Research Article, J Regen Med Vol: 11 Issue: 4
The Effectiveness of Bone Marrow Aspirate Injection for Osteoarthritis of the Hip
Nicholas Tsitsilianos1*, Zainab Shirazi1, Jaspal Ricky Singh2, Jessica Lu2
1Department of Rehabilitation and Regenerative Medicine, Columbia-Cornell, 1320 York Avenue, New York
2Department Rehabilitation Medicine, Weill Cornell Medical College, 1320 York Avenue, New York
*Corresponding Author: Nicholas Tsitsilianos
Regenerative Medicine, Columbia-Cornell, 1320 York Avenue, New York
E-mail: Nicholas_tsitsilianos@outlook.com
Received: 02-Jul-2022, Manuscript No. JRGM-22-68361;
Editor assigned: 04-Jul-2022, PreQC No. JRGM-22-68361(PQ);
Reviewed: 18-Jul-2022, QC No. JRGM-22-68361;
Revised: 20-Jul-2022, Manuscript No. JRGM-22-68361(R);
Published: 26-Jul-2022, DOI: 10.4172/2325-9620.1000220
Citation: Tsitsilianos N, Shirazi Z, Ricky J, Lu J (2022) The Effectiveness of Bone Marrow Aspirate for Osteoarthritis of the Hip. J Regen Med 11:4
Abstract
Objectives: Bone Marrow Aspirate (BMA) intra-articular injection is a minimally invasive orthobiologic treatments option for Osteoarthritis (OA). Hip OA affects a significant amount of the population and has a paucity of data surrounding orthobiologic treatments. The primary objective of this study was to delineate the clinical impact of bone marrow aspirate intra-articular injections on decreasing pain and improving function in patients with hip OA.
Methods: Here we present a single-centre, retrospective analysis of thirty-one subjects, aged 32 to 83 (62.4 ± 16.5), with Kellgren- Lawrence (KL) Hip OA grading of 2-4 (mean 2.9 ± 0.7), who underwent intra-articular bone marrow aspirate injection into the hip and were followed for twelve months. Outcome measures were at baseline, twelve weeks, six months, and twelve months using the Visual Analog Scale (VAS) for pain and the Hip Disability and Osteoarthritis Outcome Score Jr (HOOS-Jr) for function. The proportion of responders, as defined by a ≥ 50% reduction in VAS pain score, was assessed at 12 weeks, six months and twelve months.
Results: At six and twelve months follow up, there was a statistically significant improvement in VAS scores (P<0.05). Stratifying by KL grade, subject with KL grades 2 and 3, experienced statistically significant improvement in VAS scores at six and twelve months. KL grade 4 showed significant improvement in pain at twelve months. Twenty-three present of responders at six months and 61% at twelve months reported ≥ 50% reduction in pain. When stratifying by KL grade, 80% and 71% of KL2 and KL3 grades respectively were responders by 12 months. Subjects experienced statistically significant improvement in HOOS-Jr scores at six-month and twelve-month.
Conclusion: Our study suggests that BMA may improve pain and function for patients with mild, moderate, and severe hip OA from 12 weeks to 12 months.
Keywords: Autologous bone marrow concentrates; Knee osteoarthritis; Knee pain; Cell therapy.
Introduction
Osteoarthritis (OA) is the most common form of arthritis and is defined as a progressive degenerative process affecting the joints in our body [1]. In 2017, OA was estimated to have affected over 300 million people on a global scale [2]. The knee is the most common joint diagnosed with OA. However, hip OA also affects a significant amount of the population with studies reporting a prevalence of symptomatic hip OA as high as 9.2% among adults age 45 years and older in the United States in 2009 [3,4]. The prevalence of hip OA is higher in men before age 50, but becomes higher in women over the age of 50 [5]. Classically, treatment includes physical therapy, anti-inflammatory medication, joint injection with either steroid or viscosupplementaction, and joint replacement. The majority of hip replacements are occurring in patients over 65 years of age however as the prevalence of hip OA increases, studies over the last decade indicate that more than 50% of total hip arthroplasties will be performed in patients younger than 65 by 2030 [6].
Many argue that there is a large gap in the treatment options available for OA between that of conservative management and surgical intervention. In an attempt to address this gap and offer more options to patients with this condition, there has been increasing interest and investigation into the field of Regenerative Medicine. There are several biologically based treatments of interest for OA. Platelet rich plasma (PRP), Bone Marrow Aspirates (BMA), and adipose derived stem cells are three of the most common treatments currently available and being studied. These products attempt to harness the body’s own innate healing potential to relieve pain, improve function, and potentially alter the biologic environment within the joint to promote healing and regeneration. PRP is an autologous product derived from whole blood and processed to contain concentrated platelets [7]. These platelets contain growth factors that can help signal a healing cascade for various tissues.
