Case Report, J Pharm Drug Deliv Res Vol: 6 Issue: 6
An Observation on Vancomycin Concentrations in Cerebrospinal Fluid and Serum of a Chinese Critically-Ill Patient with Intravenous Drug Injection
Mo-Han Dong1,2, Ying Wu1, Bei-Yu Chen3* and Ai-Dong Wen1
1Department of Pharmacy, Xijing Hospital, Fourth Military Medical University, Xi’an, 710032, China
2Department of Medical Administration and Education, Xijing Hospital, Fourth Military Medical University, Xi’an, 710032, China
3Department of Orthopedics, Xijing Hospital, Fourth Military Medical University, Xi’an, 710032, China
*Corresponding Author : Bei-Yu Chen, PhD
Department of Orthopedics, Xijing Hospital
Fourth Military Medical University, Xian, 710032, P.R. China
Tel: 86-29- 84773636
E-mail: chenby@fmmu.edu.cn; adwen2017@fmmu.edu.cn
Received: September 04, 2017 Accepted: September 18, 2017 Published: September 25, 2017
Citation: Dong MH, Wu Y, Chen BY, Wen AD (2017) An Observation on Vancomycin Concentrations in Cerebrospinal Fluid and Serum of a Chinese Critically-Ill Patient with Intravenous Drug Injection. J Pharm Drug Deliv Res 6:6. doi: 10.4172/2325-9604.1000169
Abstract
Background: The blood-brain-barrier (BBB), which is characterized with the selective permeability, has major impact on distribution, concentration and biological effects of most drugs in the central nervous system (CNS).
Methods: In this study, we chose one critically-ill patient in year 2015 and monitored drug concentrations in the cerebrospinal fluid (CSF) and serum during vancomycin therapy, in order to observe vancomycin permeability of BBB or clinical application potential under CNS infection state.
Results: During a period of 48h after intravenous drug injection, the peak level was 3.45 µg/ml, lowest level 0.13 µg/ml, and average level 1.46 µg/ml in the CSF. In the serum, the peak level was 64.23 µg/ml, lowest level 3.91 µg/ml and average level 21.56 µg/ml. In all nine time-points detected during 48h, CSF drug concentrations were lower than that in serum, but CSF drug levels in six of nine time-points showed above MIC (1.0 µg/ml) or effective for MRSA, indicating that BBB had larger influence on drug permeability or distribution of vancomycin in the CNS. However, intravenous injection could reach concentration effective for MASA of CNS infection.
Conclusions: This was difficult to obtain case and it has provided new evidence on vancomycin usage in CNS for MASA infections in a Chinese critically-ill patient.
Keywords: Vancomycin; Cerebrospinal fluid; MRSA; Central nervous system
Introduction
It is well known that the blood-brain barrier (BBB) exists in the central nervous system (CNS) of mammals, which is structurally composed with the endothelial cells, base membrane and abundant astroglial process foots end on them [1,2]. The BBB is functionally characterized with the selective permeability that then greatly influences distribution of most drugs and biological effectiveness in CNS tissue [3,4]. However, the BBB opening or permeability might be changed or regulated by various factors. Among them, the infection state or inflammatory stimuli remarkably enhanced BBB infiltration of antibiotics such as vancomycin in the CNS [5-8].
In recent years, with increasing cases of MRSA infection in hospital, the strong antibiotic vancomycin is required for severe CNS infections. Previous studies have indicated that vancomycin has low BBB permeability under physiological condition, thereby decreasing distribution and concentration in CNS. Being a glycopeptide antibiotic discovered in 1958, vancomycin has been used for clinical treatment of severe infections of drug-resistant gram positive bacteria such as methicillin-resistant Staphylococcus Aureus (MRSA) [9,10]. It is a viable option when other common-used antimicrobial drugs are ineffective. Vancomycin is even considered to be “the last line of defense” against those resistant strains of bacteria especially MRSA. Questions regarding vancomycin dosing regimens, administration modes and related rationales are still much concerned for its clinical application in the CNS diseases [9,10]. In this study, an observation on drug concentrations in the cerebrospinal fluid (CSF) and serum was made in a critically-ill patient treated with vancomycin injection, in order to examine its permeability and effectiveness for MASA under infection stat of CNS.
