Research Article, J Health Inform Manag Vol: 4 Issue: 4
The Impact of the Workflow Re-Engineering of Outpatient Operation In a Hospital Pharmacy: A New Model with Advanced Screening by Pharmacist.
Wilson W Chu1*, Eugene Leung1, Ronnie F Tsang1, Shirley W Yip2, Jacob K So2, Kathy H Fung2, Zac Wong1, Jessica Chan1 and Charles Lo31 Department of Pharmacy, Pharmacist, United Christian Hospital, Hospital Authority, Hong Kong, China.
2 Department of Pharmacy, Resident Pharmacist, United Christian Hospital, Hospital Authority, Hong Kong, China.
3 Department of Pharmacy, Senior Pharmacist, United Christian Hospital, Hospital Authority, Hong Kong, China.
*Corresponding Author : Wilson W Chu
BPharm, MBA, MClinPharm, BCGP
Pharmacist, Pharmacy Department, United Christian Hospital, Hospital Authority,
Hong Kong, China
E-mail: www.chu@yahoo.com
Received: September 02, 2020 Accepted: October 10, 2020 Published: October 17, 2020
Citation: Wilson WC. Eugene L et al., (2020) The Impact of the Workflow Re-Engineering of Outpatient Operation In a Hospital Pharmacy: A New Model with Advanced Screening by Pharmacist. J Health Inform Manag 2020 4:4.
Abstract
Objective: The primary objective of this study is to investigate the impact on pharmacists’ pre-prescription vetting advanced screening on the effectiveness of identifying drug-related problems (DRPs). The aim is to evaluate the impact of the pharmacists’ pre-vetting advanced screening on the enhancement of dispensing workflow and to reduce unnecessary uplication of effort in the dispensing procedures.
Methods: This is a prospective interventional cohort study, with a control group, carried out in United Christian Hospital Hong Kong. In the control arm, all prescriptions were processed according to the standard procedures from 19th June to 06th July 2020. In the intervention arm, it involved a workflow re-engineering having two clinical pharmacists to screen the rescriptions and to perform clinical checking before the data entry process from 05th June to 18th June 2020. A standardised checklist was used to guide the clinical checking. The number of prescriptions with potential DRPs requiring clarification, identified at different stages, were compared between the intervention and control arms.
Results: In the control group, 152 interventions were made with 56% of them were made in the vetting stage by dispensers and 44% of interventions were made at the checking and issuing stages by pharmacists or senior dispensers. In the intervention group, a total of 216 interventions were captured with 76% of interventions were made by pharmacists at the early dispensing stage before data entry.
Conclusion: Workflow re-engineering having clinical pharmacists to screen the prescriptions and to perform clinical checking before the data entry process could provide an effective way to detect drug-related problems at early stages of the hospital pharmacy dispensing process.
Keywords: Pharmacists, Hospitals, Pharmacy Service, Outpatients, Drug Prescriptions, Workflow
Introduction
Preventable patient harm is prevalent globally across medical settings, with drug-related problems (DRP) accounting for a significant portion of all patient harm cases, causing substantial health and economic impact [1-4]. According to the Pharmaceutical Care Network Europe Foundation (PCNE) definitions, a DRP is an event or circumstance involving drug therapy that actually or potentially interferes with desired health outcomes [5]. There is little evidence on how DRPs lead to patient harm, but the general data suggests that DRPs are relatively common and mostly preventable [6,7,8]. Severe harm and death associated with DRPs are also reported, but an unbiased estimation on its prevalence has yet to be successful due to heterogeneity across studies [1,2,6]. The exact cost of DRPs on healthcare systems is unknown. However, the cost of adverse drug reactions, which were considered a proxy of DRPs, had been estimated to be in millions, if not billions per annum [9]. In 2012, a study in Germany extrapolated the national total treatment cost of adverse drug events to be €1.058 billion per annum, while a 2013 national literature review on medication safety in Australia estimated $1.2 billion per annum for managing medication-related hospital admissions [10,11]. The 2018 report published by the Policy Research Unit in Economic Evaluation of Health & Care Interventions (EEPRU) in the United Kingdom concluded an estimated cost of £98.5 million to the NHS was resulted from “definitely avoidable adverse drug reactions” [9].
