Research Article, J Soil Sci Plant Nutr Vol: 7 Issue: 3
The Study of Microbiological Methods for Cleaning Soil Contaminated with Heptyl in Kazakhstan
Murat Toktar*
Department of Soil Science and Agrochemistry, Kazakh Research Institute of Soil Science and Agrochemistry, Almaty, Kazakhstan
- *Corresponding Author:
- Murat Toktar
Department of Soil Science and Agrochemistry,
Kazakh Research Institute of Soil Science and Agrochemistry,
Almaty,
Kazakhstan;
E-mail: murat-toktar@mail.ru
Received date: 27 January, 2020, Manuscript No. JSPH-23-6800;
Editor assigned date: 30 January, 2020, PreQC No. JSPH-23-6800 (PQ);
Reviewed date: 13 February, 2020, QC No. JSPH-23-6800;
Revised date: 29 June, 2023, Manuscript No. JSPH-23-6800 (R);
Published date: 27 July, 2023, DOI: 10.4172/jsph.1000191.
Citation: Toktar M (2023) The Study of Microbiological Methods for Cleaning Soil Contaminated with Heptyl in Kazakhstan. J Soil Sci Plant Nutr 7:3.
Abstract
The aim of the study is to determine microorganisms capable of absorbing heptyl from soils of gray-brown desert sandy soil and loamy soil, selected in the positional area of the Baikonur cosmodrome and compilation of optimal associations from cultures of microorganisms that have the ability to digest heptyl as the sole carbon source. UDMH is a chemically highly active substance and as shown above, its transformation leads to the formation of multiple connections of different classes. Task of establishing existing transformation mechanisms in such complex situations this is a question that requires an application efforts. Scientists put forward many theoretical assumptions research on the mechanisms of UDMH transformation reactions.
Keywords: Soil; Heptyl, Microorganisms, Nutrient medium, Degradation of hepty
Introduction
For instance, during the period of intensive exploitation by the Baikonur cosmodrome, 27 falls of the first stages of the proton LV were registered in the territory of the Yu-2 zone with an area of 0.1 million hectares. On the fields of Kazakhstan’s territory, subject to long-term exposure to the SRA as a SLV SP IA, the soil takes the main share of the technogenic load in the form of mechanical and pyrogenic deformation of soil horizons, disturbance of balanced development of soil biocenosis. With the development of the space industry in Kazakhstan interest of state structures, scientific-technical and educational divisions, as well as broad public circles in information about the environmental situation on the territory of the objects of the Baikonur rocket and space complex BaiNura. Special resonance in society is caused by issues related to with an assessment of the ecological state of the sites in Kazakhstan territories that are subject to long-term exploitation as areas of falling of separating parts of launch vehicles [1].
Taking into account the risk of negative consequences for the environment, the implementation of the SRA in the Yu-2 zone remains a potential factor of external technical impact on the ecosystem, despite that 7 years have passed since the last fall of the first stage of the proton LV. This aspect provides the basis for monitoring of the natural ecosystem in the Yu-2 zone with subsequent assessment of its environmental sustainability [2].
For the studies regularly carried out in the Kazakhstani SLV SP IA, the criteria of environmental sustainability are used, which reflect factors that can disturb the equilibrium state of natural ecosystems as abiotic (weather and climate conditions, terrain topography, morphogenetic and physicochemical properties of land cover, etc.) and anthropogenic (SRA, household activities, grazing on pastures and etc.). The key subject of environmental assessment are biocenosis, since environmental sustainability can be explained only on the basis of biotic compensation of all fluctuations and directed abiogenic changes [3].
Materials and Methods
The seeding was made from 30 soil samples taken from the upper horizon of the proton booster rocket drop zone (drop zone 15, 25) and areas of the emergency fall of the proton-M rocket fragments. For the isolation of microorganisms, soil samples were sown on nutrient agar (beef-extract agar) of the following composition (g/l): Peptone-5.0; sodium chloride-5.0; meat extract-1.5; yeast extract-1.5; agar-20.0 and Starch and Ammonium Agar (SAA), g/l: Potassium phosphate-2- substituted-1.0; ammonium sulfate-1.0; magnesium sulfate-1.0; sodium chloride-1.0; calcium carbonate-1.0; insoluble starch-10.0; and agar-20.0.