Materials and Methods
Patient information
Patient Shan XX, male, 43 years old, 72 kg in body weight, was admitted into the Department of Neurosurgery, Xijing Hospital in June 25, 2015. He reported to have continual fever for one month and up to 39°C after operation on brain stroke. The patient reported suffering from high blood pressure for six years. One month ago, he suffered from brain cerebral hemorrhage, and operation was done to remove brain blooding blocks and followed by lateral ventrical drainage. Lumbar vertebral puncture was performed to collect suitable volume of CSF sample, and result of laboratory assay of CSF confirmed intracranial infection. The diagnoses were recognized as the intracranial infection, hydrocephalus, multiple cerebral infarcts, and post-operation of brain stroke.
Preparation of vancomycin standard solution
| Vancomycin serum concentrations (µg/ml) | 0.5 | 1 | 2 | 5 | 10 | 20 | 50 |
| Volume of standard solutionï¼Ã‚ˆµl) | 10 | 10 | 10 | 10 | 10 | 10 | 10 |
| Concentrations of standard solution (µg/ml) | 10 | 20 | 40 | 100 | 200 | 1000 | 1000 |
The patient received intravenous injection vancomycin to treat intracranial MRSA infection after admission into hospital. During 48h of vancomycin therapy, the drug concentration in CSF and serum was monitored. The ethic approval was obtained from the Fourth Military Medical University ethics committee. According to ethic management, a clear explanation regarding purpose vancomycin monitoring in serum and CSF was made to patient’s relatives, the agreement and consent for vancomycin monitoring and possible publication was obtained by signing agreement sheet.
Reagents and vancomycin standard solution
Vancomycin (Standard product, 100% purity, Lot No.130360- 201302), tinidazole (Standard product, 99.8% purity, Lot No. 100336-200703), commercially purchased from The Food and Drug Qualifying Institute of China. Methanol (fisher scientific), Ethylnitrile (fisher scientific), phosphoric acid, Potassium dihydrogen phosphate, Magnesium sulphate, Purified water (Milli-Q purify water Maker).
For assaying vancomycin concentration, the vancomycin standard solution and quality-control solution were prepared as following.
Preparation of quality-control sample
| Vancomycin serum concentrationsï¼Ã‚ˆµg/ml) | 1 | 8 | 40 |
| Volume of standard solutionï¼Ã‚ˆµl) | 10 | 10 | 10 |
| Concentrations of standard solution (µg/ml) | 20 | 200 | 1000 |
Vancomycin antibiotic sensitivity or resistance assay
Bacterial culture was done to detect the MRSA in CSF samples. After MRSA was detected, antibiotic sensitivity was then performed. The bacterial culture and antibiotic sensitivity assays were assisted by Department of Laboratory Inspection. Vancomycin sensitivity or resistance assay and MIC was verified by agar gel dilution method in the following procedure: I) preparation of agar gel, 10 g peptone, 5 g yeast extract and 10 g sodium chloride dissolved in 1L distilled water. The pH value was adjusted to 7.2 by 5 mol/L sodium oxyhydrogen and sterilized under high-pressure steam heating; II) When the temperature of agar gel was cooled down at 60°C, vancomycin was added in the concentration gradients, i.e. 16 μg/ml, 8 μg/ml, 4 μg/ml, 2 μg/ml, 1 μg/ml, 0.5 μg/ml, 0.25 μg/ml, 0.10 μg/ml, 0.05 μg/ml and 0.01 μg/ml, and plated in culture dishes one by one and labeled; III) The bacterial culture medium with CSF sample was mixed and planted in agar gel with above vancomycin concentration gradients and kept for 24 h culture at 35°C; IV) observation of bacteria growth was followed, and minimum inhibitory concentration (MIC) was identified as the lowest vancomycin concentration in which no bacterial clone was formed in the agar gel. According to guide line used currently, when MIC <2.0 μg/ml, a priority of vancomycin was recommended for treatment of MRSA infection.