Medication management and reconciliation are one of the clinicallevel intervention recommended by the Organisation for Economic Co-operation and Development (OECD) as one of the value-based approaches to reducing patient harm [12]. The hospital pharmacists play an important role and are equipped with the expertise to lead collaborative, multidisciplinary efforts to prevent DRPs that could result in patient harm. Through periodic clinical checking of medical cases, pharmacists could identify pharmacotherapeutic problems after the comprehensive evaluation of all relevant information including patient characteristics, disease states, medication regimen, and laboratory results [14,15].During the dispensing process in the hospital pharmacy, early identification and clarification of suspected problems could potentially prevent DRPs, reduce overall workflow disruptions, and reduce drug cost [16].
In 2017 a local pilot study was conducted to show that up to 65% of DRPs were identified at the downstream section of the whole dispensing process, during the later stages of the checking and issuing process [5]. The conclusion was drawn that “The majority of DRPs were identified at the downstream of the dispensing flow…” and recommendations were given including “pharmacist screening before prescription vetting could be considered to avoid delay of DRP identification” [17].
This study aims to investigate the impact of pharmacists’ preprescription vetting advanced screening on the effectiveness of identifying drug-related problems (DRPs) and on the efficiency of dispensing workflow.
Methods
This is a prospective interventional cohort study, with a control group, carried out in United Christian Hospital Hong Kong. The interventional study period was from 05th June to 18th June 2020 while the control study period was from 19th June to 06th July 2020, from Monday to Friday 0900 to 1700 except public holidays and lunch break from 1300 to 1400. All Specialist Out-patient Clinics’ prescriptions were included in the study. Exclusion criteria were prescriptions with only one medication, those issued by the accident and emergency department, and those from the staff clinic. Confidentiality of the information was maintained by not disclosing the patient name, patient ID, name of the doctor who prescribed, and name of the pharmacist who did the interventions. British National Formulary, Lexicomp Drug Information Handbook, UpToDate the electronic clinical resource tool, and an in-house checklist for medication order review were used as standards to substantiate correct interventions by the pharmacists.
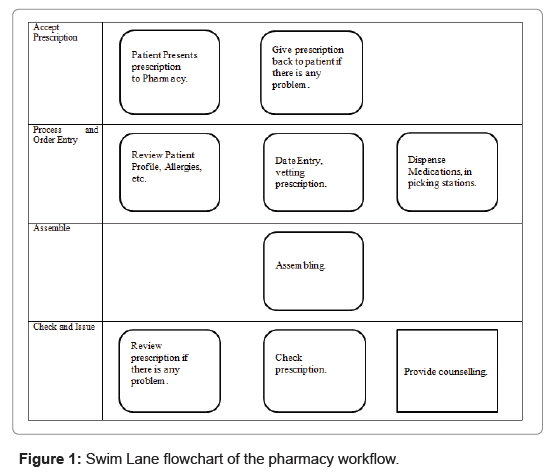
The standard operating procedures (SOP) to process a prescription in United Christian Hospital consist of the followings:
1. Receiving a prescription. Pharmacy staff to check that the prescription is valid.
2. Data entry process. A member of the pharmacy team to review the patient profile and uses the hospital computer system to process the prescription.
3. Dispensing. Pharmacy dispensing staff to dispense the medications from different picking stations.
4. Assembling. Pharmacy staff to assemble the medications.
5. Checking. The pharmacist or senior dispenser to perform a clinical check of the medications as well as a technical check on the accuracy of the information entered into the pharmacy software system, the label, and the contents of the vial or package.
6. Issuing and counselling. The pharmacist or senior dispenser to provide counselling to the patient on the medications.
According to the SOP, pharmacy staff will contact the prescribers for clarification when DRPs are identified during any step of the above procedures. A red sticker with numbered code will be affixed on that prescription to indicate the stage (1=Vetting; 2=Checking; 3=Issuing) at which the possible DRP is identified.
In the control arm, all prescriptions were processed according to the above procedures. In the intervention arm, the workflow was reengineered so that two clinical pharmacists were stationed to screen the prescriptions and to perform clinical checking with the access of both the Pharmacy Medication System (PMS III) and the electronic Patient Record (ePR) before the data entry process. As using a checklist for medication order review by pharmacists could encourage standardization of care [18], a standardised checklist as shown in Table 1 was developed to guide clinical checking. After checking, an “approved” stamp was chopped on the screened prescription. If a DRP was identified, a red sticker with the numbered code “0” was affixed on the prescription. Therefore, the coding was 0: Clinical pharmacist screening; 1=Vetting; 2=Checking; 3=Issuing. All the other procedures were the same in both arms.
