Research Article, Jor Vol: 8 Issue: 2
An Update on the Criteria for Diagnosing Allergic Fungal Rhinosinusitis: A Review of the Literature
Faisal A Arshad1*, Adam Kara1 and Showkat Mirza2
1Department of ENT, Oxford University Hospitals, UK
2Department of ENT, Sheffield Teaching Hospital NHS Foundation Trust, Sheffield, England
*Corresponding Author : Faisal A Arshad
TIG Fellow in Head & Neck Surgical Oncology, Department of ENT, Oxford University Hospitals, UK
Tel: +44 07495956612
E-mail: drfaisalarshad@doctors.net.uk
Received: April 04, 2019 Accepted: May 18, 2019 Published: May 25, 2019
Citation: Arshad FA, Kara A, Mirza S (2019) An Update on the Criteria for Diagnosing Allergic Fungal Rhinosinusitis: A Review of the Literature. J Otol Rhinol 8:2. doi: 10.4172/2324-8785.1000369
Abstract
Allergic fungal rhinosinusitis (AFRS) is a type of hypersensitive inflammatory response that causes chronic, recurrent, and noninvasive hypertrophic sinus disease. The aim of this paper is to report the latest information and development in the diagnostic conundrum that allergic fungal rhinosinusitis can present. A literature review was conducted of PubMed English language search of current diagnostic criteria for allergic fungal rhinosinusitis, from 1951 to 2017. The diagnosis of allergic fungal rhinosinusitis needs to be considered in all patients with severe chronic rhinosinusitis with nasal polyposis. Patients often have asthma and complain of thick ‘wallpaper glue’ like nasal secretions that can also often be seen on nasal endoscopy. CT scanning often shows heterogeneity and MRI scanning may also help to identify cases. The diagnostic role of total and fungus-specific IgE in AFRS requires further prospective evaluation. The role of genetic and immunological testing in AFRS requires developing as diagnostic tools. The diagnosis is therefore often a clinical one supported by investigations such as radiology
Keywords: Allergic fungal rhinosinusitis; Diagnostic criteria; Nasal polyps; Bent and Kuhn criteria
Introduction
There are a number of causes of chronic rhinosinusitis (CRS) with nasal polyposis (CRSwNP) including allergy, mucociliary disorders, systemic conditions and fungal disease. Fungal rhinosinusitis (FRS) includes a number of conditions which can be broadly divided into 2 main categories; invasive and non-invasive. Invasive FRS includes acute invasive fungal rhinosinusitis, chronic invasive fungal rhinosinusitis and chronic invasive granulomatous fungal rhinosinusitis. Non-invasive FRS includes fungus ball and allergic fungal rhinosinusitis (AFRS).
AFRS, a type of hypersensitive inflammatory response, causes chronic, recurrent, and non-invasive hypertrophic sinus disease that affects immunocompetent hosts [1]. AFRS falls into the category of CRSwNP associated with eosinophilic inflammation [2,3]. The percentage of AFRS in patients with CRS ranges widely from 5 to 10% in some studies to a much higher percentage in others studies, such as in a by Ponikau et al. [4]. In the latter study the authors concluded that 93% of patients that underwent endoscopic sinus surgery for CRS met the published criteria for AFRS and speculate that this condition is the principal cause of CRS.
The clinical entity of AFRS was first described by Safirstein et al. in 1976 after noting similar pathological appearances to that of allergic bronchopulmonary aspergillosis [5]. AFRS has characteristic clinical, radiological and histopathological features. The most recognised criteria for AFRS are those presented by Bent and Kuhn in 1994 [6]. In this literature review we aim to highlight the latest information in relation to the diagnostic criteria for AFRS. A literature review was conducted of PubMed (MEDLINE® and the NLM® database of indexed citations) English language search of current diagnostic criteria for AFRS, from 1951 to 2017.