Administration of vancomycin
Since July 2, 12:00 am, 2015, vancomycin injection was given to this critically-ill patient by vancomycin injection solution (Wenkexin, Japan) at 1 g/per time/1h, twice a day, and two injections were carried out within 48 h.
Collection of serum and cerebrospinal fluid samples
At total nine time-points of before and during vancomycin therapy (July 2 to July 3, 2015), i.e. 0.5h, 1h, 2h, 3h, 6h, 10h, 12h, 24h, 48h after first vancomycin injection, the CSF and serum and samples were collected for detection of vancomycin concentrations. Assay of vancomycin levels in CSF and serum was performed as follow: 200 μl of serum or cerebrospinal fluid was placed in a 1.5 ml centrifugetube, followed by adding vancomycin standard solution and 10 μl internal control standard tinidazole solution (400 μg/ml). After mixed completely for 30 seconds, 30 μl 10% Zinc sulfuric acid was added for precipitation, and mixed by strong vibration for 3 min. The centrifugation was carried out at 1600 rpm for 10 min; supernatant was picked for HPLC-UV analysis by placing into automatic sample bottle at 20 μl volume for each sample. The vancomycin concentrations in the CSF and serum were measured at each time point and timeconcentration curve was demonstrated.
Laboratory detection of hepatic and kidney function items
To value the hepatic and kidney functions or possible drug toxicity, the blood serum was further taken for detecting ALT, AST, AST/ALT, total proteins, albumin, globulin, percentage of albumin/ globulin, Total bilirubin (Tbil), DBil, IBil, alkaline phosphatase, r-glutamine-transferase, liucine-rich amino-peptidase, Urea, Bun levels or abnormal changes during vancomycin therapy.
Results
Bacterial culture of patient CSF sample and antibiotic sensitivity
Bacterial culture indicated positive MRSA infection of CSF sample collected at June 26, 2015. Besides, the serum was also collected for detection of IL6 and PCT by electro-chemical method at June 30, 2015 as a collateral index of CNS infection state. It showed that IL6 was 7.10 (reference value <7 pg/ml) and PCT was 0.023 (reference value<0.05 ng/ml). This result also suggested that the patient suffered from local intracranial MRSA infection. The antibiotic sensitivity to MRSA was detected afterwards by index of minim inhibition concentration (MIC in μg/ml or mg/L). It showed that the cultured bacteria was sensitive to vancomycin (MIC</=1 μg/ml), minocycline (MIC=20 μg/ml) and cyclobenshaxin (MIC</=0.25 μg/ml). On the other hand, the bac
teria appeared relative resistance to antibiotic Erythromycin, Cyclomycin, Lifupin, L-Ofloxamin, Amxoicllin, Pennecillin, Ampicillin, Tobramycin and Gentamycin (Table 1).
| Antibiotics sensitivity or resistance of MASA (Jun 30, 2015) | |||
|---|---|---|---|
| Antibiotics | methods | Results | sensitivity |
| Minocyclines | KB | 20 | S |
| Vancomycin | MIC | <=1 | S |
| Cyclobenshaxin | MIC | <=0.25 | S |
| Erythromicn | MIC | >4 | R |
| cyclomycin | MIC | >8 | R |
| Lifupin | MIC | >2 | R |
| L-oflioxacin | KB | 11 | R |
| Amoxicillin | MIC | >4/2 | R |
| Penicillin | MIC | >0.25 | R |
| Ampicillin | MIC | >8 | R |
| Tobramycin | MIC | >8 | R |
| Gentamycin | MIC | >8 | R |
S, sensitivity; R, resistant; KB unit=mm; MIC unit=mg/l (µg/ml)
Table 1: Laboratory result on antibiotic sensitivity or antibiotic resistance of MASA cultured in the cerebrospinal fluid (CSF) sample.
Clinical treatment outcome of vancomycin therapy
In reference to MRSA bacterial positive result and antibiotic sensitivity assay, intravenous injection vancomycin was started at July 2, 12:00 and continued to July 5, 2015. The outcome of treatment was acceptable with obvious improvement of illness, and patient checked out at July 6, 2015 due to willingness of patient relatives. Checkout medical receipt was given to the patient: Continuing antibiotic treatment and supporting therapy after transfer to other hospital.