| Aspects | Remarks | |
|---|---|---|
| Verification of patient’s identity | Name | / |
| Age | Doses may be different for specific age ranges (geriatric and paediatric populations) | |
| Sex | / | |
| Pregnancy | E.g. angiotensin converting enzyme inhibitors, Valproate, Finasteride should be avoided in pregnancy | |
| Lactation | Codeine in lactating mothers increases risk of opioid toxicity in infants | |
| Allergies | Especially ‘free text’ allergies | |
| Adverse drug reactions | E.g. edema caused by amlodipine | |
| Therapeutic review | Formulary/restrictions/product availability | E.g. QV cream is restricted to paediatric patients only according to local drug formulary |
| Compliance with medication order writing standards | E.g. avoid trailing zeroes “5.0” after decimal place, avoid (N) and write “nocte” and “noon” instead. | |
| Comorbidities | E.g. avoid Metoclopramide and Cinnarizine in Parkinsonism patients | |
| Contraindications | E.g. Pioglitazone is contraindicated in NYHA Class III/IV heart failure | |
| Laboratory tests/levels | E.g. serum creatinine, potassium | |
| Renal function | In line with internal renal dosage checking guideline, involving new oral anticoagulants, low molecular weight heparins, aminoglycosides, cephalosporins, antivirals, and digoxin | |
| Dose | Weight, renal function, hepatic function, normal range | |
| Dosage form | E.g. sustained release preparations should not be given to patients with feeding tubes | |
| Route | E.g. intravenous or subcutaneous, but not intramuscular for Darbepoetin alfa; intramuscular route avoided for patients on anticoagulants | |
| Schedule/frequency | Spacing out with other medications | |
| Duration (number of days) | E.g. duration of Clopidogrel prescribed should match with the intended duration of dual antiplatelet therapy (DAPT); Resonium A usually intended for short course of treatment | |
| Start date/start time | ||
| Stop date/stop time | ||
| Duplication | Same drug or therapeutic duplication |
Table 1: The checklist for clinical medication order review by pharmacists
The number of prescriptions with potential DRPs requiring clarification, identified at different stages, was compared between the intervention and control arms. Furthermore, all the prescriptions with DRPs identified at the clinical pharmacist screening stage were analysed and categorised into three groups as follows:
• Group A: prescriber was contacted and the amendment was made.
• Group B: prescriber was contacted for clarification but no amendment was made.
• Group C: prescriptions were checked against PMS III or ePR but no action was taken.
| Control | Intervention | |
|---|---|---|
| Total number of prescriptions handled as described in SOP. | 11635 (100%) | 11349 (100%) |
| Total number of prescriptions screened at Location 0. | - | 8883/11349 (78%) |
| Total DRPs identified. | 177 | 216 |
| DRPs identified at Advanced screening, location (0). | - | 165 / 216 (76%) |
| DRPs identified at Data Entry, location (1). | 102 / 177 (58%) | 12 / 216 (6%) |
| DRPs identified at Checking, location (2). | 30 / 177 (17%) | 11 / 216 (5%) |
| DRPs identified at Issuing, location (3). | 45 / 177 (25%) | 28 / 216 (13%) |
| DRPs identified at Checking + Issuing, location (2) + (3). | 42% | 18% |
Table 2: Outcome of study
Results
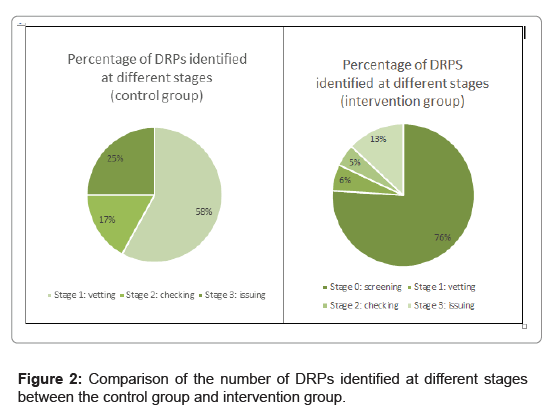
In the control group, a total of 11,635 prescriptions were processed by the pharmacy and 177 interventions were made. 58% of interventions were made in the vetting stage by dispensers and 42% of interventions were made at the checking and issuing stages by pharmacists or senior dispensers. In the intervention group, 11,349 prescriptions were processed and 78% of the prescriptions were screened by the pharmacists before the prescription vetting stage. A total of 216 DRPs were captured. 76% of interventions were made at the advanced screening stage and only 24% of interventions were made at later stages by pharmacists, senior dispensers, or dispensers.