There is growing evidence that PRP is safe and effective in improving patients’ pain and function when used to treat OA via intra-articular injection. Recently, PRP was shown to be superior to other commonly used methods of conservative treatment. In the 2017 and 2020 meta-analyses by Shen et al. and Migliorini et al. respectively, the authors found PRP to be superior to both viscosupplementation and steroid for improving pain and function in knee OA for up to 12 months post injection [8,9]. Though less data exists for the use of PRP in the hip, recent studies have demonstrated similar results. Singh et al found PRP to be effective in reducing pain and improving function in a retrospective analysis of 36 patients who received a single intraarticular injection of PRP for hip OA [10].
While PRP is a known source of various beneficial growth factors, cells known as Mesenchymal Stem Cells (MSCs) may offer a more robust signaling cascade and healing process. Bone marrow and adipose tissue are two areas of the body that are currently being utilized to harvest autologous MSCs for the treatment of musculoskeletal conditions. Bone marrow aspirate can be further concentrated with centrifugation to produce bone marrow aspirate concentrate BMAC which is more widely studied compared to BMA alone. From the work of Caplan et al. MSCs are now thought to act more as medicinal signaling cells in the in vivo environment. MSCs are derived from pericytes and it is thought that they establish a regenerative environment by having anti-apoptotic, anti-scarring, mitotic and angiogenic affects [11]. At this point, it is unclear if one source is superior to the other, as Mautner et al found that both Bone Marrow Aspirates Concentrate (BMAC) and microfragmented adipose tissue resulted in a similarly significant improvement in pain and function for symptomatic knee OA [12].
By harvesting bone marrow, most commonly from the posterior iliac crest, we are able to concentrate the product to contain an increased amount of MSCs, platelets, and various other growth factors. Unfortunately, there is a paucity of data reporting the effect of BMAC on OA in the hip, as the majority of available data centers around the knee. The aim of this investigation is to report the clinical effectiveness of image-guided BMA injections for the treatment of hip OA.
Materials and Methods
This study was performed at a single-center outpatient rehabilitation office at a large tertiary care hospital. After institutional review board approval was obtained, the records of patients diagnosed with hip osteoarthritis and treated with BMA between January 2017 and January 2021 were obtained using International Classification of Diseases (ICD-9) codes and Current Procedural Terminology (CPT) codes. Inclusion criteria were age 18 to 99 years, hip pain for at least four months, at least one positive physical exam maneuver(s) including Internal Rotation Over Pressure (IROP) and hip Flexion Adduction and Internal Rotation (FADIR), diagnosis of hip osteoarthritis on plain radiograph, failure to improve satisfactorily (defined by the patient as intolerable pain and functional limitations) with physical therapy (minimum three months), and oral pain medications +/- intra-articular steroid injections. Exclusion criteria were patients with a prior history of hip surgery and those who refused BMA. In addition, patients who received a steroid injection into the hip within three months, were taking NSAIDs or antiplatelet medications, or had any signs of infection were also excluded from the study. After a thorough review of the medical records, thirty-one patients fulfilled the inclusion criteria.
Hip Osteoarthritis Classification
The Kellgren and Lawrence system is a method of classifying the severity of knee osteoarthritis (OA) using five grades [13].
• Grade 0: no radiographic features of OA are present;
• Grade 1: doubtful joint space narrowing (JSN) and possible osteophytic lipping;
• Grade 2: definite osteophytes and possible JSN on anteroposterior weight-bearing radiograph;
• Grade 3: multiple osteophytes, definite JSN, sclerosis, possible bony deformity;
• Grade 4: large osteophytes, marked JSN, severe sclerosis, and definite bony deformity.
Bone Marrow Aspirate: Preparation
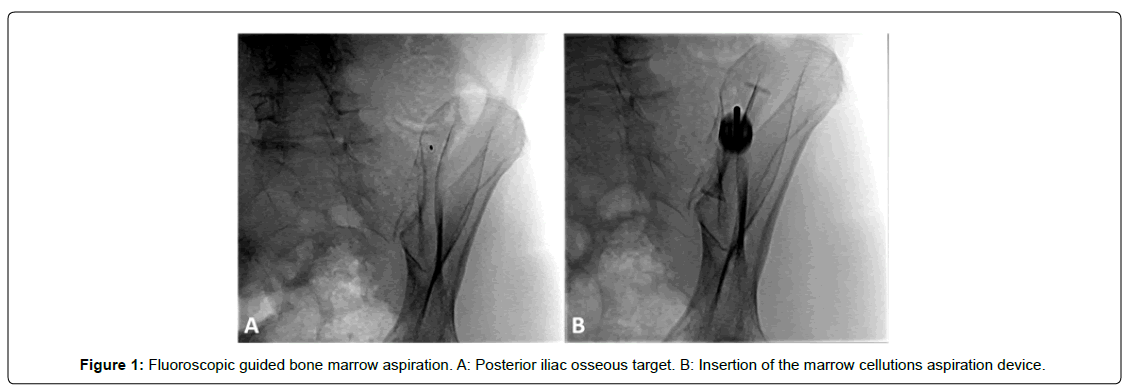
A single, fellowship-trained, board-certified physiatrist (JRS) performed the procedure. For the aspiration, the patient was placed in the prone position. The fluoroscope was used to maximally profile the posterior iliac bone utilizing an oblique anterior-posterior projection with the image detector obliquely rotated towards the contralateral iliac bone [Figure 1]. Once the posterior iliac bone is profiled, a skin needle entry site (osseous target site) is selected and marked along the middle third posterior iliac bone at the central medullary space. Once subcutaneous and periosteal anesthesia is achieved, intermittent fluoroscopy is used to ensure that the biopsy needle follows a “bull’s eye” trajectory, parallel to the X-ray beam, into the posterior iliac bone at the planned osseous entry site and then along the long axis of the iliac bone in anterior-posterior plane.