Dynamics of vancomycin concentrations in the serum and cerebrospinal fluid
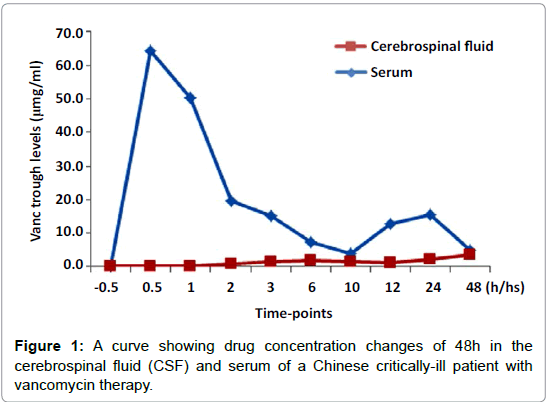
By detection of drug concentrations at nine time-points, i.e. 0.5h, 1h, 2h, 3h, 6h, 10h, 12h, 24h, 48h after intravenous injection of vancomycin, dynamic changes of drug levels were observed in both CSF and serum. During a period of 48 h, the peak level was 3.45 μg/ ml, lowest level was 0.13 μg/ml, and average level was 1.46 μg/ml in the CSF, while in the serum, the peak level was 64.23 μg/ml, lowest level was 3.91 μg/ml and average level was 21.56 μg/ml. In nine timepoints detected, CSF drug concentrations were all lower than that in serum, but, the CSF drug levels in six of nine time-points observed in 48 h, showed above MIC (<=1.0 μg/ml) or concentrations effective for target bacteria MRSA (Figure 1 and Table 2).
| Serum and CSF trough level during vancomycin therapy | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Time-points | -0.5h | 0.5h | 1h | 2h | 3h | 6h | 10h | 12h | 24h | 48h |
| CSF trough level (µg/ml) | 0 | 0.13 | 0.13 | 0.93 | 1.57 | 1.98 | 1.4 | 1.31 | 2.19 | 3.45 |
| Serum trough level (µg/ml) | 0 | 64.23 | 50.28 | 19.61 | 15.2 | 7.47 | 3.91 | 12.78 | 15.5 | 5.09 |
| -0.5h, .05h before vancomycin administration; CSF, Cerebrospinal fluid | ||||||||||
Table 2: The drug concentrations of 48h in the cerebrospinal fluid (CSF) and serum of a Chinese critically-ill patient treated with intravenous injection of vancomycin.
The hepatic and kidney functions during vancomycin administration
The hepatic and kidney function was detected in the serum samples for 11 days from June 26, 26 to July 6, 2015. Data indicated that before and during vancomycin therapy, major hepatic and kidney functional index (e.g. ALT/AST, BUN) remained basically stable, and no obvious drug toxicity on liver and kidney function was detected (Table 3).
| Detection of hepatic and kidney functions during vancomycin therapy | |||||||
|---|---|---|---|---|---|---|---|
| Factors | 26-Jun | 28-Jun | 30-Jun | 2-Jul | 4-Jul | 6-Jul | Reference Values |
| ALT | 33 | 41 | 52 | 55 | 46 | 38 | 9-50 IU/L |
| AST | 17 | 18 | 25 | 19 | 16 | 16 | 15-40 IU/L |
| AST/ALT | 0.5 | 0.04 | 0.5 | 0.3 | 0.3 | 0.3 | |
| TP | 65.6 | 60.6 | 63.2 | 56.3 | 62.7 | 60.6 | 65-85 g/L |
| ALB | 38.6 | 39.9 | 37.8 | 33.2 | 38.7 | 37.7 | 40-55 g/L |
| GLO | 27.1 | 20.7 | 25.4 | 23.1 | 24 | 22.9 | 20-40 g/L |
| ALB/GLO | 1.4 | 1.9 | 1.5 | 1.4 | 1.6 | 1.6 | 1.2-2.4 |
| TBIL | 7.1 | 5.9 | 8.7 | 4.5 | 6.4 | 9 | 3.4-2.5 (µmol/L) |
| DBIL | 3 | 2.7 | 3.8 | 2 | 2.9 | 3.9 | 0.0-6.8(µmol/L) |
| IBIL | 4.1 | 3.2 | 4.9 | 2.5 | 3.5 | 5.1 | 6.8-12 (µmol/L) |
| BUN | 1.43 | 1.56 | 1.28 | 1.67 | 1.12 | 2.29 | 2.5-6.3 (µmol/L) |
| CR | 67 | 72 | 63 | 69 | 60 | 65 | 53-115 (µmol/L) |
Table 3: Laboratory assay of hepatic and kidney functions of a Chinese critically-ill patient before and during vancomycin therapy.
