Research Article, J Vet Med Sci Vol: 11 Issue: 5
The First Report of Eurasian Bearded Vulture (Gypaetus Barbatus) Sex Identification Using CHD1 Gene Markers in Comparison to Alexandrine Parakeet (Psittacula Eupatria), Kestrel (Falco Tinnunculus) and Ring Necked Parakeet (Psittacula Krameri)
Nastaran Sadat Sadrshirazia1*, Mohammad Pakdaman Jirkola1, Iman Memarianb2 and Ruben Khachatryanb2
1Department of Molecular Biology, Azimi Mehdi Molecular biological Institute, Babolsar, Iran
2Department of Veterinary Science, Agricultural and Yerevan Zoo Veterinary Institute, Yerevan, Armenia
*Corresponding Author:Nastaran Sadat Sadrshirazia , Department of Molecular Biology, Azimi Mehdi Molecular biological Institute, Babolsar, Iran, Tel: 00989122022841; E-mail: nshirazi1985@gmail.com
Received date: 23 February, 2022, JVSMD-22-55337; Editor assigned date: 25 February, 2022, PreQC No. JVSMD-22-55337 (PQ); Reviewed date: 10 March, 2022, QC No. JVSMD-22-55337; Revised date: 25 April, 2022, Manuscript No. JVSMD-22-55337 (R); Published date: 02 May, 2022, DOI: 10.4172/2325-9590.1000021
Citation: Sadrshirazia NS, Jirkola MP, Memarianb I, Khachatryanb R (2022) The First Report of Eurasian BeardedVulture (Gypaetus Barbatus) Sex Identification Using CHD1 Gene Markers in Comparison to Alexandrine Parakeet (Psittacula Eupatria), Kestrel (Falco Tinnunculus) and Ring Necked Parakeet (Psittacula Krameri). J Vet Med Sci 11:5.
Abstract
Molecular identification methods based on DNA analysis are essential useful techniques with high sensitivity for sex identification of monomorphic birds. One of the most endangered bird species that has no sexually dimorphism is Bearded vulture (Gypaetus barbatus) which its abundance and breeding range have drastically declined in recent years. In this study comparative PCR sexing assays based on amplification of CHD-1 encoding sequence including 6 different primer sets were used in AMI molecular biological lab institute in Iran to identify the unknown sub adult Bearded vulture sex that is kept and protected in FPWC center in Armenia, Yerevan. Some of the assays were also used on samples taken from Alexandrine parakeet (Psittacula eupatria), Kestrel (Falco tinnunculus) and Ring necked Parakeet (Psittacula krameri). Results showed that P2/P8 and P2/NP primer sets are useful for sex identification of Bearded vulture (Gypaetus barbatus).
Keywords: Bearded vulture; Bird; Sex identification; PCR; CHD-1
Introduction
There are many endangered species of animals including various kind of ornamental poultry and pray birds because of destruction of their natural environment and illegal hunt all over the world. Many of these bird species (almost 60 percent) are sexually monomorphic and there is no way to morphologically distinguish between both sexes [1]. Molecular identification methods based on DNA analysis are essential useful techniques with high sensitivity for sex identification of monomorphic birds [2]. There are useful PCR techniques based on amplification of partial parts of the encoding CHD-1 gene sequence located on both Z and W sex chromosomes in birds, which are two Z chromosomes in male and one Z and one W chromosome in female [3-8]. One of the most endangered bird species is Bearded vulture (Gypaetus barbatus) which its abundance and breeding range have drastically declined in recent years [9]. The bird has been classified into two sub species of GB barbatus or the Eurasian Homa which lives in mountainous regions of the Middle East including the Zagros and Alborz Mountains of Iran, the Caucasus region, the Koh-i-Baba in Bamyan, Afghanistan, the Altai Mountains, the Himalayas, Ladakh in northern India, western and central China. And the sub-species of GB meridionalis or the African Homa [10].
The female usually lays a clutch of 1 to 2 eggs (rarely 3) which are incubated for 53 to 60 days and the chicks are dependent to their parents for up to two years. It usually takes 5 years to reach full maturity [11]. Since this species has no morphological sexual dimorphism and according to the low rate of breeding, a reliable sex identification test is essential for breeding strategies of captive birds for recovery process in animal preservation’s organizations and further release them into their natural environment.
