain, hepatomegally and gastrointestinal disturbances) results from the destruction of liver tissue by the migrating immature flukes and can lead to abnormal liver function tests. Eosinophilia and mild or moderate anemia as well as sudden death are also common features of acute liver fluke infection [26].
Sub-acute fasciolosis is characterized by an infectious dose of 800-1000 ingested metacercariae. Animals become lethargic, anemic and may die. Weight loss is the dominant feature of sub-acute fasciolosis. Chronic fasciolosis results due to the presence of the mature adult flukes in the bile ducts accumulate over time and eventually reach a pathogenic number or infectious doses (200-800 metacercariae). The symptoms are hepatic fibrosis and hyperplasic cholangitis, progressive anemia, hypoproteinemia, weakness and general depression, loss of appetite, submandibular edema (also known as “bottle jaw”), ascites, diarrhea, weight loss and in severe cases, death.
In heavy infection where anemia and hypoalbuminaemia are sever, submandibular edema frequently occurs, with smaller fluke burden, the clinical effect is minimal and the loss of productivity is difficult to differentiate from inadequate nutrition. It must be emphasized that diarrhea is not a feature of bovine fasciolosis unless it is complicated by the presence of Ostertagia species. Combined infection with these two parasites has been referred to as fasciolosis/ostertagiosis complex. Fasciola infections may cause a loss of production in milking cows.
Diagnosis
History and clinical examination: A tentative diagnosis of fasciolosis may be established based on prior knowledge of epidemiology of the disease in a given environment, observation of clinical sign, information on grazing history, seasonal occurrence, and identification of snail habitats. Infection with F. hepatica is usually associated with herds and flocks grazing wet and marshy land. On the other hand, F. gigantica uses water, livestock drinking from snail infected watering places as well as with grazing wet land. In acute cases of fasciolosis, sudden death and severe anemia occurs due to the migratory young flukes through the liver; however no fluke eggs are passed in the feces. In sub acute cases signs of rapid loss of conditions, severe anemia, high fluke egg count, death occurs 12-20 weeks after infection, and in chronic fasciolosis, gradual wasting, severe anemia with ascites, edema, bottle jaw and very high fluke eggs counts may lead to death more than 20 weeks after infestation.
Post mortem examination: The most direct, reliable and cost effective technique for the diagnosis of fasciolosis is liver examination at slaughter or necropsy. In acute fasciolosis, there may be peritonitis, particularly on the visceral surface of the hepatic capsule. The migration of the flukes in the liver leaves dark hemorrhagic streaks and foci. The liver is swollen, friable and has capsular perforations marked by hemorrhagic tags.
Calcification of the bile duct and enlargement of the gall bladder are characteristics lesions observed in chronic cases of fasciolosis. Progressive biliary cirrhosis, which ultimately produces a hard fibrotic liver in which the bile ducts are prominent, thickened fibrous and in cattle, often calcified.
Fecal examination: Diagnosis of fasciolosis in ruminants caused by Fasciola species has been solely by the detection of Fasciola eggs in the feces of infected animals. Although the procedure is simple and confirmatory, it is however not a useful diagnostic tool at low levels of adult fluke burden. Also, it cannot detect infection at the prevalent period, because eggs are found in feces when the flukes are already matured (usually between 10 and 14 weeks of infection.
Chronic fasciolosis is diagnosed by finding the eggs in the feces by using sedimentation techniques. However they must be distinguished from the eggs of other flukes, especially the large eggs of paraphistomum. The Fasciola egg has a yellow shell with indistinct operculum and the embryonic cells are indistinct, while, the paraphistomum eggs have transparent shell and distinct operculum, their embryonic cells are clear and have a small knob at the posterior pole, and the eggs are larger than those of the liver fluke. The number of eggs in fecal samples is not an accurate to indication of the number of parasites in the liver, nor of the amounts of damage being done to the host.