Following optimal fluoroscopic positioning, 10cc of 1,000 units/ mL heparin were withdrawn into a 10-mL syringe. After the syringe was connected to the introducer needle, heparin was injected until the introducer needle was fully rinsed and then aspirated back into the syringe. This process was repeated for the longer aspiration needle. All stylets were then rinsed with heparin. Following this, 1.0 mL heparin was added to the 10-mL collection syringe. Under fluoroscopic guidance, bone marrow was aspirated from the posterior iliac crest using the Marrow Cellution Bone Marrow Harvesting Device (Ranfac Corp., Avon, MA) and consistent with best practice guidelines and expert consensus technique. Once proper localization was confirmed by attaching the syringe and drawing 1 mL, the syringe was removed, and the blunt stylet was inserted to drive the access needle to the necessary depth. When the outer housing reached skin level, the blunt stylet was removed, the aspiration cannula was attached to the access needle, and the syringe was attached. The physician then held the outer housing in place while rotating with the opposite hand 360° to raise the cannula tip 0.75 cm into a new location. This rotation/ aspiration technique was repeated 5-6 times to obtain approximately 6-8 mL BMA [14].
BMA Injection Technique
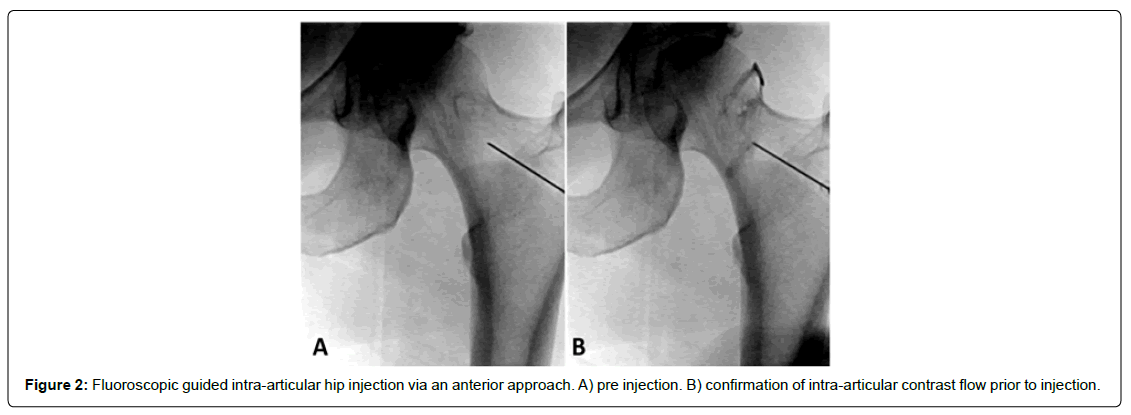
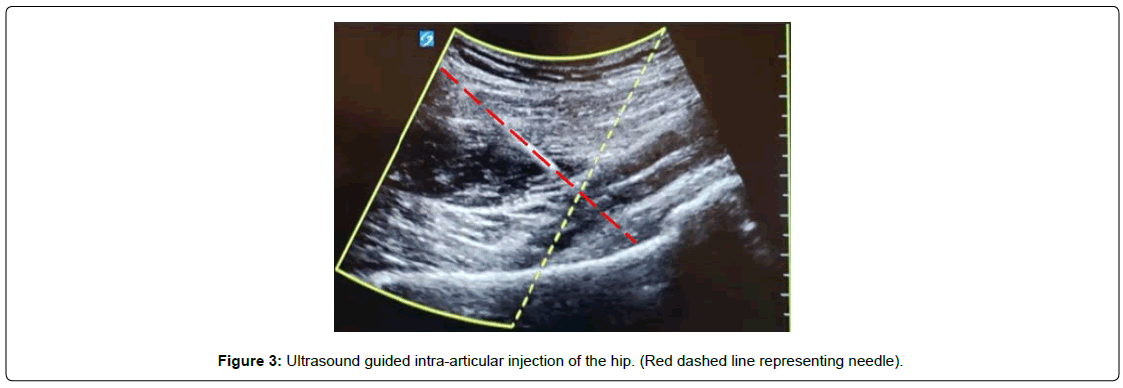
Injection of BMA was performed using two (fluoroscopic or ultrasound) imaging-guided techniques. For the fluoroscopically guided procedure, the patient was placed in a supine position and prepped and draped in typical sterile fashion. Using antero-posterior fluoroscopic imaging, the skin was marked at a spot over the centre of the femoral neck. A skin wheal using a 25-gauge needle was made, and deeper structures were anesthetized using local anesthetic. Once anesthetized, a 22-gauge, 3.5-inch spinal needle was directed toward the junction of the femoral head and neck [Figure 2]. Once osseous contact was made, radio-opaque contrast medium was injected to confirm intra-articular flow. Reasons for using the anteroposterior fluoroscopic approach include it ability to allow the anterior musculature to relax offering a procedure advantage, as well as for comfort when patients cannot tolerate the lateral decubitus position for a lateral approach. Using ultrasound guidance, the anterior hip joint was directly visualized by placing the transducer longitudinally at the femoral head-neck junction [Figure 3]. Ultrasound offers the advantage of minimizing radiation exposure as well as visualization of other structures to avoid such as surrounding vasculature. Although depending on the patient’s body habitus it can become difficult to visualize deeper structures. Following the sterile prep, the skin and subcutaneous tissues were anesthetized, and through this anesthetized track, a syringe containing 3 mL of 1% lidocaine attached to a 3.5-inch 22-gauge spinal needle was inserted approximately 3 cm. Using sterile ultrasound gel, the needle was guided toward the anterior joint capsule. Once the capsule was penetrated, the syringe containing the BMA was attached, and the injectate was delivered. A detailed depiction of the procedural technique with images has been previously described by Yasar et al. [15]. BMA was injected into the intra-articular and subcapsular space until resistance was met; between 6-8 cc of bone marrow aspirate was injected into the joint and extracapsular space. Immediately after the procedure, the needle was removed, and a sterile Band-Aid was placed over the injection site.