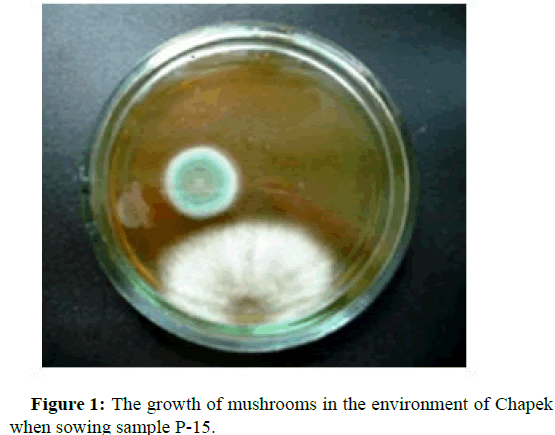
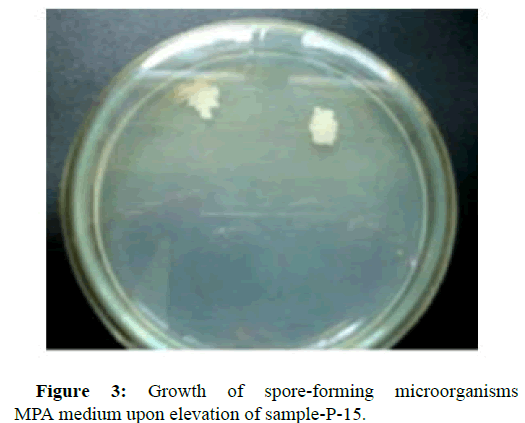
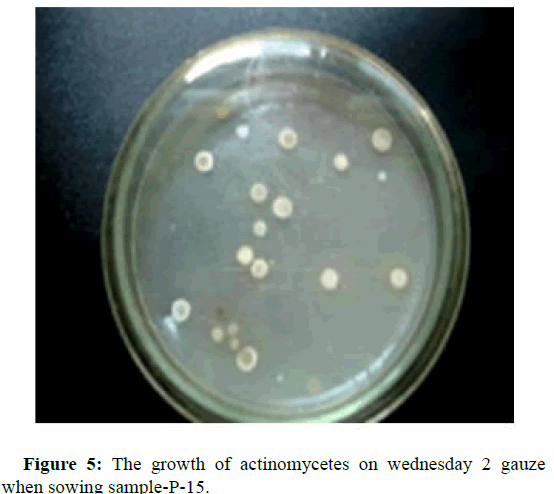
To isolate spore-forming microorganisms, the soil sample was sown after its preliminary heating in a water bath for 15 minutes at a temperature of 87°С. For the isolation of actinomycetes, gauze medium 2 (g/l) was used: Hottinger broth-50 ml; peptone-5; sodium chloride-5; glucose-10; for mushrooms-the Czapek’s medium (g/l): Sucrose-30; sodium nitrite-2; potassium phosphate-2-substituted-1; magnesium sulfate-0.5; potassium chloride-0.5 and iron sulfate-0.01 [4].
The sifting of grown colonies was made on the swarms of nutrient agar of the same composition. Microorganisms were grown in a thermostat at a temperature of 28°C-30°С for 3 days (bacteria) and 7 days (actinomycetes, micromycetes). Subsequently we counted the number of microorganisms by a range of serial dilutions in sterile tap water and cultured them in an agarized nutrient medium followed by counting the grown colonies.
To determine the intensity of soil respiration, an absorption method was used, in which the amount of carbon dioxide, released from soil samples during a certain time, was determined by neutralizing the alkali solution.
To isolate microorganisms capable of assimilating heptyl, cultures of microorganisms were grown on a dense and liquid nutrient medium, with the addition of heptyl, as the sole carbon source in concentrations corresponding to 0.25; 0.5; 0.75; 1.0; 1.25; 1.5; 1.75; 2.25; 2.5; 5.0; 10.0 maximum permissible level in soil equal to 0.1 mg/kg. The morphological, cultural and biochemical properties of microorganisms were studied by standard methods. According to the Bergey's manual, the identification of microorganisms was conducted. The shaker was used to produce biomass of microbial cultures. It was found that in the studied soil samples there are various groups of microorganisms. Figures 1-5 show pictures of the growth of microorganisms [5].