Biochemical methods: This method is used for the first estimation of plasma levels of enzymes released by damaged liver cells. Two enzymes are usually measured GGT (Gamma Glutamate Transferase) and GLDH (Glutamate Dehydrogenase). Elevation of GGT (Gamma Glutamate Transferase) indicates damage to the epithelial cell lining of the bile ducts. Elevation of these enzymes takes place after the flukes reach the bile ducts and levels are maintained for a long period. GLDH (Glutamate Dehydrogenase) is released when parenchyma cells are damaged and levels become elevated within the first weeks of infection. They indicate damage to hepatic cells, related to parasite migration. Their levels are increased during the migration of F. hepatica [27].
Public health significance
Fasciolosis is emerging as an important disease in man particularly in countries such as Bolivia, Peru, and Egypt. F. hepatica may be acquired by man, but not directly from cattle. A person must ingest metacercaria in order to become infected. Human cases of fasciolosis have been reported South America, Europe, Africa, Australia and Far East within estimated 2.4 million cases worldwide. The number of people infected with Fasciola hepatica has increased significantly since in 1980. Several geographical areas have been described as endemic for the disease in human. Human acquire infection through ingestion of metacercaria adhering to certain aquatic plants and vegetables. Infection may also be acquired by consumption of water or ingestion of food items washed with such contaminated water.
The most common transmission route in humans is the ingestion of water cress contaminated with metacercaria, although, depending up on the geographical location, and a variety of edible aquatic plants can be vehicles of transmission. Water containing floating metacercaria has also been implicated in transmission. To date, the majority of reported human cases of fasciolosis are due to infections of F. hepatica. However, some reports indicate a rise in human infections due to F. gigantica in Vietnam [28]. Global estimate prevalence is between 1.7 and 2.4 million human infection worldwide and a further 180 million at risk of infection.
Economic importance
Fasciolosis causes major monetary losses in sheep, goat, buffaloes and cattle. Both types of flukes (Fasciola hepatica and F. gigantica),causes substantial monetary loss, which includes loss in carcass weight, reduction in milk yield, decline in production, reproductive performance and death due to predisposing of animals to other diseases, cost of treatment expenses and liver condemnation at slaughter houses. Fasciola hepatica infects more than 300 million cattle and 250 million sheep worldwide and, together with F. gigantica, causes significant monetary losses to global agriculture, estimated at more than USD 3 billion annually through lost productivity, such as a reduction of milk and meat yields. The effects of clinical fasciolosis are well known but those of sub clinical fasciolosis are often unnoticed leading to marked economic losses; reduced live-weight gains, milk yields and fertility. Fasciolosis is considered as an important limiting factor for ovine and bovine production. In general infection of ruminants with F. hepatica (temperate liver fluke) and F. gigantica (tropical liver fluke) causes significant monetary loss estimated to over USD 200 million per annum to agricultural sector worldwide with over 600 million animals infected.
The status of bovine fasciolosis in Ethiopia
Bovine fasciolosis exists in almost all region of Ethiopia. However, the prevalence, epidemiology and Fasciola species involved vary with locality. This is mainly attributed to the variation in the climate and ecological condition such as altitude, rainfall and temperature and livestock management system. Various reports indicated that Ethiopia is one of the countries with suitable climatic condition for the existence of fasciolosis.
The disease causes various problems in livestock population of the country. Both species F. hepatica and F. gigantica are found in Ethiopia and transmitted by the snail called L. truncatula and L. natalensis, respectively. Available reports strongly suggests that fasciolosis exists in almost all parts of the Ethiopia. It is regarded as one of the major setbacks to livestock productivity incurring huge direct and indirect losses in the country. In Ethiopia the prevalence of bovine fasciolosis due to F. hepatica and F. gigantica has been known by different workers. The prevalence based on coprology and abattoir results varies from place to place in different parts of Ethiopia (Table 1).
| Administrative region | Fecal (%) | Abattoir (%) |
|---|---|---|
| Dessie | - | 25.2 |
| Assela | 45.25 | 34.97 |
| Addis Ababa | - | 20.3 |
| Debre Zeit | - | 28.6 |
| Nekemte | - | 21.9 |
| Adwa | - | 32.3 |
| Bahir Dar | - | 31.5 |
| Hossana | 34.9 | 30.5 |
| Hawassa | - | 28.63 |
| Jimma | - | 53.48 |
| Wolaita Sodo | 15.67 | 25.33 |
| Mekelle | - | 35.2 |
| Woreta | 41.41 | - |
Table 1: The Status of Bovine Fasciolosis in Ethiopia.