Discussion
The Bent and Kuhn diagnostic criteria are historically the standard for diagnosis. The 5 major criteria include: (1) nasal polyposis, (2) presence of fungi on direct microscopy or culture of sinus content, (3) eosinophilic mucin without fungal invasion into sinus tissues, (4) type I (Ig E medicated) hypersensitivity to fungi demonstrated by skin testing or in vitro testing and (5) characteristic CT findings, including sinus expansion or heterogeneous opacification. The Minor criteria include asthma, unilateral disease, bone erosion, fungal cultures, Charcot Leyden crystals and serum eosinophilia [6]. DeShazo et al. proposed to remove type I hypersensitivity as a diagnostic criterion for AFRS given that identical clinical forms of CRS but without type I hypersensitivity to fungi have been described [3].
The Bent and Kuhn diagnostic criteria for AFRS remain controversial, it may take months to years to establish all 5 of the major criteria. In 2009 the international Society for Human and Animal Mycology convened a working group to attempt to build a consensus on terminology and disease classification of fungal rhinosinusitis. They concluded that they are imprecise and require better definition [7]. The European position paper on rhinosinusitis and nasal polyps from 2012 acknowledges that of the Bent and Kuhn diagnostic criteria, only type I hypersensitivity combined with characteristic CT findings are unique to AFRS and distinguish it from other forms of inflammatory sinus disease [8].
Clinical features
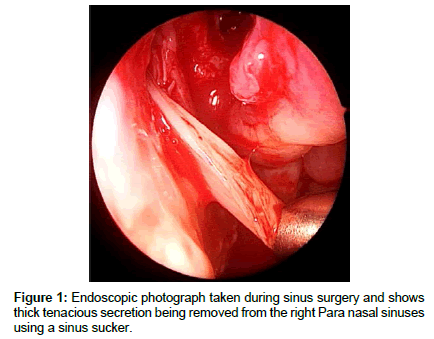
Clinical features of AFRS include a history of sinus disease relatively resistant to medical therapy in an immunocompetent patient. The patient may complain of thick ‘wallpaper glue’ secretions with more severe sinonasal symptoms than with ‘simple’ CRSwNP (Figure 1). These patients may well have had multiple nasal polypectomies and endoscopic sinus procedures in the past with only a short lived improvement in symptoms. Two-thirds of patients are atopic and half suffer from Asthma [9]. Patients may well have concurrent Samter’s triad (nasal polyposis, asthma, and aspirin sensitivity) which is associated with a more severe form of sinonasal polyposis but part of the severity of the patient’s symptoms may be due to the associated AFRS. Therefore, AFRS should be considered and looked for in all patients with Samter’s triad and treated appropriately. The condition is more common amongst young adults and in geographical areas of high humidity. AFRS can lead to late complications of proptosis, visual disturbance and facial dysmorphism e.g. telecanthus formation, if not diagnosed and managed appropriately.
Radiological features
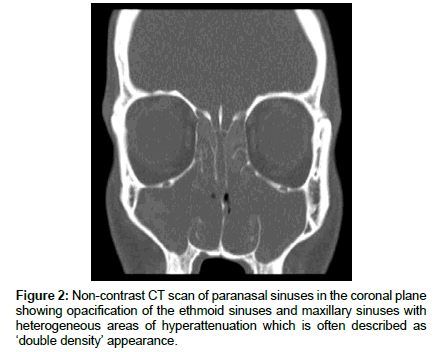
In AFRS Computer Tomography (CT) findings include pansinus opacification (Figure 2). Due to the deposition of heavy metals, such as iron and manganese, allergic mucin provides the heterogeneous signal intensity that is characteristic of AFRS. However, the heterogeneity may be subtle and missed on bone window setting but may be more apparent on a soft tissue window setting. Local bony involvement such as bone destruction and thinning of bony structures secondary to expansion of accumulated mucus, is 10 times more common in AFRS than in other forms of chronic rhinosinusitis (CRS) [6]. This can cause, for example, widening of the nasal bones.
Magnetic resonance imaging (MRI) has been shown to demonstrate a high specificity for AFRS, especially when combined with CT [10]. The high protein concentration of allergic mucin results in crosslinking and slows macromolecular motion, giving rise to T1 central hypointensity and T2 central signal void. Both T1 and T2 series demonstrate peripheral enhancement [10]. Furthermore MRI is an important adjunct when considering dural involvement or intracranial expansion.