Selected cultures of microorganisms were grown on stubble nutrient agar. After that, microorganism cultures were sown on solid nutrient medium containing heptyl, as the sole carbon source, at concentrations corresponding to 10.5; and 2.5 remote controls. Cultivation was carried out for 7 days in a thermostat at a temperature of 30°С. Grown colonies of microorganisms (32 in total) were sown in nutrient broth. These associations were tested for biomass accumulation by using heptyl as the sole carbon source.
Results and Discussion
According to the results of a comparative analysis of the biomass accumulation of associations, with growth on the heptyl medium, 2 associations of cultures of microorganisms were selected 7+15+26+29 and 7+26. The works on the accumulation of biomass of microorganism cultures of 7, 15, 26, 29 was undertaken. Associations were compiled of individual cultures in the above ratios.
In order to study the biological properties of the selected crops, their identification was carried out as well. Culture No. 15 forms rounded colonies, which are convex, cream-colored, smooth edged, with smooth surface, shiny and pasty consistency. The colony size is 1 mm-3 mm. Bacterial cells are rod-shaped with rounded ends, sporeforming and gram-positive. It is aerobic and it has positive reaction to catalase. Its optimum temperature of growth is 28°C-30°С. Such signs as bacilli-shaped bacteria, positive gram stain, positive reaction to catalase and ability to form spores, allowed the microorganism to be attributed to Bacillus sp [6].
Culture No. 26 is represented by gram-positive, fixed and very short sticks with rounded ends. In the one-day culture on the mineral medium, prevail the cells that did not disperse during division and which have a characteristic arrangement at an angle to each other. The cell size is 1.3 × 0.8 microns and its shortening is observed with age. Colonies on potato agar are of creamy color and round, with a diameter of 1 mm-1.5 mm and they are convex with a smooth edge and shiny. Its consistency is soft, easily removed from the surface of the agar and easily smeared.
The catalase culture is positive, non-acid-resistant, gelatin does not liquefy, casein does not decompose and starch is not hydrolyzed. It assimilates well hydrocarbons, acetate, propionate, ethanol, butanol, propanol and glucose. It grows well on yeast extract and it uses ammonium and nitrate nitrogen. According to its morphological, physiological and biochemical characteristics, the culture is attributed to Rhodococcus sp.
Culture No. 7 has a spherical cell, immobile, with a diameter of 0.6 μm-1.0 μm; and it forms irregular groups or occurs singly. The colonies are round with a smooth edge, warm colored, opaque, smooth and shiny, with a diameter of 2 mm-5 mm [7]. It assimilates ammonium and nitrate, catalase-positive, it forms a yellowish pigment and it does not hydrolyze starch nor it form an indole and it forms a hydrolase. It uses glucose, L-valine, alanine and hydrocarbons as its sources of growth. Its optimum temperature is 25°C-37°С. According to its morphological, physiological and biochemical characteristics, culture is referred to Micrococcus sp.
Culture No. 29 is a rod with a size of 0.9 μm-1.6 μm × 1.5 μm-2.5 μm and it becomes spherical in the stationary phase, usually in pairs and chains of various lengths. It does not form spore. Its gram stain is negative and it is aerobe. It forms catalase. It does not form indole and HS. It grows at 20°C-30°С and its optimum temperature is 33°C-35°С. The culture is related to Acinetobacter sp.
Figure 5 shows individual episodes of studying the biological properties of selected cultures of microorganisms [8]. The laboratory experiment was conducted to clean-up gray-brown desert sandy soil and loamy soil from UDMH contamination at a concentration of 800 mg/kg. Soils were selected in the position area of the cosmodrome. In the first variant, all four cultures were introduced to the soil 7+15+26+29, in the second two cultures of microorganisms, namely 7+26. The drug was not introduced to the control soil.