Treatment
The optimum treatment of hepatic fasciolosis must destroy the migrating immature flukes as well as adults in the bile ducts. Triclabendazole (Fasinex) is considered as the most common drug due to its high efficacy against adult as well juvenile flukes. It is effective against adult F. hepatica at a dose rate of 10 mg/kg in sheep and 12 mg/kg in cattle are highly effective against all stages of flukes from one week old and is a drug of choice in outbreak of acute fasciolosis [29].Control and prevention
Seasonal treatment: Prophylaxis by drugs consists of eliminating flukes by regular treatments. Since local climatic conditions influence infection, they should be considered when determining the time of treatment. Two treatments are usually recommended per year. The first is given at the end of the rainy season (October-November) to eliminate the adult parasite so that the animals pass the dry season in good condition and also to avoid contamination from the dry season water wholes. The second treatment should be planned for the end of the dry season (March, April or May rarely later) when the immature flukes migrate through the hepatic parenchyma.
Reduction of snail population: Control of parasitic disease is crucial to improve the productivity of the animals. In most fasciolosis endemic areas, control of intermediate snail host population offers a good opportunity for reduction of transmission and is generally effective when combined with one or more other methods such as chemotherapy or environmental sanitation. Before any scheme of snail control is under taken a survey of the snail habitats should be made to determine whether they are localized or wide spread. The best long term method for reducing mud snail populations such as L. truncatula is drainage since it ensures permanent destruction of snail habitat. Other useful method of fluke control of the intermediate host is fencing the waterlogged area or treats annually with a molluscide. Copper sulphate (CuSo4) is most widely used; although more efficient molluscide, such as N-trityl morpholine, have been developed.
Materials and Methods
Description of the study area
The study will be conducted from November 2017 to April 2018 in Chole district in East Arsi zone of Oromia Regional State, southern east Ethiopia. Chole is one of the East Arsi zone wareda which located 242 km from Finfinne (Addis Ababa) east to Asella town. Geographically, the district lies between 8.1385° north, 39.9009° east. The total area of the district is estimated at 68200 hectares. The altitude of the district ranges rarely exceeds 1500 meters above sea level with the annual rainfall of 650 to 750 mm and the mean annual maximum and minimum temperature of 25°C 18 respectively. Chole is bordered on the south by Amigna, on the southwest by Sude, on the northwest by Merti, on the north by Aseko, and on the east by Gololcha. It is inhabited by a human population of about 124,789 population. 116,441 (93.31%) rural population and 8348 (6.69%) urban population respectively. The climatic condition of the woreda is divided into high land 48%, wayena dega -18%, lowland -22% and werch- 12% respectively. The topography of these areas includes plates, mountains and hills.
The agricultural system and husbandry practice of the communities specially the farmers is mainly mixed farming system and extensive management system respectively. Even though, chole woreda contains a huge livestock population there are different types of livestock disease which includes the production and productivity rates of animals. The main crops grown in chole wareda are mainly wheat, maize, barley, sorghum, teff, chickpea, sesame and the main animal feeds include crop residues, grass, and some improved forages. According to the total livestock population in data The woreda have a huge livestock population such as cattle 102,506, sheep 42,603,Goat 40,961, Horse 12,443, Donkey 10,387, Mule 4,526, poultry 70,149.