Materials and Methods
In this study three blood samples were taken from one Bearded vulture (Gb. barbatus) or Home bird with sex known as male, one sub mature unknown sex and another unknown sex mature Home bird in FPWC center in Armenia, Yerevan, and sent to the AMI molecular biological lab institute in Iran for sex identification. Two blood samples of Alexandrine parakeet (Psittacula eupatria) from both sexes, a male Kestrel and a female Ringed neck Parakeet (Psittacula krameri) were already taken from the birds in Eram Zoo in Tehran, and were used as comparative samples in the study. DNA was purified from the blood samples using DNA purification kit (Sinaclon, Iran) according to the manufacturer protocol. Since the mature Home with unknown sex was released in nature and the DNA purification process of its blood sample was failed due to clot formation, the sample was eliminated from the study. Primers used in this study for amplification of CHD1-Z and CHD1-W encoding gene sequences were as below.
PCR amplification with two primer sets of P2 [5'-TCTGCATCGCTAAATCCTTT-3'] and P3 [5'-AGATATTCTGGATCTGATAGTGA-3'] [4] and P8 [5'-CTCCCAAGGATGAGRAAYTG-3'] and P2 [5'-TCTGCATCGCTAAATCCTTT-3'] [7] using annealing temperature 48°C PCR was performed in 50 μL volumes including 10 μg genomic DNA, 250 nM of each primer, 1.5 mM MgCl2, 200 μM dNTP, and 0.5 or 1 unit Taq polymerase (Sinaclon, Iran, Tehran). The settings for the thermal cycler were the following: hot start at 94°C (5 min) followed by 40 cycles including denaturing at 94°C (45s), primer annealing at 46°C temperature (1 min), elongation at 72°C (55s), and final extension at 72°C (5 min).
All PCR products were stained using DNA safe dye (Sinaclon, Iran, Tehran) and separated by electrophoresis on 1.5% and 3% agarose gel then visualized in illuminator under UV light.
The PCR amplification with four different primer sets (Table 1) were done on DNA samples taken from the two mentioned Home birds, a male Kestrel (Falco tinnunculus) and one female Ringed parakeet (Psittaculata krameri) for comparison the results. In addition to P2 and P8 primers, the two primer sequences were FNP [5'-GAGAAACTGTGCAAAACA-3'] and Rmp [5'-AGTCACTATCAGATCCGGAA-3'] [7,12]. The later primer sets were used to determine the female bird because the primer set can only detect female-specific CHD1W gene by including 3'-terminal mismatch primer MP [13]. Alternatively, another primer set of P2 and P8 (5'-CTCCCAAGGA TGAGRAAYTG-3') was used for bird species whose sex cannot be determined by the former primer set [7,13]. Four different PCR amplification was done as shown in table 1 in an automated thermo cycler under PCR program as following: 50 μL volumes including 10 μg genomic DNA, 250 nM of each primer, 1.5 mM MgCl2, 200 μM dNTP, and 0.5 or 1 unit Taq polymerase (Sinaclon, Iran, Tehran). The settings for the thermal cycler were the following: hot start at 94°C (5 min) followed by 40 cycles including denaturing at 94°C (45s), primer annealing at 46°C temperature (1 min), elongation at 72°C (55s), and final extension at 72°C (5 min). All PCR products were stained using DNA safe dye (Sinaclon, Iran, Tehran) and separated by electrophoresis on 3% agarose gel then visualized in illuminator under UV light.
Results and Discussion
In contrast with mammals, the sex determination system in birds is different and the females have heterogametic chromosomes (ZW) in comparison to male birds whose sex chromosomes are identical (ZZ). As more than 60% of all bird species are sexually monomorphic, and the necessity of precise method for sex identification of birds, today various molecular techniques based on sex-specific genetic markers are used due to sex identification of birds [13-16]. Griffiths reported that 27 species of birds could be sexed successfully using one set of primers, P2/P8 by changing PCR conditions annealing temperature and MgCl2 concentration. In this study six different PCR primer sets were tested for amplification of CHD1-Z and CHD1-W encoding gene sequence for sex identification of Eurasian Bearded vulture for the first time.
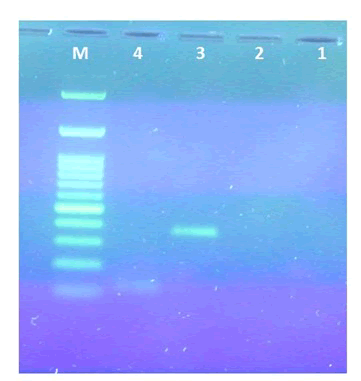
Samples from Alexandrine parakeet, Ringed neck parakeet and Kestrel were also tested. As shown in Figure 1, PCR amplification of male Alexandrine parakeet genomic DNA sample with P2/P8 and P2/P3 primer sets led to a PCR product length of about 400 bp and 150 bp respectively. In this study in spite of changes in PCR program and PCR set concentrations no PCR product was amplified using DNA of Female Alexandrine parakeet as template.