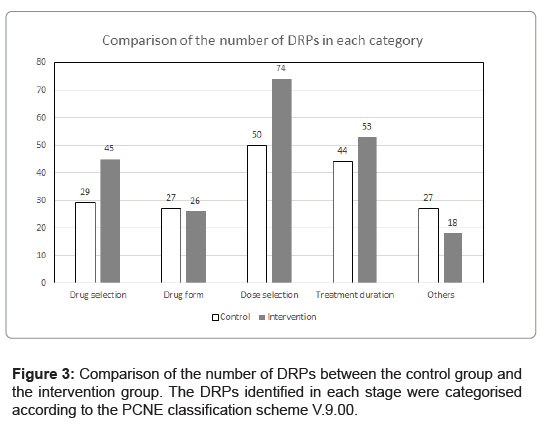
Figure 3 showed that the total number of DRPs identified at all stages and classified according to the PCNE classification scheme V.9.00. For the numbers of DRPs about drug selection and dose selection, there was around 50% increase after the advanced screening by pharmacists. There was an increase of 20% in the number of DRPs about treatment duration. The number of DRPs involving drug form was similar. Here are some examples of DRPs identified; 1. Dabigatran 150mg bd was prescribed for a patient with estimated creatinine clearance below 30 mL/min, which was subsequently changed to Apixaban 2.5mg bd by the prescriber after discussing it with the pharmacist. 2. Both Tenofovir alafenamide and Tenofovir disoproxil fumarate were prescribed for a patient. 3. Discharge patients using Insulin penfill were prescribed with Insulin vial. 4. Triple therapy for Helicobacter pylori eradication was prescribed to a patient on Gefitinib. Due to the drug-drug interaction with the proton pump inhibitor, the physician made remarks to withhold Gefitinib during triple therapy instead of spacing out administration time. 5. Calcitriol 7.5 microgram daily was prescribed but the intended dosage was 0.75 microgram daily. 6. Dexamethasone 10 mg was prescribed for the overnight dexamethasone suppression test but the intended dosage was 1 mg. 7. Azithromycin was prescribed for long-term immunomodulation, but only 3 days were prescribed. 8. Clopidogrel as part of a dual-antiplatelet therapy was intended to be continued for 37 more days, but 20 weeks was prescribed.
501 suspected problems were identified in the intervention group at the advanced screening stage which did not require the amendment of prescription. These problems encompassed practical, non-clinical issues that normally could be questioned at the checking and/or issuing stage and could be confirmed to proceed by checking against ePR or asking the patient directly. An example of said problems include the rectification of outdated, drug-specific remarks written by previous prescribers, such the remark “dose increased” attached to a chronic drug, where the actual dose increase was already performed months before the current dispensing episode. Others include checking the dispensing history on whether the patient was using insulin vial (10 mL preparation) and syringe instead of the pen-fill form, confirming the colonoscopy date to ensure sufficient expiry date of the dispensed bowel preparation kit, and checking of inconsistent prescription duration against the appointment date. Identification of these suspected problems and their rectification at the advanced screening stage minimized the labour required for doing these at the later checking and issuing stages, reducing disruptions to those pharmacists so that they could focus on the actual clinical checking and counseling.
For refill prescriptions, ePR was checked to identify any unintentional medical consultations or hospital admissions during the period between the original supply and refill supply dates. These consultations and admissions could result in amendments to the usual drug treatment regimen. For each of such identified cases, the original prescriber was informed, the “best possible medication history” updated and the involved drug item rectified. Through the removal of unnecessary drugs resulted from these cases, there was a total drug cost savings of HKD $1933 during the study period. Here are some examples: 1. The refill item was amlodipine 5 mg daily, but the dosage was increased to 7.5 mg daily by another physician during an episode of unintentional consultation between two dispensing dates. 2. Famotidine was to be refilled, but the patient obtained lansoprazole initiated by another specialty. 3. The refill item was potassium chloride SR tablet 600 mg daily, but it was discontinued previously by the physician as the patient had hyperkalemia (potassium level of 5.2 mmol/L).
Discussion
In the control arm, we found that 42% of DRPs were identified at the later stage of the dispensing process during the checking at location 2 and issuing at location 3 by pharmacists or senior dispensers. In the intervention arm, the DRPs identified at locations 2 and 3 were greatly reduced to 18%. From the above results, this study showed that the new workflow could “re-engineer” the point of DRP identification from the downstream to upstream of our operation.