Study animal
A total of 384 heads of cattle were randomly selected and subjected to qualitative coprological examination. The selected animals were from both local and cross breeds of different age, body condition and sex groups. Cattle were randomly selected for fecal sample collection from localities of Chole woreda of chole 01 kebele, moye 01 kebele and waregu jawi kebele based on the availability of a suitable habitat for the snail intermediate hosts data obtained from Chole wereda veterinary clinics and chole werada livestock and fishery development office.
Sample size determination
Since there is no similar work done in the area previously, expected prevalence was taken as 50% and the confidence interval was chosen as 95% and precision 50%. By substituting these values in the formula, the sample size was 384. Thus, the sample size is calculated according to Thrusfield (2005) as follows:
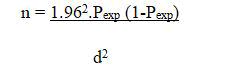
Where, n=required sample size
Pexp=expected prevalence=50%
d=desired absolute precision=5%. Hence, d=0.05 and p=0.5 (50%).
Z=1.96, value for the 95% confidence level
Thus, by using the given formula the sample size was estimated to be 384.
Data collection and laboratory diagnosis
A total of 384 fecal samples were collected during the entire period of the study, directly from the rectum of selected animal using a gloved hand and place into a universal bottle containing 10% formalin. Samples will be soon taken to the Asella regional veterinary Laboratory as fresh as possible. Sedimentation technique will be used to detect the presence or absence of fluke eggs in the fecal sample collected.
The sedimentation technique is a qualitative method for detecting trematode eggs in feces. The majority of trematode eggs are too large and heavy to float reliably in the flotation fluids normally used for nematode eggs. They do however sink rapidly to the bottom of a fecal/ water suspension and this is the basis of the fecal sedimentation technique.
Sedimentation technique procedure
• A 3g portion fecal sample was weighed out using a balance and put in beaker1.
• Pour 45 of tap water into beaker1
• Mix (stir) thoroughly with a stirring device
• Filter the fecal suspension through a tea strainer into beaker 2
• Pour the filtered material into a test tube
• Allow to sediment for five minutes
• Remove the supernatant very carefully
• Resuspend the sediment for five minutes
• Discard the supernatant very carefully
• Stain the sediment by adding one drop of methylene blue.
• Transfer the sediment to a microscope, cover with a cover slip (104 magnification power).
To differentiate between eggs of Paramphistomum species and Fasciola species, a drop of methylene blue solution was added to the sediment where eggs of Fasciola species show yellowish colour while eggs of Paramphistomum species stain by methylene blue. During sampling information on sex, breed, health status, body condition and approximate age of individual animals were recorded. Age was classified as young (<5 years) and adult (>or =5 years). Samples that were not processed within 24 h were stored in a refrigerator at 4°C.
Study design
A cross sectional study will be conducted from November 2017 to April 2018 in Chole district in East Arsi zone of Oromia Regional State of Ethiopia. Active data was generated from randomly selected cattles with regard to age, breed, sex and body condition was considered as risk factors to test for occurrence of fasciolosis.
Data management and analysis
All raw data generated from this study were coded and entered in MS Excel database system. Using Statistical Package for Social Science (SPSS) version 20 computer program, data were analyzed. The prevalence of fasciolosis was calculated as the number of infected individuals divided by the number of cattle examined x 100.
Categorical data were analyzed with the Pearson's Chi-square (χ2) test to measure the association between prevalence of the parasite with the potential risk factors as a statistical tool. For all analysis, P<0.05 was considered as significant differences between the parameters measured.
Results
Coprological result
From the total of fecal sample examined for fasciolosis 37.5 % ( 144/384) were found to be positive for Fasciola egg by coprological examination (Table 2).
| Species | No of animal examined | No of positive | Prevalence |
|---|---|---|---|
| Bovine | 384 | 144 | 37.50% |
Table 2: Prevalence of bovine fasciolosis on species.