Post BMA Protocol
Study subjects conducted a standard post procedure protocol which includes progressive and evolving percautions, therapy goals, home exercises from Day 1 to 28 post procedure, as detailed in appendix A below.
Outcome Measures
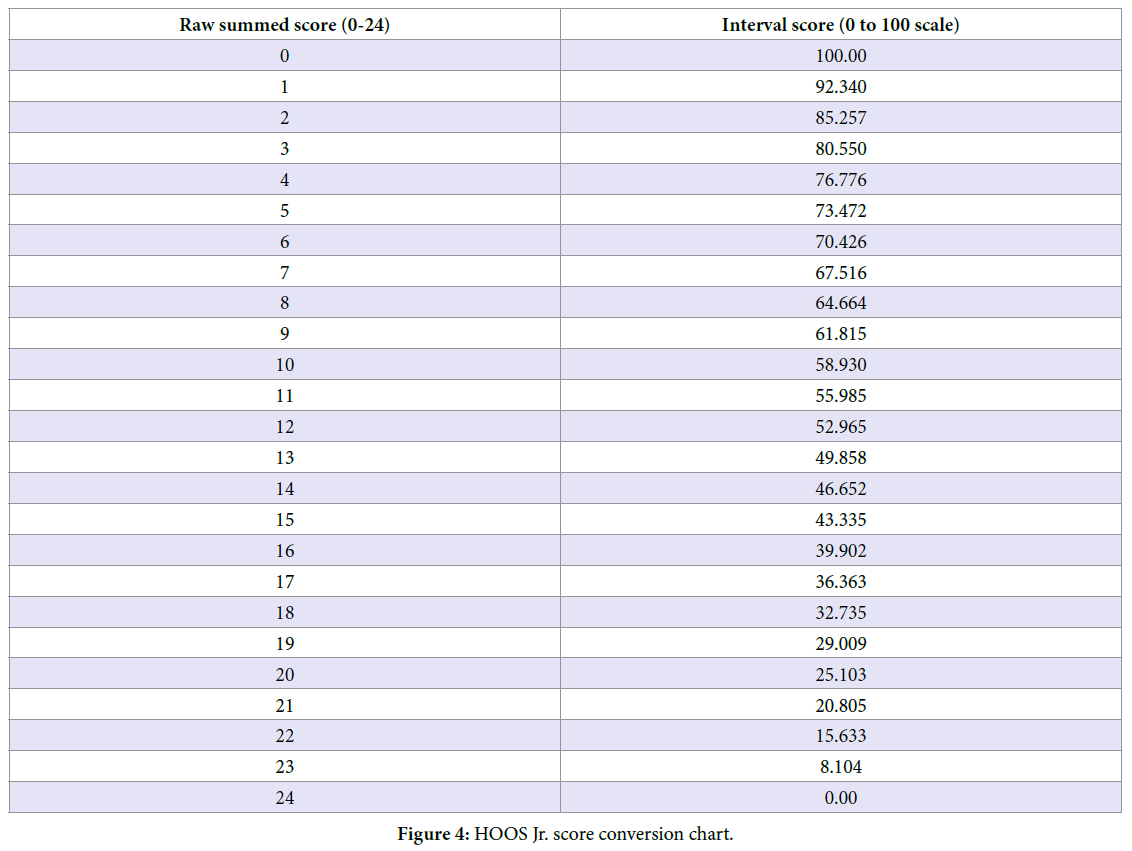
The primary outcome measure of this study was reduction in hip pain, as quantified by a scale of 1-10 Visual Analog Scale (VAS) for pain intensity; lower scores were indicative of less pain. The Hip Disability and Osteoarthritis Outcome Score Jr (HOOS-Jr) was used as a primary outcome measure. The HOOS-Jr is modified from the longer HOOS score which was designed as a means to evaluate the opinion of adults with hip disability, regardless of the presence of osteoarthritis [16]. The HOOS-Jr is a 6 item questionnaire which focuses on 3 subcategories: joint pain, stiffness, and function. Each item is answered on a scale of 0-4. Sums of the raw score (0-24) are then converted to an interval score ranges from 0 to 100 using the chart below [Figure 4], where 0 represents total hip disability and 100 represents perfect hip. A secondary outcome measure was to analyze the proportion of responders in this study. Responders were defined as patients who reported a >50% improvement on pain scores assessed at each time interval.