In the course of the experiment, the soil was loosened and moistened to 60%, in one case with tap water and in the other, with mineral additives dissolved in water. After 14 days, the selected soil samples were analyzed by liquid ion chromatography for their UDMH content. The results of analyzes on the effect of various microorganisms on the process of decomposition of heptyl are shown in (Table 1). The experimental conditions were as following: The initial figure concentration of UDMH 0.8 g/kg of soil and duration-14 days (Figure 6).
| No | Sample code | The composition of the introduced microcultures | UDMH, mg/kg | The degree of extraction of UDMH, % |
|---|---|---|---|---|
| 1 | K-1 (Control) | - | 0.393 | 99.95 |
| 2 | K-2 (Control) | - | 5068 | 99.36 |
| 3 | 4.714286 | 7+15+26+29 | 0.759 | 99.90 |
| 4 | 1 26/7М | 7+26 | 1.192 | 99.85 |
| 5 | 1 4 | 7+15+26+29 | 0.658 | 99.91 |
| 6 | 1 4М | 7+26 | <0.05 | 100 |
| 7 | 2 26/7 | 7+15+26+29 | 7.297 | 99.08 |
| 8 | 2 26/7М | 7+26 | 5.310 | 99.33 |
| 9 | 2 4 | 7+15+26+29 | 5.738 | 99.28 |
| 10 | 2 4М | 7+26 | 6.138 | 99.23 |
Table 1: Effect of various microorganisms on the decomposition process of UDMH within 2 weeks (2 types of soil, the initial concentration of UDMH 800 mg/kg).
The results showed that the greatest degradation of heptyl occurred in soil sample No. 6 and No. 1, the association of four strains 7+15+26 +29 was added to the latter and during the experiment the soil was moistened with mineral additives dissolved in water. The experiment was carried out at an ambient temperature of 10°C-15°С. The content of heptyl in the specified sample after 14 days was 0.05 mg/kg of soil, compared to 0.393 mg/kg in control [9].
It was established that after catalytic detoxification of soil, even during an emergency spill (10 g/kg of soil), the use of biological method allows to reduce concentration of heptyl to 50 mg/kg of soil within 2 weeks [10].
The research results showed that the greatest degradation of heptyl occurred in soil sample No. 1, in which an association of four strains was introduced 7+15+26+29 and during the experiment the soil was moistened with mineral additives dissolved in water. The experiment was carried out at an ambient temperature of 10°C-15°С. The content of heptyl in the specified sample after 10 days was 0.05 mg/kg of soil, compared to 0.393 mg/kg in the control [11].
Of the 32 cultures of microorganisms, re-seeded on a dense nutrient medium containing heptyl as the sole carbon source, 4 cultures showed a good growth 7, 15, 26, 29. The rest of the cultures had weak growth or were absent. 5 passages of selected crops on synthetic nutrient medium with heptyl were carried out. The study found also the possibility of assimilation by selected cultures of microorganisms of a lower concentration of heptyl, namely, 0.25, 0.5, 0.75, 1.0, 1.25, 1.5 and 1.75 remote controls [12].
In order to isolate the larger number of cultures of microorganisms capable of assimilating heptyl, experience was conducted to isolate them from accumulative cultures obtained from 30 soil samples cultured in Voroshilova-Dianova liquid with 2% heptyl. The cultivation was carried out for 14 days in a thermostat at a temperature of 30°С. Then a picture of the growth of microorganisms was observed [13].
The soil samples of P-15; P-8 dated 07/01/19; P-10, P-20, P-21, RB-4-C-1 during this time showed an increase. From these samples, surface seeding was carried out on a dense nutrient medium with 2% heptyl. After cultivation in a thermostat, 20 colonies of microorganisms capable of absorbing heptyl were isolated. The results of a comparative analysis of selected microorganisms demonstrated that four initially isolated cultures of microorganisms 7, 15, 26, 29 are the most active in biomass accumulation in the nutrient medium with heptyl, which allowed to select them for use in cleaning the soils contaminated with heptyl.