Based on sex of the animal the prevalence of bovine fasciolosis was found to be 33.8% and 42.1% in male and female respectively. Even though, there was no statistical significant association (p>0.05), the prevalence was higher female than male animals (Table 3).
| Sex | Animal examined | Positive animal | Prevalence | X2 | Df | p-value |
|---|---|---|---|---|---|---|
| Male | 213 | 72 | 33.80% | |||
| Female | 171 | 72 | 42.10% | 2.79 | 1 | 0.95 |
| Total | 384 | 144 | 37.50% |
Table 3: Prevalence of bovine fasciolosis on sex basis.
The prevalence of bovine fasciolosis between ages was found to be 42.5% and 30.1% in adult and young respectively. The higher prevalence was observed in adult as compared to young animals. There was statistically significant association (P<0.05) in prevalence of bovine fasciolosis between ages (Table 4).
| Age | Animal examined | Positive animals | Prevalence | X2 | df | p.value |
|---|---|---|---|---|---|---|
| Adult | 228 | 97 | 42.50% | |||
| Young | 156 | 47 | 30.10% | 6.092 | 1 | 0.014 |
| Total | 384 | 144 | 37.50% |
Table 4: Prevalence of bovine fasciolosis between Ages.
The prevalence of bovine fasciolosis among body condition of animals was found to be 26.6%, 45.5% and 69.7% in good, medium and poor body conditions animals respectively. The highest prevalence was observed in poor body condition as compared to good and medium body conditions. There was statistically significant association (P<0.05) in prevalence of fasciolosis among the three body conditions of the animals (Table 5).
| BCS | Animal examined | Positive animal | prevalence | X2 | Df | p-value |
|---|---|---|---|---|---|---|
| Good | 218 | 58 | 26.60% | |||
| Medium | 123 | 56 | 45.50% | 33.525 | 2 | 0 |
| Poor | 43 | 30 | 69.70% | |||
| Total | 384 | 144 | 37.50% |
Table 5: Prevalence frequency of bovine fasciolosis among body condition.
The prevalence of bovine fasciolosis between breeds was found to be 35.3% and 56.6% in local and cross breeds respectively. The highest prevalence was observed in cross breeds as compared to local breeds. There was statistically significant association (P<0.05) in prevalence of fasciolosis between breeds (Table 6).
| Breed | Animal examined | Positive animals | prevalence | X2 | df | p-value |
|---|---|---|---|---|---|---|
| Local | 354 | 127 | 35.80% | |||
| Cross | 30 | 17 | 56.60% | 5.101 | 1 | 0.024 |
| Total | 384 | 144 | 37.50% |
Table 6: Prevalence of bovine fasciolosis on different breeds.
The prevalence of bovine fasciolosis between animals showing overt clinical sign and apparently healthy animals was found to be 34.9% and 39% respectively. The higher prevalence was observed in apparently healthy animals than animals with clinical sign. However, there was no statistically significant association (P>0.05) in prevalence of fasciolosis between animals showing overt clinical sign and apparently healthy animals (Table 7).
| Animals | Animal examined | Positive result | Prevalence in % | X2 | p-value |
|---|---|---|---|---|---|
| With clinical signs | 143 | 50 | 34.9 | ||
| Apparently normal | 241 | 94 | 39 | 0.758 | 0.384 |
| Total | 384 | 144 | 100 |
Table 7: Prevalence of bovine fascioliasis in relation to clinical signs of liver fluke infection based on coproscopic examination.
Age determination (young, adult and old)
Young <5 years old
Adult >=5 years (Table 8)
| Age (year) | characteristic change |
|---|---|
| 1 ½-2 | I1 erupts |
| 2-2 ½ | I2 erupts |
| 3 | I3 erupts |
| 3 ½ -4 | I4 erupts |
| 5 | All incisor and canine are in wear |
| 6 | I1 is level and the neck has emerged from the gum |
| 7 | I2 is level and the neck is visible |
| 8 | I3 is level and the neck is visible I4 may be level |
| 9 | I4 is level and the neck is visible |
| 10 | The dental stare is square in I2 and in all teeth by 12 years |
| 15 | The teeth that are not fallen out are reduced small round. |
| Remark: Canine of ruminants is usually considered as fourth incisor. | |
Table 8: Age determination based on dental formula.