Statistical Analysis
Descriptive statistics (including mean, standard deviation, median, range, frequency, and percentage) were calculated to characterize the patient population. The one-sample paired t test was used to compare VAS and HOOS values between 1) pre-injection (baseline) and twelve weeks after the procedure, 2) pre-injection (baseline) and six months after the procedure, and 3) pre-injection (baseline) and twelve months after the procedure. In addition, patients were stratified by Kellgren-Lawrence classification, and a one-sample paired t test was used to compare VAS values between 1) pre-injection (baseline) and twelve weeks after the procedure, 2) pre-injection (baseline) and six months after the procedure, and 3) pre-injection (baseline) and twelve months after the procedure.
For responder analysis, a chi-square test was used to compare the proportion of responders to nonresponders in terms of radiographic grade of hip arthritis, defined by the Kellgren-Lawrence scale. Statistical significance was defined at a P value of <5% (P<0.05).
Results
A total of thirty-one subjects qualified for this study and underwent bone marrow aspirate injection for osteoarthritis of the hip. The average patient age (range) was 62.4 ± 16.5 (32 to 83) years, with 52% of subjects being female and 48% male. The baseline VAS as a group was 6.2 ± 2.0. Demographic data, along with stratification based on Kellgren-Lawrence scale, can be found in Table 1.
| Age, mean ± SD, y | 62.5 ± 16.5 |
| Gender, No. (%) | |
| Male | 15 (48.4) |
| Female | 16 (51.6) |
| Pain at baseline, mean ± SD | 6.2 ± 2.0 |
| Kellgren-Lawrence Hip Grading, mean ± SD | 2.9 ± 0.7 |
| Kellgren Lawrence Hip Grading, No. (%) | |
| 0 | 0 (0) |
| 1 | 0 (0) |
| 2 | 10 (32) |
| 3 | 14 (45) |
| 4 | 7 (23) |
Table 1: Baseline demographic information (N = 31).
Visual Analog Scale Scores (Cohort)
The average VAS score for the group was 6.2 ± 2.0 at baseline. At twelve weeks, six months, and twelve months, it was 5.9 ± 2.1, 3.8 ± 2.6, and 3.0 ± 1.4 respectively. Although there was no significant improvement in pain at twelve weeks (P=0.4), there was a statistically significant improvement at both six-month and twelve-month follow up (P<0.05) Table 2.
| Baseline | 12 wk | Baseline vs 12 wk, 95% CI; P | 6 mo | Baseline vs 6 mo, 95% CI; P | 12 mo | Baseline vs 12 mo, 95% CI; P |
|---|---|---|---|---|---|---|
| 6.2 ± 2.0 | 5.9 ± 2.1 | -0.5 to 3.3; 0.4 | 3.8 ± 2.6* | 0.7 to 4.1; <0.05* | 3.0 ± 1.4* | 3.2 to 4.8; <0.05* |
| CI = confidence interval; VAS = Visual Analog Scale. *Statistically significant | ||||||
Table 2: Change in VAS from baseline.
Visual Analog Scale Scores (Stratified by Kellgren-Lawrence)
No patients showed significant improvement in pain at twelve weeks. However, in patients whose radiographic hip arthritis grade was KL grades 2 and 3, there was a significant improvement in pain at both six months and twelve months [Tables 3 and 4]. Conversely, patients who suffered from severe hip arthritis (KL grade 4) only showed significant improvement in pain at twelve months [Table 5].
| Baseline | 12 wk | Baseline vs 12 wk, 95% CI; P | 6 mo | Baseline vs 6 mo, 95% CI; P | 12 mo | Baseline vs 12 mo, 95% CI; P |
|---|---|---|---|---|---|---|
| 6.5 ± 2.0 | 5.1 ± 1.7 | 0.5 to 3.3; 0.1 | 3.0 ± 2.8 | 0.7 to 6.3; <0.05* | 3.0 ± 1.8 | 1.6 to 5.4; <0.05* |
Table 3: Change in VAS from baseline (Kellgren/Lawrence Grade 2)
| N = 14 | ||||||
| Baseline | 12 wk | Baseline vs 12 wk, 95% CI; P | 6 mo | Baseline vs 6 mo, 95% CI; P | 12 mo | Baseline vs 12 mo, 95% CI; P |
| 6.4 ± 2.1 | 6.4 ± 2.3 | -1.6 to 1.5; 0.9 | 3.6 ± 2.6 | 1.3 to 4.2; <0.05* | 2.5 ± 1.0 | 2.4 to 5.3; <0.05* |
Table 4: Change in VAS from baseline (Kellgren/Lawrence Grade 3).