Routine and biochemical analysis during vancomycin administration
In addition, the routine and biochemical assay of CSF sample was performed for 11 days from June 26, 26 to July 6, 2015, for the purpose to demonstrate appearance of CSF, protein or globulin content, white and red blood cells. The muddy CSF appearance, increase of white and red blood cells, decrease of glucose and Cl content, increase of proteins and globulin were observed, also indicating the existence of intracranial infection state (Table 4).
| Routine and biochemical analysis of CSF during vancomycin therapy | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Factors | Jun-26 | Jun-29 | Jun-30 | Jul-01 | Jul-02 | Jul-04 | Jul-05 | Reference Values | Factors | Jun-26 |
| CSF Appear | Yellow clear | Yellow clear | Yellow muddy | Yellow muddy | Yellow muddy | Yellow muddy | CSF Appear | Yellow clear | ||
| Globulin | + | ++ | ++ | ++ | ++ | ++ | + | Globulin | + | |
| RBC | 0 | 0 | 0 | 100 | 161 | 600 | 800 | X10*6/L | RBC | 0 |
| WBC | 668 | 373 | 442 | 241 | 36 | 4152 | 1105 | x10*6/L | WBC | 668 |
| LC(%) | 14 | 18 | 24 | 27 | 36 | 5 | 7 | % | LC(%) | 14 |
| NC(%) | 86 | 82 | 76 | 73 | 64 | 95 | 93 | % | NC(%) | 86 |
| Glucose | 1.98 | 2.28 | 1.5 | 2.91 | 3.02 | 0.52 | 1.11 | 2.1-3.9mmol/L | Glucose | 1.98 |
Table 4: Laboratory assay of routine and biochemical index in the cerebrospinal fluid (CSF) of a Chinese critically-ill patient with vancomycin therapy.
Discussion
This study has shown that BBB might have obvious influence on permeability and distribution of vancomycin in the central nervous system. During a period of 48h after intravenous injection of vancomycin, the peak level was 3.45 μg/ml and average at 1.46 μg/ml in the CSF, while peak level was 64.23 μg/ml and average at 21.56 μg/ ml in the serum. It indicated that CSF drug concentrations were lower than that of serum, but CSF drug level reached and remained effective (above MIC) for MASA at 3hours after injection. Vancomycin is now recognized as a viable antibiotic for MRSA among antibiotics used currently [9,10]. This study has further provided new valuable evidence for vancomycin therapy against MASA infections of CNS in a critically-ill patient.