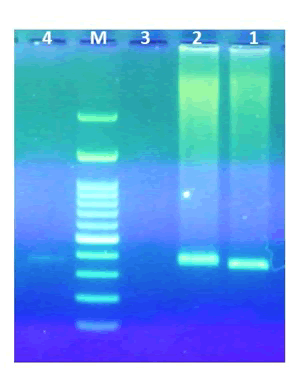
Figure 2 and Figure 3 shows PCR sexing assays of male and sub adult Bearded vulture in comparison with male Alexandrine parakeet using P2/P8 and P2/P3 primer sets. As shown below, the sub-adult Bearded vulture determined as female bird because of two different PCR products on 1.5% agarose gel electrophoresis.
Figure 3: PCR sexing assays P2/P3. P2/P8 primer sets: 1) Male Bearded vulture (Gypaetus Barbatus) P2/P3; 2) Male Bearded vulture (Gypaetus Barbatus) P2/P83) Female Bearded vulture (Gypaetus Barbatus) P2/P3; 4) Female Bearded vulture (Gypaetus Barbatus) P2/P8; 5) Female Alexandrine parakeet (Psittacula eupatria) P2/P8; 6) Male Alexandrine parakeet (Psittacula eupatria) P2/P8.
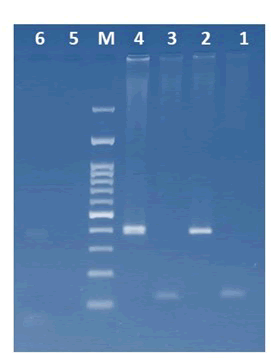
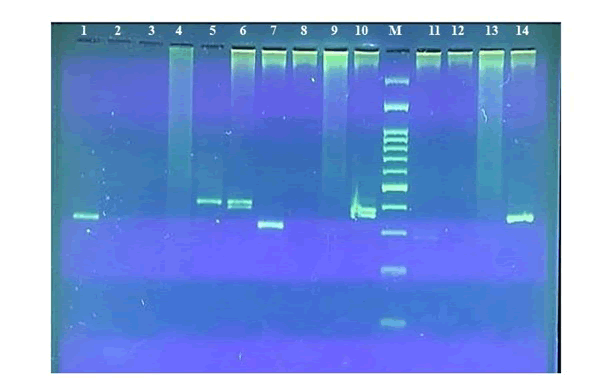
Since the sub-adult’s two PCR products were blur, another PCR assay were done using four different primer sets on two male and sub-adult Bearded vulture, Female Ringed neck Parakeet, and male Kestrel. The PCR products were electrophoresed on 3% agarose gel (Figure 4). The comparative results are summarized in Table 1.
Figure 4: PCR sexing assays using four sets of primer pairs: 1) Female Ring necked parakeet (Psittacula krameri) P8/RMP; 2) Male kestrel (Falco tinnunculus) P8/RMP; 3) Male kestrel (Falco tinnunculus) P2/RMP; 4) Male kestrel (Falco tinnunculus) FNP/RMP; 5) Male kestrel (Falco tinnunculus) FNP/P2; 6) Female Bearded vulture (Gypaetus Barbatus) P2/P8; 7) Female Bearded vulture (Gypaetus Barbatus) P8/RMP; 8) Female Bearded vulture (Gypaetus Barbatus) P2/RMP; 9) Female Bearded vulture (Gypaetus Barbatus) FNP/RMP; 10) Female Bearded vulture (Gypaetus Barbatus) FNP/P2; 11) Male Bearded vulture (Gypaetus Barbatus) P8/RMP; 12) Male Bearded vulture (Gypaetus Barbatus) P2/RMP; 13) Male Bearded vulture (Gypaetus Barbatus) FNP/RMP; 14) Male Bearded vulture (Gypaetus Barbatus) FNP/P2.
| Sex | Species | P2/P3 | P2/P8 | P8/MP | P2/MP | NP/Mp | Np/P2 |
|---|---|---|---|---|---|---|---|
| M | Bearded vulture | + | + | + | - | - | + |
| F | Gypaetus barbatus | + | + | + | - | - | + |
| M | Alexandrine parakeet | Not tested | + | Not tested | Not tested | Not tested | Not tested |
| F | Psittacula eupatria | Not tested | - | Not tested | Not tested | Not tested | Not tested |
| M | Kestrel | Not tested | Not tested | - | - | - | + |
| Falco tinnunculus | |||||||
| F | Ring necked Parakeet | Not tested | Not tested | + | Not tested | Not tested | Not tested |
| Psittacula krameri |
Table 1: Summarized results of PCR sexing assays using six different primer sets based on amplification CHD1-Z and CHD1-W encoding gene sequences.