Immunological testing
The total immunoglobulin E (IgE) levels are generally elevated, often to more than 1,000 U/mL. The importance of type I hypersensitivity in AFRS pathophysiology is not clear. Humoral immunity and immunoglobulin pathways may contribute. After in-depth examination of humoral immune response to fungal antigens, Pant et al. found that there was no difference in the levels of total IgE, ratio of mould-mix specific IgE to total IgE and absolute levels of IgE to specific fungi between AFRS patients and patients with allergic rhinitis and fungal allergy (without CRS). This challenges the central pathogenic importance of elevated fungal-specific IgE levels in AFRS, suggesting that immune mechanisms other than fungal allergy are involved and that elevated fungal-specific IgE may simply represent concurrent rhinosinusitis and fungal allergy [11].
Collins et al. demonstrated that most patients with AFRS had fungal-specific IgE in sinus mucin, and a minority of chronic sinusitis patients with “allergic, eosinophilic fungal-like” sinus mucin and no evidence of systemic fungal allergy also had mucin antifungal IgE [12]. This suggested that a local type I hypersensitivity response against fungal antigens may be present even in those patients without evidence of systemic allergy and irrespective of the identification of fungi in eosinophilic mucin [11]. In the absence of systemically detectable allergen-specific IgE or a positive prick test, the pathogenic importance of locally produced IgE in allergenspecific hypersensitivity responses is yet to be established [11]. The evidence suggests that an IgE-mediated process is involved in the pathogenesis of AFRS, it being a primary factor however is still not clear [12]. A study by Wise et al. in 2008 showed a significant increase in total and fungus-specific IgE staining in AFRS sinus epithelium and subepithelium as compared to controls and to CRSsNP patients [13]. Chang and Fang demonstrated that a group of AFRS patients all had a negative serum IgE response to Aspergillus, but 85.7% of the same patients had maxillary sinus tissue-specific immunoglobulin E (sIgE) to Aspergillus [14].
In relation to immunology, Ebert et al. found enhanced production of protease-activated receptors (PARs) in AFRS patients as compared to non-diseased controls [15]. PARs cause an increased production of inflammatory cytokines and potentiate Th2 responses, which are characteristic findings in AFRS [16]. Ayers et al. demonstrated that patients with AFRS and CRSwNP have an increased number of local dendritic cells in sinus mucosa samples as compared to controls without CRS [17]. Laury et al. found significantly increased levels of periostin, which is an extracellular matrix protein associated with eosinophil accumulation, in AFRS sinus tissue samples as compared to CRSsNP and control samples by both immunofluorescence (p<0.001) and PCR (p=0.011) [18].
Histopathology
The histopathologic findings in AFRS of mucosal specimens on hematoxylin-eosin (H&E) staining show a typical inflammatory infiltrate composed of eosinophils, lymphocytes, and plasma cells. The mucosa is hypertrophic and hyperplastic but does not have evidence of necrosis, giant cells, granulomas, or invasion into surrounding structures [19].
AFRS is associated with a unique allergic fungal mucin that is thick and highly viscous. Microscopically, the mucin often takes on a chondroid appearance with sheets of eosinophils, frequently with the presence of eosinophilic breakdown products or Charcot-Leyden crystals that can be seen with H&E staining [20]. Fungi themselves do not stain with H&E staining. Silver containing stains (e.g. Grocott silver or Gomori methamine silver) are usually needed to appreciate the branching, non-invasive fungal hyphae. Montone et al. in their study observed 74.4% of AFRS patients having histological evidence of fungi [19].
Microbiological testing
Thick, eosinophilic mucus rich in inflammatory debris should alert the surgeon and pathologist to search for the presence of fungus, although the presence of fungus may not be the defining characteristic to confirm a diagnosis of AFRS. Some experts postulate the first stage of the disease is the allergic reaction which prompts eosinophilic mucus production. The second stage is the antigenic reaction to the fungi trapped by this allergic reaction.