Previous evidences showed that BBB had low permeability to vancomycin and difficulty in achieving adequate concentrations under physiological condition, thereby decreasing its distribution or concentration in CNS. Vancomycin concentrations might be subtherapeutic in critically-ill patients based on a prospective study [11]. Aouinti et al. report a case of a 74-year-old man with post traumatic meningitis with vancomycin concentration in CSF [12] but it is lack of extensive observations on drug concentrations during vancomycin therapy for CNS infections. In present study, we observed nine time-points during a period of 48 h after intravenous injection of vancomycin. The drug concentrations in the CSF were lower than that in serum, but CSF drug levels showed above MIC (<=1.0 μg/ ml) for MRSA in six of nine time-points detected. Our data thus confirmed that BBB had large influence on vancomycin permeability and distribution in CNS, which is basically consistent with previous studies. Interestingly, intravenous injection of vancomycin could reach drug levels effective for MASA in this critically-ill patient. The illness of patient was improved and no toxicity on liver and kidney was detected, suggesting application potential of intravenous vancomycin injection against MASA in CNS infection. It is well known that therapeutic drug monitoring of CSF and serum is critical in assisting clinician to decide vancomycin dosing [13]. Kim et al. compared vancomycin pharmacokinetics in the neurosurgical and non-neurosurgical groups of patients [14]. Neurosurgery might damage the BBB to allow more vancomycin distribution into CSF after intravenous injection [15,16]. On the other hand, neurosurgery also enhance vancomycin clearance significantly [14,17]. Moreover, vancomycin concentrations were influenced by various other factors such as age, body weight and severity of disease. Sheng et al. established a vancomycin PPK model to estimate individual P
K parameters in Chinese infant patients [18]. Kishk et al. studied vancomycin AUC/MIC in a pediatric population [19]. Guideline suggested an area under the curve/minimum inhibitory concentration (AUC/MIC)>400 corresponded to vancomycin trough serum concentration of 15-20 mg/L for MASA in adult. Correspondingly, AUC/MIC of 400 in children should be a trough concentration of 11 mg/L. More studies recommended vancomycin 10 mg/kg TID as initial dosage regimens for low birth weight neonates with infected bacteria MIC ≤1 μg/ml [20]. In the children with cerebral ventricular shunt infections, the vancomycin CSF concentrations ranged from 0.06 to 9.13 mg/L, and were greater than MIC at the end of the dosing [21]. Kane and Hanes saw an unexplained increase of serum concentration in a morbidly obese patient [22]. In the pediatric patients with cystic fibrosis, a positive correlation was seen between dosage and trough drug concentration [23]. Therefore, vancomycin dosage regimens in CNS infections should be individualized for these neurosurgical patients, infants, low-body weight or obese and critically-ill patients clinically. Although low drug permeability of vancomycin is greatly concerned due to BBB blockage, inflammatory stimuli might enhance BBB opening and permeability under CNS infection state. An increase of vancomycin permeability and CSF concentration was reported in those patients with pneumococcal meningitis. Mean levels of vancomycin in CSF and serum were 7.2 and 25.2 mg/L. The trough levels of CSF and serum showed positive linear correlation, and vancomycin reached effective concentration in the CSF during treatment of meningitis [24]. Ricard et al. observed concentrations of vancomycin in CSF of patients with pneumococcal meningitis receiving combined dexamethasone, latter might reduce vancomycin penetration into CSF. Mean levels of vancomycin in CSF was 7.2 mg/L and effective concentrations could be obtained even when concomitant steroids were used [25]. Recently, Wu et al. also carried out an observation on concentrations of norvancomycin, a new developed drug in China, in the CSF and serum of patients after craniotomy, and demonstrated its therapeutic capability against MASA infections. The peak levels were 8.82-16.31 mg/L in the CSF, remained in 6.12-6.24 mg/L at 24 h. The peak serum levels were 55.52-59.22 mg/L, and remained in 8.01-8.21 mg/L at 24 h. The CSF concentration reached or exceeded 90% MIC (2 mg/L) of MRSA by administration of norvancomycin post-neurosurgery in conventional and continuous dosing methods [26]. Finally, vancomycin dosing and administration mode can be manipulated to reach or sustain concentrations effective for MRSA infections in CNS. To maintain a sufficient CSF levels, intraventricular administration was utilized in combination with transvenous administration of vancomycin. The combined administration of 5-10 mg reached CSF concentration of 33 mg/L at 24 h or 11.7 mg/L at 72 h after injection, and CSF concentration could be prolonged in newborns [27]. Popa et al. also explored CSF concentrations by intraventricular vancomycin dosing for meningitis. Vancomycin was injected at 10 mg/12-48 h and continued for 15 days, CSF concentrations sustained around 10- 30 mg/L [28]. In addition, prolonged vancomycin release of novel drug-loaded bone-like hydroxyapatite/poly amino acid scaffold was observed in vitro and in vivo. Drug release was more rapid during the first 48 h and then followed by a period of sustained slow release of vancomycin [29]. The BBB intervention strategies such as microwave and supersonic stimulation were also supposed to increase permeability of drug in the CNS tissue, which merit for further extensive investigation on clinical vancomycin application for MASA infections of the central nervous system.