Conclusion
PCR assays using P2/P8 and NP/P2 primer sets led to amplification of two PCR product length in female Bearded vulture DNA samples in comparison to one PCR product in male bird as expected. These results shows that P2/P8 and NP/P2 primer sets are useful for sex determination of male and female Bearded vulture bird.
Acknowledgments
Authors would like to thank AMI molecular biological Institute for financial support of the work, we would also thank FPWC (Foundation for the Preservation of Wildlife Cultural Assets) and Eram Zoo (Tehran,Iran) for supplying the blood samples.
References
- Bermudez-Humarani LG, Garcia Garcia A, Humberto Leal-Garza C, Riojas –Valdes VM, Jaramillo-Rangel G, et al. (2002) Molecular sexing of monomorphic endangered ara birds. J Exp Zool 292:677-680.
- Romanov MN, Betuel AM, Chemnick LG, Ryder OA, Kulibaba RO, et al. (2018) Widely Applicable PCR Markers for Sex Identification in Birds. Anim Genet 55:220–231.
- Griffiths R, Korn RM (1997) A CHD1 gene is Z chromosome linked in the chicken Gallus domesticus. Gene 197:225–229.
- Griffiths R, Tiwari B (1995) Sex of the last wild Spix’s macaw. Nature 375:454.
- Griffiths R, Tiwari B, Becher S (1992) The identification of sex in the Sturnus vulgaris using a molecular DNA technique. Mol Ecol 3:191-194.
- Griffiths R, Daan S, Dijkstra C (1996) Sex identification in birds using two CHD genes. Proc Roy Soc London B 263:1251–1256.
- Griffiths R, Double M, Orr K, Dawson R (1998) A DNA test to sex most birds. Mol Ecol 7:1071–1075.
- Saitoh Y, Saitoh H, Ohtomo K, Mizuno S (1991) Occupancy of the majority of DNA in the chicken W chromosome by bent repetitive DNA sequences. Chromosome 101:32–40.
- Gavashelishvili A, McGrady M J (2006) "Breeding site selection by bearded vulture (Gypaetus barbatus) and Eurasian griffon (Gyps fulvus) in the Caucasus". Anim Conserv 9:159–170.
- Gavashelishvili A, McGrady MJ (2006) "Geographic information system-based modelling of vulture response to carcass appearance in the Caucasus". J Zool 269:365–372.
- Gavashelishvili A, McGrady MJ (2007) "Radio-satellite telemetry of a territorial Bearded Vulture Gypaetus barbatusin the Caucasus". Vulture News 56:4–13.
- Itoh Y, Suzuki M, Ogawa A, Munechica K, Murata K, et al. (2001) Identification of the sex of a wide range of Carinatae birds by PCR using primer sets selected fromchicken EE0.6 and its related sequences. J Hered 92:315-321.
- Mu-Yeong Lee, Yoon-Jee Hong, Sun-Kyung Park, Young-Jun Kim, Tae-Young Choi, et al. (2008) Application of Two Complementary Molecular Sexing Methods for East Asian Bird Species. Genes Genom 30:365-372.
- Bryja J and Konecny A (2003) Fast sex identification in wild mammals using PCR amplification of the Sry gene. Folia Zool. Brno 52:269-274.
- Williams CL, Breck SW and Baker BW (2004) Genetic methods improve accuracy of gender determination in beavers. J Mammal 85:1145-1148.
- Yamamoto K, Tsubota T, Komatsu T, Katayama A, Murase T, et al. (2002) Sex Identification of Japanese Black Bear, Ursus thibetanus japonicus, by PCR based on Amelog. J Vet Med Sci 64:505-508.
 Spanish
Spanish  Chinese
Chinese  Russian
Russian  German
German  French
French  Japanese
Japanese  Portuguese
Portuguese  Hindi
Hindi