When comparing the downstream clinical checking in the control with the upstream clinical checking according to the new workflow would provide three benefits; Firstly, it smoothened the whole dispensing process by reducing the unnecessary effort needed to process prescriptions. During the dispensing operation according to SOP with downstream clinical checking, any amendments made upon a prescription would be performed after all the medications were dispensed and assembled. The pre-amendment medications would have to be discarded and returned to the drug stock, while a new batch of medications would have to be dispensed and assembled according to the amended prescription after the intervention, thus duplicating the effort of processing the prescription. Early identification of DRPs could also facilitate successful intervention since the prescriber could be contacted earlier before he/she went off work.
Secondly, upstream clinical checking would potentially improve medication safety by reducing disruptions to the checking and issuing processes. Similar to above, as fewer amendments would be performed at checking and issuing, there would be fewer distractions burdening the pharmacists and senior dispensers at the checking and issuing stage, potentially improving medication safety.
Thirdly, if there was no clinical screening before vetting, some of the DRPs might be missed and thus jeopardising the treatment outcome of the patients. As pharmacists possess professional clinical knowledge and are an indispensable part of medication reconciliation, the re-engineering could increase the extent of participation of pharmacists in DRP identification. Upon thorough medication review, pharmacists could intervene and ask the doctor to discontinue or reduce the dose of all medications with an unfavourable balance of benefits and harms, as well as any inappropriate prescribing from doctors, e.g. drug duplication & drug without indication.
Based on another study conducted locally in June 2020, it showed that 21% of our patients are 80-year-olds or above and 33% of total prescriptions have 5 items or above [19]. According to the World Health Organization, polypharmacy is the concurrent use of multiple medications [20]. Although there is no standard definition, polypharmacy is often defined as the routine use of five or more medications [13]. These include over-the-counter, prescription, traditional, and complementary medicines used by a patient [21]. Another systematic review study suggested that the most commonly reported definition of polypharmacy was the numerical definition of five or more medications daily [22]. To combat polypharmacy, supervised withdrawal of an inappropriate medication could reduce some of the problems associated with polypharmacy [17]. The process is described as “Deprescribing” which can reduce overall medication burden i.e. possible side effects suffering in patients and the medication regimen complexity [23]. Ultimately, all these goals relate to better drug compliance and improving quality of life [24]. Moreover, deprescribing can prevent any unnecessary drug prescribing and thus reduce the drug expenditure of our healthcare system [15]. A new service such as pharmacist-led medication assessment and deprescribing clinic for patients with polypharmacy is suggested.
The majority (66%) of the prescriptions screened in location 0 by pharmacist falls into the category of “Rx checked against ePR, no amendment”. These efforts are not redundant but can prevent potential DRPs. It is common in our practice to assess the patient’s health record and physician’s consultation notes via the in-house electronic system (ePR). When we encounter suspected DRPs such as treatment duration and renal dosage adjustment, we can refer to the consultation notes and/or laboratory test results to justify the drug regimen. This can smoothen the screening process without overwhelmed phone-calls to doctors for confirmation.
According to the comparison of the causes of DRPs between the control group and the intervention group (Figure 3), we can see that the greatest difference are C1 (Drug selection, 55%), C3 (Dose selection, 48%), and C4 (Treatment duration, 20%). The re-engineering may further elaborate on a shift of pharmacist manpower in the dispensing process. According to the current standard operation procedure, the main role of pharmacists is drug checking and drug issuing. In view of the study results, putting pharmacists in the upstream of the dispensing process can discover and rectify more DRPs in an earlier stage.
While 76% of the interventions were done at the pharmacist screening stage (location=0), there were still 13% of the interventions at drug issuing (location 3). These are mainly triggered by the patients upon counselling of medications, such as finding out that the patient was being non-compliant, request of additional drugs, or refusal of drugs.
There are some limitations to this study. Due to the constraint of manpower, the new workflow was not implemented from 1300 to 1400 and after 1700. Neither the A&E prescriptions nor single-item prescriptions were included in this study. Therefore, the total number of prescriptions screened and DRPs identified at Location 0 may be undercounted. This may result in under-estimate the number of cases under clinical screening made by pharmacists. Moreover, the workload of the control and the intervention period may be different and subject to seasonal changes which may affect the accuracy of the results. Besides, the prescribing practice of different physicians in these two periods may also lead to deviation of study outcomes. Also, the pharmacists who participated in the screening process may have different clinical judgment leading to different intervention results.