Conclusion
In this study, vancomycin concentrations were observed in cerebrospinal fluid and serum after intravenous drug administration. The peak level was 3.45 μg/ml and average level 1.46 μg/ml in the CSF, respectively. The CSF levels showed above MIC (≤ 1.0 μg/ml) at 3h after vancomycin injection. Data thus indicated that intravenous injection of vancomycin could obtain concentration effective for MASA from CSF sample and it has provided new evidence on clinical application of vancomycin in CNS for MASA infections in a Chinese critically-ill patient.
Acknowledgement
This work is supported by grants from Shaanxi Social Developmental Program for Science and Technology (No. S2016YFSF0706) and National Natural Science Foundation of China (No. 31600830).
Ethical approval: The study was approved by the Research Ethics Committee of Xijing Hospital, the Fourth Military Medical University, China.
References
- Keaney J, Campbell M (2015) The dynamic blood-brain barrier. FEBS J 282: 4067-4079
- Haseloff RF, Dithmer S, Winkler L, Wolburg H, Blasig IE (2015) Transmembrane proteins of the tight junctions at the blood-brain barrier: structural and functional aspects. Semin Cell Dev Biol 38: 16-25.
- Bujak R, Struck-Lewicka W, Kaliszan M, Kaliszan R, Markuszewski MJ (2015) Blood-brain barrier permeability mechanisms in view of quantitative structure-activity relationships (QSAR). J Pharm Biomed Anal 108: 29-37.
- Gupta S, Basant N, Singh KP (2015) Qualitative and quantitative structure-activity relationship modelling for predicting blood-brain barrier permeability of structurally diverse chemicals. SAR QSAR Environ Res 26: 95-124
- Wang LF, Li X, Gao YB, Wang SM, Zhao L, et al. (2015) Activation of VEGF/Flk-1-ERK pathway induced blood-brain barrier injury after microwave exposure. Mol Neurobiol 52: 478-491.
- Bae MJ, Lee YM, Kim YH, Han HS, Lee HJ (2015) Utilizing ultrasound to transiently increase blood-brain barrier permeability, modulate of the tight junction proteins, and alter cytoskeletal structure. Curr Neurovasc Res 12: 375-383.
- Troletti CD, de Goede P, Kamermans A, de Vries HE (2016) Molecular alterations of the blood-brain barrier under inflammatory conditions: The role of endothelial to mesenchymal transition. Biochim Biophys Acta 1862: 452-460
- Shawahna R (2015) Physical and metabolic integrity of the blood-brain barrier in HIV infection: A special focus on intercellular junctions, influx and efflux transporters and metabolizing enzymes. Curr Drug Metab 16: 105-123.
- Dong MH, Wang JW, Wu Y, Chen BY, Yu M, et al. (2015) Evaluation of body weight-based vancomycin therapy and incidence of nephrotoxicity: A retrospective study in Northwest of China. Int J Infect Dis 37: 125-128
- Wang J, Dong M, Lu Y, Zhao X, Li X, Wen A (2015) Impact of pharmacist interventions on rational prophylactic antibiotic use and cost saving in elective cesarean section. Int J Clin Pharmacol Ther 53: 605-615.
- Bakke V, Sporsem H, Von der Lippe E, Nordøy I, Lao Y, et al. (2017) Vancomycin levels are frequently subtherapeutic in critically ill patients: a prospective observational study. Acta Anaesthesiol Scand 61: 627-635.
- Aouinti I, Charfi R, Trabelsi S, Gaies E, Salouage I (2014) Vancomycin therapeutic drug monitoring in cerebrospinal fluid. Therapie 69: 529-530.