| N = 10 | ||||||
| Baseline | 12 wk | Baseline vs 12 wk, 95% CI; P | 6 mo | Baseline vs 6 mo, 95% CI; P | 12 mo | Baseline vs 12 mo, 95% CI; P |
| 5.6 ± 1.6 | 5.9 ± 2.0 | -1.7 to 1.1; 0.6 | 5.3 ± 1.9 | -1.3 to 1.9; 0.7 | 3.9 ± 1.2 | 0.4 to 3.0; <0.05* |
Table 5: Change in VAS from baseline (Kellgren/Lawrence Grade 4).
Responder Analysis
Response to bone marrow aspirate for the treatment of hip arthritis was defined by a ≥ 50% reduction in pain scores. When analyzing the group as a whole, 23% of the group at six months and 61% of the group at twelve months reported ≥ 50% reduction in pain [Table 6]. However, stratifying the proportion of responders by the Kellgren-Lawrence scale revealed more specific sub-set data. Even though there were more responders in the KL2 and KL3 grades compared to the KL4 group at twelve weeks and six months, there were no statistically significant differences at these time intervals [Table 7 and 8]. However, at twelve months, there was a statistically significant difference between the 80% and 71% of responders in the KL2 and KL3 grades, respectively, compared to the 14% of responders in the KL4 grade. Of note, only 1 patient out of 7 with KL4 grade arthritis ever responded to treatment, reporting a ≥ 50% pain reduction at 12 months [Table 9].
| VAS 12 wk, No. (%) | 95% CI | VAS 6 mo, No. (%) | 95% CI | VAS 12 mo, No. (%) | 95% CI | |
| Responders | 5/31 (16) | 1.0 to 9.0 | 7/31 (23) | 2.5 to 11.7 | 19/31 (61) | 13.6 to 24.2 |
| Nonresponders | 26/31 (84) | 22.0 to 30.0 | 24/31 (77) | 19.3 to 28.5 | 12/31 (39) | 6.8 to 17.4 |
Table 6: >50% VAS reduction from baseline.
| Responders at 12 wk | ||||
| Kellgren-Lawrence Grade 2 | Kellgren-Lawrence Grade 3 | Kellgren-Lawrence Grade 4 | ||
| Responders (<50% reduction on VAS) | 3 | 2 | 0 | 5 |
| 1.61 | 2.25 | 1.12 | ||
| -1.19 | -0.029 | -1.13 | ||
| Nonresponders | 7 | 12 | 7 | 26 |
| 8.39 | 11.74 | 5.87 | ||
| -0.23 | -0.0057 | -0.22 | ||
| 10 | 14 | 7 | 31 | |
Table 7: Responder characteristics based on K-L Grade at 12 weeks.
| Responders at 6 mo | ||||
| Kellgren-Lawrence Grade 2 | Kellgren-Lawrence Grade 3 | Kellgren-Lawrence Grade 4 | ||
| Responders (<50% reduction on VAS) | 2 | 5 | 0 | 7 |
| 2.25 | 3.16 | 1.58 | ||
| -0.029 | -1.07 | -1.58 | ||
| Nonresponders | 8 | 9 | 7 | 24 |
| 7.74 | 10.84 | 5.42 | ||
| -0.0086 | -0.31 | -0.46 | ||
| 10 | 14 | 7 | 31 | |
Table 8: Responder characteristics based on K-L Grade at 6 months.
| Responders at 12 mo | ||||
| Kellgren-Lawrence Grade 2 | Kellgren-Lawrence Grade 3 | Kellgren-Lawrence Grade 4 | ||
| Responders (<50% reduction on VAS) | 8 | 10 | 1 | 19 |
| 6.13 | 8.58 | 4.29 | ||
| -0.57 | -0.24 | -2.52 | ||
| Nonresponders | 2 | 4 | 6 | 12 |
| 3.87 | 5.42 | 2.71 | ||
| -0.9 | -0.37 | -4 | ||
| 10 | 14 | 7 | 31 | |
Table 9: Responder characteristics based on K-L Grade at 12 months.
Hip Disability and Osteoarthritis Outcome Score, Joint Replacement
When analysing functional outcomes, the HOOS, JR scale was used. The group as a whole revealed statistically significant improvement in function from baseline at the six-month and twelvemonth follow up, but no statistically significant improvement at the twelve-week follow up [Table 10].
| Baseline | 12 wk | 6 mo | 12 mo | |
| HOOS, JR Score | 17.9 ± 3.9 | 16.1 ± 4.6 | 11.6 ± 4.6* | 9.2 ± 5.1* |
Table 10: Change in HOOS, JR score from Baseline.
Discussion
The primary objective of this study was to delineate the clinical impact of BMA on decreasing pain and improving function in patients with hip OA. There was no loss to follow up in this retrospective analysis. A complete set of data was collected from each participant and allowed for a comprehensive statistical analysis.