During the study period, body condition of animals was classified in to poor, medium and good based on the following body condition scoring method (Table 9).
| Body condition score 1 | The individual Spinous process is sharp when to touch and easily distinguished. |
| Body condition score 2 | The Spinous process can be identified individually when touched but feel round rather than sharp. |
| Body condition score 3 | The Spinous process can only felt with very firm pressure and area of either side of the tail and head have some fat cover. |
| Body condition score 4 | Fat cover around tail and head is easily seen as slight mouds, soft to touch, the Spinous process cannot be felt. |
| Body condition score 5 | The bone structure of the animal is no longer noticeable and the tail and head are almost completely buried in fatty tissue. |
Table 9: Body condition score of the animal.
Body condition score 1 and 2=Poor
Body condition score 3=Medium
Body condition score 4 and 5=Good
Discussion
The present total prevalence of bovine fasciolosis during coprological examination was found to be 37.5%. This result is very close to the findings of (37.2%) by Solomon Woldemariam and Abebe Wossene in Mecha and Fogera, (33.42%) by Yilma Jobre and Mesfin Ali in Gondar and 34.9% by Bekele, et al., in Lemo district, Southern Ethiopia. This finding was lower than the findings of 41.41% in and around Woreta, North Western Ethiopia by Tsegaye, et al., 45.25% by Ayalew and Endalkachew, at Bahir Dar municipal abattoir, Northern Ethiopia, Bahru and Ephraim in kaffa (86%), Yadeta in Western Showa (82.5%), Dagne in and around Debre Berhan (80%), Fekadu around Bahir Dar (60.2%), Wondwossen in Arsi Administration region (53.72%) and (49.55%) by Ahmadi and Meshkehkar in Iran. In contrary, the result was higher than the findings of 4.9% recorded in Soddo and (8.6%) Mellau, et al., at Arusha, Tanzania.
The reason behind the variation of the present results with the above findings might be attributed to the differences in ecology of the study areas, management systems of animals, the intervention of nearby veterinary pharmacies, sample size as well as the present study were conducted during the dry period of the year when the infection rates of fasciolsis is expected to be low, since one of the most important factors that influence the occurrence of fasciolosis in an area is the availability of a suitable habitat for the snail intermediate hosts and essential for the development of fluke eggs, miracidiae searching for snails and dispersal of cercariae [30].
Analysis of the fecal egg detection result didn’t show statistically significant difference between two sexes as risk factor (P>0.05). Suggesting sex seems have no difference in the infection rates of fascioliasis. Both male and female animals are equally susceptible and exposed to the disease (Table 3). Similar results that support the present findings are reported by Dagne, Rahmato, Abie et al., 2012, Solomon Woldemariam and Abebe Wossene. This could be associated with similar management given to both male and females cattle. In communal grazing areas, both females and males graze on the same pasture and move in searching of food and water together, which expose to the same risk of infection. On the contrary Woldemariam S and Wossene A, revealed high prevalence rates in the male than female. This is probably related to the management system with longer exposure of male out door while females are kept in door during pregnancy and at the beginning of lactation.