- Lonsdale DO, Udy AA, Roberts JA, Lipman J (2016) Antibacterial therapeutic drug monitoring in cerebrospinal fluid: difficulty in achieving adequate drug concentrations. J Neurosurg 118: 297-301.
- Kim AJ, Lee JY, Choi SA, Shin WG (2016) Comparison of the pharmacokinetics of vancomycin in neurosurgical and non-neurosurgical patients. Int J Antimicrob Agents 48: 381-387.
- Li X, Wu Y, Sun S, Mei S, Wang J, et al. (2015) Population pharmacokinetics of vancomycin in postoperative neurosurgical patients. J Pharm Sci 104: 3960-3967.
- Li X, Wu Y, Sun S, Zhao Z, Wang Q (2016) Population pharmacokinetics of vancomycin in postoperative neurosurgical patients and the application in dosing recommendation. J Pharm Sci 105: 3425-3431.
- Lin Wu FL, Liu SS, Yang TY, Win MF, Lin SW, et al. (2015) A larger dose of vancomycin is required in adult neurosurgical intensive care unit patients due to augmented clearance. Ther Drug Monit 37: 609-618.
- Sheng XY, Chen CY, Ma LY, Liu YO, Zhou Y, et al. (2017) Population pharmacokinetics of vancomycin in Chinese infants. Int J Clin Pharmacol Ther 55(7):558-566.
- Kishk OA, Lardieri AB, Heil EL, Morgan JA (2017) Vancomycin AUC/MIC and Corresponding Troughs in a Pediatric Population. J Pediatr Pharmacol Ther 22: 41-47.
- Kato H, Hagihara M, Nishiyama N, Koizumi Y, Mikamo H (2017) Assessment of optimal initial dosing regimen with vancomycin pharmacokinetics model in very low birth weight neonates. J Infect Chemother 23: 154-160.
- Autmizguine J, Moran C, Gonzalez D, Capparelli EV, Smith PB (2014) Vancomycin cerebrospinal fluid pharmacokinetics in children with cerebral ventricular shunt infections. Pediatr Infect Dis J 33: 270-272.
- Kane SP, Hanes SD (2017) Unexplained increases in serum vancomycin concentration in a morbidly obese patient. Intensive Crit Care Nurs 39: 55-58
- Durham SH, Garza KB, Eiland LS (2016) Relationship between vancomycin dosage and serum trough vancomycin concentrations in pediatric patients with cystic fibrosis. Am J Health Syst Pharm 73: 969-974.
- Shokouhi S, Alavi Darazam I (2014) Determination of vancomycin trough level in serum and cerebrospinal fluid of patients with acute community-acquired meningitis: a prospective study. J Infect 69: 424-429.
- Ricard JD, Wolff M, Lacherade JC, Mourvillier B, Hidri N, et al. (2007) Levels of vancomycin in cerebrospinal fluid of adult patients receiving adjunctive corticosteroids to treat pneumococcal meningitis: a prospective multicenter observational study. Clin Infect Dis 44: 250-255.
- Wu Y, Kang J, Wang Q (2017) Drug concentrations in the serum and cerebrospinal fluid of patients treated with norvancomycin after craniotomy. Eur J Clin Microbiol Infect Dis 36: 305-311.
- Matsunaga N, Hisata K, Shimizu T (2015) An investigation into the vancomycin concentration in the cerebrospinal fluid due to vancomycin intraventricular administration in newborns: a study of 13 cases. Medicine (Baltimore) 94: e922.
- Popa D, Loewenstein L, Lam SW, Neuner EA, Ahrens CL (2016) Therapeutic drug monitoring of cerebrospinal fluid vancomycin concentration during intraventricular administration. J Hosp Infect 92: 199-202.
- Cao Z, Jiang D, Yan L, Wu J (2017) In vitro and in vivo drug release and antibacterial properties of the novel vancomycin-loaded bone-like hydroxyapatite/poly amino acid scaffold. Int J Nanomedicine 12: 1841-1851.
 Spanish
Spanish  Chinese
Chinese  Russian
Russian  German
German  French
French  Japanese
Japanese  Portuguese
Portuguese  Hindi
Hindi