At 12 weeks post-injection, there was no statistically significant improvement in VAS or HOOS Jr. In contrast, subsequent followup at 6 and 12 months revealed a significant improvement in both outcome measures. This demonstrates the time-dependent effect of BMA in improving pain and function in patients with hip OA. Additionally, stratification of patients by KL grades showed that patients with severe hip OA (KL grade 4) did not experience a significant improvement in pain (quantified by VAS) until 12 months post-injection. This highlights the additive effects of hip OA severity (quantified by KL grading) and post-injection time, suggesting that patients who have progressed to severe disease will experience a greater delay in clinically significant pain reduction following BMA injection.
The results of this study have several clinical implications. First, the data suggests that BMA injection can provide long-term pain relief and functional restoration (as much as 6 to 12 months) while avoiding the complications/risks, prolonged recovery time, and added cost associated with surgical intervention. Second, patients with KL grades 2-3 experienced a sooner reduction in pain (compared to KL grade 4), which suggests that earlier intervention with BMA can significantly improve quality of life in patients with hip OA. Finally, these findings may encourage clinicians to shift toward using BMA rather than intra-articular Corticosteroid Injections (CSI).
Although CSI are commonly used to relieve pain and restore function in patients with OA, they only provide short-term benefits and may contribute to cartilage degeneration and disease progression [17]. In contrast, studies have shown that orthobiologics (such as HA, PRP, and BMAC) regulate inflammation and promote cartilage healing, which would improve the joint complex itself rather than simply mitigating pain [18-23]. According to a recent meta-analysis, intra-articular injections of PRP resulted in the best overall outcome (with regards to both pain and function) compared to CSI, HA, and placebo for patients with knee OA from 3 to 12 months postinjection [24]. A 2021 retrospective analysis concluded that BMAC was safe and superior when compared with PRP in knee OA [25]. In another retrospective analysis of 505 patients with knee OA, BMAC was shown to be superior to both HA and Autologous Conditioned Serum (ACS) in reducing pain and improving functional outcomes. Furthermore, only BMAC demonstrated improved functional outcomes even in patients with more severe degenerative changes [26]. Although these findings are limited to OA in the knee, it would be logical to extrapolate the same effects to the hip.
Current research regarding the therapeutic efficacy of BMAC for symptom management in patients with hip OA is severely lacking. To our knowledge, there is only one study that investigated the role of BMAC in pain and function, which showed a statistically significant improvement in both outcome measures for up to 6 months [27]. However, the sample size was limited to 18 hips and a shorter duration of follow-up compared to our study’s duration of 1 year.
A unique aspect of this study was our use of a single site ‘Marrow Cellutions’ Bone Marrow aspiration system (MC system) which did not involve concentration of the aspirate as is done in BMAC preparations. Scapone et al showed that the MC system produced concentration of CFU-fs, CD34+ cells and CD117+ cells that were comparable or greater to BMAC [14]. There have been no studies documenting the superiority of BMAC vs BMA without concentration; however BMA is less costly and easier to institute. Therefore, our study highlights the potential for BMA without concentrate to serve as an alternative injectate that is more feasible in clinical practice, though further research is needed to investigate the comparative efficacy.
One limitation of this study was its retrospective design, which by nature prevents blinding and establishment of a control group. This has the potential to introduce recall bias into the study. In addition, we were unable to quantify the harvested cell counts used, which limited further analysis and identification of a potential dose response correlation.
Conclusion
Further research is required to demonstrate the efficacy of intraarticular injection of BMA for hip OA. Our study suggests that BMA may be an effective treatment for patients with not only mild to moderate, but also severe hip OA in regards to improving pain and function from 12 weeks to 12 months. This data suggests that BMA can potentially delay or prevent invasive and expensive joint replacement surgery. A larger prospective randomized controlled trial is warranted in order to further characterize the efficacy of BMA for the treatment of hip OA.
Acknowledgment
The authors would like to thank Mrs. Althea Dursten and Mr. Norman Eig for their generosity in providing funding for this study.
References
- Prevention CFDCA (2020) Osteoarthritis (OA).
- Kloppenburg M, Berenbaum F (2020) Osteoarthritis year in review 2019: epidemiology and therapy. Osteoarthritis Cartilage, 28(3): 242-248.
- Felson DT (1998) Preventing knee and hip osteoarthritis. Bull Rheum Dis, 47(7): 1-4.
- Lespasio MJ (2018) Hip Osteoarthritis: A Primer. Perm J, 22: 17-84.
- Jordan JM (2009) Prevalence of hip symptoms and radiographic and symptomatic hip osteoarthritis in African Americans and Caucasians: the Johnston County Osteoarthritis Project. J Rheumatol, 36(4): 809-815.
- Patel A, Pavlou G, Mujica-Mota RE, Toms AD (2015) The epidemiology of revision total knee and hip arthroplasty in England and Wales: A comparative analysis with projections for the United States. A study using the National Joint Registry dataset. Bone Jt J, 97: 1076-1081.
- Arnoczky SP, Sheibani-Rad S, Shebani-Rad S (2013) The basic science of platelet-rich plasma (PRP): what clinicians need to know. Sports Med Arthrosc Rev, 21(4): 180-185.