Age of animals is one factor investigated in this study. Our results showed that the rate of bovine fasciolosis was 30.1% in age of less five years and 42.5% in age of more than and equal five years. The results showed a significant association between bovine fasciolosis and age of animals examined (P value=0.014). These results are consistent with other studies regarding young animal’s low rate of infection than old animals. This finding is not surprising due to the fact that bovine fasciolosis is a chronic disease, the higher age reflects a much longer period of exposure to infection
The reason for significantly high prevalence of fasciolosis (P<0.05) in cross breed compared to local breed could be due to lower resistance of cross than local breed. The association between the prevalence of fasciolosis and body condition of the animals was found to be statistical significant. The present study was revealed highest prevalence in poor body condition 69.7% as compared to good and medium body conditions. In support of this finding, a study was conducted in Adwa, Ethiopia by Mihreteab, et al., in Wolaita Sodo, Ethiopia by Edilawit, et al., in Jimma, Ethiopia by Abie, et al., and in Hossana, Southern Ethiopia by Bekele, et al., revealed highest prevalence in poor body conditions of cattle than good and medium body conditions. The probable reason could be due to the fact that animals with poor body condition are usually less resistant and are consequently susceptible to various diseases including fasciolosis and due to reduced performance of the animals created by luck of essential nutrients and poor management by the animal owner. This study showed that the prevalence of bovine fascioliasis by coproscopic examination was (34.9%) in animals showing overt clinical signs, than those which are apparently normal (39%). The result didn’t show statistically significant association (p>0.05) between bovine fasciolosis and health status of the animal.
Conclusion
In general fasciolosis was found prevalent in the study areas. This has been hindrance to the livestock production by causing remarkable direct or indirect losses in the study areas. Moreover, the study area is suitable for the survival of the snail which worsened the situation for the future. Therefore, strategic application of fluckicide and avoiding animals grazing on marshy land plays considerable success for the control of fasciolosis in these study areas.
References
- CSA (Central Statistical Agency) (2009) Agricultural and Veterinary parasitology, survey. Report on livestock, poultry and bee hives population, private peasant holdings. Addis Ababa, Ethiopia, 2.
- Juyal P, Singal L (2011) Herbal immunomodulatory and therapeutic approaches to control parasitic infection in livestock. Department of veterinary parasitology college of veterinary science Punjab agricultural university, Ludhiana, India, 1-8.
- Shitaye J, Tsegaye W, Paulik I (2007) Bovine tuberculosis infection in animal and human population in Ethiopia: a review. Vet Med 52:332-417.
- Ahmed E, Markvichitr K, Jumwasorn S, Koonawoothtthin S, Achoothesa J (2007) Prevalence of Fasciola species infections of sheep in the middle awash river basin, Ethiopia. Southeast Asian J Trop Med Public Health 38: 51-52.
- Graber M (1978) Helminthes and helminthiasis of domestic and wild animals of Ethiopia. Bull Anim Health Prod Afr 23:57-86.
- Dechasa T, Anteneh W, Dechasa G (2012) Prevalence, gross pathological lesions and economic losses of bovine fasciolosis at Jimma municipal Abattoir, Ethiopia. J Vet Med Anim Health 4: 6-11.
- Dawit K, Adem H (2011) Abattoir survey on the prevalence and monitory loss associated with fasciolosis in sheep and goats. Int J Livest Prod 2:138-141.
- Sandra MTM, Maria LS (2003) Fasciola hepatica infection in cattle and buffaloes in the state of Rio Grand dosul, Brazil. Parasitol Latinoam 58:169-172.
- Rahmeto A, Fufa A, Mulugeta B, Solomon M, Bekele M, et al. (2010) Fasciolosis: Prevalence, financial losses due to liver condemnation and evaluation of a simple sedimentation diagnostic technique in cattle slaughtered at Hawassa Municipal abattoir, southern Ethiopia. J Ethiop Vet 14:39-51.
- Michael A (2004): Infection prevalence of ovine fasciolosis in irrigation schemes along the upper Awash River basin and effects of strategic anthelminthic treatment in selected up stream areas. MS Thesis. Addis Ababa University, Ethiopia 79.
- Hansen I, Perry B (1994) The epidemiology, Diagnoses and Control of Helminth parasite of ruminants. A hand book for Research epidemiology. International Laboratory on Animal Disease (ILARD), Nairobi, Kenya, 171.
- Yilma J, Malone JB (1998) A geographical information system forecast model for strategic control of fasciolosis in Ethiopia. Vet Parasitol 78:103-127.