The following associations were compiled of selected cultures of microorganisms:
• All four cultures of microorganisms in equal
proportions-7+15+26+29 (1:1:1:1)
• Cultures of microorganisms in equal proportions-7+26+15 (1:1:1)
• Cultures of microorganisms in equal proportions-7+26+29 (1:1:1)
• Cultures of microorganisms in equal proportions-15+26+29 (1:1:1)
• Cultures of microorganisms in equal proportions-15+26 (1: 1)
Conclusion
In the course of the study of 30 soil samples, including gray-brown desert sandy loam soil and loamy soil contaminated with heptyl and 32 cultures of microorganisms in their cleaning-up, 4 cultures were identified and their biological properties were studied. According to the results of the comparative analysis it was determined that cultures No. 7, No. 15, No. 26 and No. 29 are the most active in terms of biomass accumulation and able to absorb heptyl. Among these selected cultures of microorganisms, 4 different associations were compiled taken in equal proportions (1: 1). The first one consists of all four cultures of microorganisms 7+15+26+29, the second of the three 7+26+15, the third culture 7+26+29 (1:1:1) and the fourth 15+26. In addition, the studies showed that the greatest degradation of heptyl occurred in the soil sample No. 1 and No. 6, in which the association of 4 strains was included, i.e. 7+15+26+2.
References
- Ul yanovskii NV, Kosyakov DS, Pikovskoi II, Khabarov YG (2017) Characterisation of oxidation products of 1, 1-dimethylhydrazine by high-resolution orbitrap mass spectrometry. Chemosphere 174: 66-75.
[Crossref] [Google Scholar] [PubMed]
- Glaser PE (1992) An overview of the solar power satellite option. IEEE Trans Micro Theor Tech 40: 1230-1238.
- Miraux L, Wilson AR, Calabuig GJD (2022) Environmental sustainability of future proposed space activities. Acta Astronaut 200: 329-346.
- Toktar M, Zhubato Z, Stepanova Y, Bekeshev E (2019) Impact of space and rocket activity on soil cover in central Kazakhstan. Web Conf 135: 01105.
- Minkina TM, Motuzova GV, Nazarenko OG, Kryshchenko VS, Mandzhieva SS (2008) Combined approach for fractioning metal compounds in soils. Eur Soil Sci 41: 1171-1179.
- Salykov A, Aimbetov A, Yesmagulova N, Jussibaliyeva A (2023) Factors and trends in the development of the space industry in the context of the digitalization of the economy of the Republic of Kazakhstan. Env Dev Sustain 12: 1-16.
[Crossref] [Google Scholar] [PubMed]
- Oesper RE (1934) Kjeldahl and the determination of nitrogen. J Chem Edu 11: 457.
- Kasimov NS, Grebenyuk VB, Koroleva TV, Proskuryakov YV (1996) The behavior of rocket-fuel components in soil water and plants. Eur Soil Sci 28: 79-95.
- Feng J, Lau PWC, Shi L, Huang WY (2022) Movement behaviors and posttraumatic stress disorder during the COVID-19 pandemic: A retrospective study of Chinese university students. J Exerc Sci Fit 20: 263-268.
[Crossref] [Google Scholar] [PubMed]
- Zhang Y, Pi DD, Liu CJ, Li J, Xu F (2022) Psychological impact of the COVID-19 epidemic among healthcare workers in paediatric intensive care units in China. Plos One 17: e0265377.
[Crossref] [Google Scholar] [PubMed]
- Sencan I, Bulut D, Sencan IH, Agalar C (2021) Global health emergencies during the pandemic and their solutions. Turk J Med Sci 51: 3194-3206.
[Crossref] [Google Scholar] [PubMed]
- Gilleen J, Santaolalla A, Valdearenas L, Salice C, Fuste M (2021) Impact of the COVID-19 pandemic on the mental health and well-being of UK healthcare workers. J Psych Open 7: e88.
[Crossref] [Google Scholar] [PubMed]
- Koroleva TV, Semenkov IN, Sharapova AV, Krechetov PP, Lednev SA (2021) Ecological consequences of space rocket accidents in Kazakhstan between 1999 and 2018. Env Pollut 268: 115711.
[Crossref] [Google Scholar] [PubMed]
 Spanish
Spanish  Chinese
Chinese  Russian
Russian  German
German  French
French  Japanese
Japanese  Portuguese
Portuguese  Hindi
Hindi