- Shen L (2017) The temporal effect of platelet-rich plasma on pain and physical function in the treatment of knee osteoarthritis: systematic review and meta-analysis of randomized controlled trials. J Orthop Surg Res, 12(1): 1-16.
- Migliorini F (2020) Comparison between intra-articular infiltrations of placebo, steroids, hyaluronic and PRP for knee osteoarthritis: a Bayesian network meta-analysis. Arch Orthop Trauma Surg.
- Singh JR (2019) The Effectiveness of Autologous Platelet-Rich Plasma for Osteoarthritis of the Hip: A Retrospective Analysis. Pain Med, 20(8): 1611-1618.
- Caplan AI (2017) Mesenchymal Stem Cells: Time to Change the Name! Stem Cells Transl Med, 6(6): 1445-1451.
- Mautner K (2019) Functional Outcomes Following Microfragmented Adipose Tissue Versus Bone Marrow Aspirate Concentrate Injections for Symptomatic Knee Osteoarthritis. Stem Cells Transl Med, 8(11): 1149-1156.
- Kellgren JH, Lawrence JS (1957) Radiological assessment of osteo-arthrosis. Ann Rheum Dis, 16 (4): 494-502.
- Scarpone M, Kuebler D, Chambers A (2019) Isolation of clinically relevant concentrations of bone marrow mesenchymal stem cells without centrifugation. J Transl Med, 17,1-10.
- Yasar E, Singh JR, Hill J, (2014) Image-guided injections of the hip. J Novel Physiother Phys Rehabil, 1-10.
- Nilsdotter A, Bremander A (2011) Measures of hip function and symptoms: Harris Hip Score (HHS), Hip Disability and Osteoarthritis Outcome Score Autologous (HOOS), Oxford Hip Score (OHS), Lequesne Index of Severity for Osteoarthritis of the Hip (LISOH), and American Academy of Orthopedic Surgeons. Arthritis Care Res, 63: 200-207.
- McAlindon TE, LaValley MP, Harvey WF (2017) Effect of intra-articular triamcinolone vs saline on knee cartilage volume and pain in patients with knee osteoarthritis: A randomized clinical trial. JAMA, 317: 1967-1975.
- Kingery MT, Manjunath, AK, Anil U, Strauss EJ (2019) Bone marrow mesenchymal stem cell therapy and related bone marrow-derived orthobiologic therapeutics. Curr Rev Musculoskelet Med, 2019; 12, 451-459.
- Centeno CJ, Pastoriza SM (2020) Past, current and future interventional orthobiologics techniques and how they relate to regenerative rehabilitation: A clinical commentary. Int J Sports Phys Ther, 15: 301–325.
- Osterman C, McCarthy MBR, Cote MP, Beitzel K, Bradley J, et al., (2015) Platelet-rich plasma increases anti-inflammatory markers in a human coculture model for osteoarthritis. Am J Sports Med, 43, 1474-1484.
- Everts P, Onishi K, Jayaram P, Lana JF, Mautner K (2020) Platelet-rich plasma: New performance understandings and therapeutic considerations in 2020. Int J Mol Sci, 21, 7794.
- Liou JJ, Rothrauff BB, Alexander PG, Tuan RS (2018) Effect of platelet-rich plasma on chondrogenic differentiation of adipose-and bone marrow-derived mesenchymal stem cells. Tissue Eng Part A, 24: 1432-1443.
- Fice MP, Miller JC, Christian R, Hannon CP, Smyth N, et al., (2019) The role of platelet-rich plasma in cartilage pathology: An updated systematic review of the basic science evidence. Arthrosc J Arthrosc Relat Surg, 35: 961-976.e3.
- Migliorini F, Driessen A, Quack V (2021) Comparison between intra-articular infiltrations of placebo, steroids, hyaluronic and PRP for knee osteoarthritis: a Bayesian network meta-analysis. Arch Orthop Trauma Surg, 141(9):1473-1490.
- El-Kadiry AE, Lumbao C, Salame N, Rafei M, Shammaa R (2022) Bone marrow aspirate concentrate versus platelet-rich plasma for treating knee osteoarthritis: a one-year non-randomized retrospective comparative study. BMC Musculoskelet Disord, 23(1):23.
- Hussein M, van Eck CF, Kregar Velikonja N (2021) Bone Marrow Aspirate Concentrate Is More Effective Than Hyaluronic Acid and Autologous Conditioned Serum in the Treatment of Knee Osteoarthritis: A Retrospective Study of 505 Consecutive Patients. Applied Sciences, 11(7): 2932.
- Whitney KE, Briggs KK, Chamness C, Bolia IK, Huard J, et al. (2020) Bone Marrow Concentrate Injection Treatment Improves Short-term Outcomes in Symptomatic Hip Osteoarthritis Patients: A Pilot Study. Orthop J Sports Med, 8(12):2325967120966162.
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
 Spanish
Spanish  Chinese
Chinese  Russian
Russian  German
German  French
French  Japanese
Japanese  Portuguese
Portuguese  Hindi
Hindi