[Crossref] [Googlescholar] [Indexed]
- Radostits OM, Gay CC, Hinchcliff KW, Blood DC, Constable PD (2007) A text book of the disease of cattle, sheep, goats, pigs and horse: Veterinary Medicine. 10th ed. Saunders Elsevier, New York, London, 1516- 1579.
- Spithill TW, Dalton JP (1998) Progress in development of liver fluke vaccines. Parasitol 14:224-228.
- Mas-Coma S, Bargues MD, Valero MA (2005) Fascioliasis and other plant borne trematode zoonoses. Int J Parasitol 35: 1255–78.
- Mas-Coma S (2004) Human fasciolosis: epidemiological patterns in human endemic areas of South America, Africa and Asia. Southeast Asian J Trop Med Public Health 35:1-11.
- Malone JB (1997) The landscape epidemiology of fasciolosis geographic determinants of disease risk. In immunology, pathophysiology and control of fasciolosis . Round Table Conference at theVIIIth International Congress of Parasitology, Izmir, Turkey. Merck AgVet, Rahway, New Jersey, 65-81.
- Mihreteab B, Haftom T, Yehenew G (2010) Bovine fasciolosis: Prevalence and its economic loose due to liver condemnation at Adwa Municipal Abattoir, North Ethiopia. Ethiop J Appl Sci technol 1: 39-47.
- Suhardono, Roberts JA, Copeman DB (2006) The effect of temperature and humidity on longevity of metacercariae of Fasciola gigantica. Trop Anim Health Prod 38: 371- 377.
[Crossref] [Googlescholar] [Indexed]
- Pedro O, Maria C, Juan J, John C, Diana W (2000) Human fasciolosis: prevalence and treatment in a rural area of Peru. Infect Dis Rev 2:42-46.
- Dusak A, Onur MR, Cicek M, Firat U, Ren T, et al. (2012) Radiological making features of Fasciola hepatica infection Pictoral review. J Clin Imaging Sci 2:2.
- Soliman MFM (2008) Epidemiological review of human and animal fasciolosis in Egypt. J Infect Dev Ctries 2:182-189.
[Crossref] [Googlescholar] [Indexed]
- Soulsby EJL (1982): Helminthes, arthropods and protozoa of omestic animals. (7th ed). Bailliere, Tindall, London, UK, 40-52.
- Solomon W, Abebe W (2007) Effects of a strategic anthihelmintic treatment intervention bovine fasciolosis: A study conducted in facility endemic area in north western Ethiopia, Ethiopia. Int J Vet Med 11: 56-68.
- Kithuka JM, Maingim N, Njeruh FM, Ombui JN (2002) The Prevalence and economic impact due to bovine fasciolosis in Kenya. An analysis of abattoir data and erestepoorl. J Vet Res South Africa. 69: 256-262.
- Garcia HH, Moro PL, Schantz PM (2007) Zoonotic helminth infections of humans: echinococcosis, cysticercosis and fascioliasis. Current Opin Infect Dis 20: 489–494.
- Ramajo V, Oleaga A, Casanueva P, Hillyer GV, Muro A (2001) Vaccination of cattle against Fasciola hepatica with homologous fatty acid binding proteins. Vet Parasitol 97:35-46.
[Crossref] [Googlescholar] [Indexed]
- Hien TV, Dung TTK, Chi NH, Dahn PH, Pham PT (2001) Fasciolosis in Vietnam. Southeast Asian J Trop Med Public Health 32:48–50.
- Ibarra F, Vera Y, Quiroz H (2004) Determination of the effective dose of an experimental fasciolicide in naturally and experimentally infected cattle. Vet Parasitol 120:65–74.
- Ayalew S, Endalkachew N (2013) Prevalence and risk factors of bovine and bovine fasciolosis and evaluation of direct sedimentation sensitivity method at Bahir Dar municipal abattoir, Northern Ethiopia. Ethiop Vet J 17: 1-17.
 Spanish
Spanish  Chinese
Chinese  Russian
Russian  German
German  French
French  Japanese
Japanese  Portuguese
Portuguese  Hindi
Hindi