Conclusion
Workflow re-engineering having the clinical pharmacists to screen the prescriptions and to perform clinical checking before the data entry process could provide an effective way to identify drug-related problems at the early stages of the hospital pharmacy dispensing process.
References
- Panagioti M, Khan K, Keers RN, Abuzour A, Phipps D, et al. (2019) Prevalence, severity, and nature of preventable patient harm across medical care settings: Systematic review and meta-analysis. BMJ 366: l4185.
- Laatikainen O, Miettunen J, Sneck S, Lehtiniemi H, Tenhunen O, et al. (2017) The prevalence of medication-related adverse events in inpatients-a systematic review and meta-analysis. Eur J Clin Pharmacol 73: 1539-1549.
- Martins AC, Giordani F, Rozenfeld S (2014) Adverse drug events among adult inpatients: A meta-analysis of observational studies. J Clin Pharm Ther 39: 609-620.
- Sutherland A, Phipps DL, Tomlin S, Ashcroft DM (2019) Mapping the prevalence and nature of drug related problems among hospitalised children in the United Kingdom: A systematic review. BMC Pediatr 486.
- Pharmaceutical Care Network Europe PCN.
- de Vries EN, Ramrattan MA, Smorenburg SM, Gouma DJ, Boermeester MA (2008) The incidence and nature of in-hospital adverse events: a systematic review. Qual Saf Health Care 17: 216-223.
- Cousins D, Crompton A, Gell J, Hooley J (2019) The top ten prescribing errors in practice and how to avoid them. The Pharmaceutical Journal.
- Alqenae FA, Steinke D, Keers RN (2020) Prevalence and nature of medication errors and medication-related harm following discharge from hospital to community settings: A systematic review. Drug Saf 43: 517-537.
- Elliott RA, Camacho E, Campbell F, Jankovic D, Martyn St James M, et al. (2018) Prevalence and economic burden of medication errors in the NHS in England. Rapid evidence synthesis and economic analysis of the prevalence and burden of medication error in the UK. Policy research unit in economic evaluation of health and care interventions. Universities of Sheffield and York, England.
- Rottenkolber D, Hasford J, Stausberg J (2012) Costs of adverse drug events in german hospitals—A microcosting study. Value in Health 15: 868-875.
- Roughhead L, Semple S, Rosenfeld E (2013) Literature review: Medication safety in Australia 2013. Australian Commission on Safety and Quality in Health Care.
- Luke S, Ane A, Nicolaas SK (2017) The economics of patient safety: Strengthening a value-based approach to reducing patient harm at national level. OECD Health Working Papers 96.
- ASHP Guidelines on Preventing Medication Errors in Hospitals.
- Clinical Checks-Royal Pharmaceutical Society. Quick reference guide.
- Clinical Check Guidance-Pharmacy Forum NI.
- Cain C, Haque S (2008) Organizational workflow and its impact on work quality. Patient safety and quality: An evidence-based handbook for nurses 31.
- Chui K, Chong G, Chu W (2017) Effectiveness of identifying drug-related problems (DRPs) at an outpatient hospital pharmacy in Hong Kong. 514.
- Meyer M, Raymond C, Rodrigue C (2011) Development and evaluation of a checklist for medication order review by pharmacists. Can J Hosp Pharm 64: 199-206.
- Chu W, Chong G (2020) Big data analytics using python-based machine learning: a challenging opportunity in hospital pharmacy. J Health Inform Manag 4: 2.
- World Health Organization. Medication safety in key action areas.
- World Health Organization (2019) Medication safety in polypharmacy.
- Masnoon N, Shakib S, Kalisch-Ellett L, Caughey GE (2017) What is polypharmacy? A systematic review of definitions. BMC Geriatrics 17: 230.
- Garfinkel D, Ilhan B, Bahat G (2015) Routine deprescribing of chronic medications to combat polypharmacy. Ther Adv Drug Saf 6: 212-233
- Duncan P, Duerden M, Payne R (2017) Deprescribing: a primary care perspective. Eur J Hosp Pharm. 24: 37-42.
 Spanish
Spanish  Chinese
Chinese  Russian
Russian  German
German  French
French  Japanese
Japanese  Portuguese
Portuguese  Hindi
Hindi