Fungal cultures in AFRS should be interpreted with caution because of their variable yield (64%–100%) [21]. A diagnosis of AFRS is possible in the context of negative cultures. Furthermore, Ponikau et al. showed that 100% of normal and healthy volunteers were positive for fungi on culture, with an average of 2.3 different organisms per volunteer [16]. Therefore, fungal culture is unlikely to be helpful in the diagnosis of AFRS and a fungal smear should instead be used.
The role of staphylococcus aureus cultures from patients with AFRS should also be considered. Clark et al. cultured significantly higher levels of Staphylococcus aureus from AFRS patients versus non-AFRS patients (63.2 and 24.1%, respectively) [12]. Dutra et al. hypothesize that staphylococcus aureus may play a crucial role in AFRS on the basis of their findings of staphylococcus aureus co-existing with Aspergillus species within the sinuses of AFRS patients, and the presence of specific IgE antibodies to the classical staphylococcus aureus superantigens in the serum of nearly all subjects with AFRS [22]. This study suggested that staphylococcus aureus synergizes with or makes use of Aspergillus species preferentially amplifying inflammatory reactions, and adds its superantigenic activities to the disease, resulting in the high total IgE concentrations typically found in AFRS. In this way, Aspergillus species and staphylococcus aureus benefit from each other’s potential to overcome the mucosal barrier, bias the immune system, and cause the fulminant characteristics of AFRS [22].
Genetic and proteomics blood testing
In relation to genetic testing, Orlandi et al. performed a DNA microarray analysis on sinus mucosa samples in AFRS and nondiseased patients and found 38 genes or potential genes expressed in the AFRS patient samples that were not expressed in the non-diseased patient samples [23]. Schubert et al. performed HLA DNA genotyping on a group of AFRS patients and found that 66% of patients with AFS carried at least one HLA-DQB1 *03 allele [24].
Proteomics blood testing is a progressing field. Das et al. through surface-enhanced laser desorption/ionization time-of- flight mass spectrometry (SELDI-TOF-MS) proteomic profiling of serum samples was able to identify patients with AFRS out of a group of patients with chronic sinusitis, with 84% sensitivity and 90% specificity [25]. In 2016, Loftus PA, et al. compared epithelial cell samples from AFRS patients and non-in inflammatory control patients and demonstrated a 41% mean decrease in transepithelial resistance in AFRS cells, a finding that was also associated with decreased expression of 2 tight junction proteins and increased expression of a leaky tight junction protein [26].
Management of AFRS patients
The mainstay of surgical treatment is extensive endoscopic debridement and establishment of effective drainage pathway of all affected sinuses. This may well include radical sphenoethmoidectomies as well as frontal sinus ‘drill out’ procedures to remove all the tenacious thick secretions and fungal debris that often require large suckers and even microdebrider and forcep removal. This is followed by medical treatment in the form of a prolonged oral course of Corticosteroid treatment ideally extending for two to three months, on a gradually reducing dose regime. There is no consensus regarding corticosteroid dosage or duration. Topical corticosteroid treatment is usually continued indefinitely. Nasal douching is also helpful. During follow up of patients with AFRS, it may be useful to monitor blood levels of IgE (specific to the relevant fungal type) as a marker of recurrence. No anti-fungal treatments have been proven to change the course of the disease. A recent Cochrane database review by Head et al. concluded that due to the very low quality of the evidence, it is uncertain whether or not the use of Topical or systemic antifungal has an impact on patient outcomes in adults with chronic rhinosinusitis compared with placebo or no treatment [27]. Furthermore, this Cochrane review stated that studies including specific subgroups (i.e. AFRS) are lacking [27].
The use of immunological agents in the treatment of AFRS appears to be a promising area. Evans et al. reports a case of recalcitrant AFRS refractory to systemic corticosteroids and multiple functional endoscopic sinus surgeries treated with anti- IgE antibody omalizumab [28]. Omalizumab is a recombinant DNA-derived human monoclonal antibody that binds to serum IgE to form complexes that prevent IgE from binding to high affinity sites on mast cells and basophils. Gan et al. assessed the efficacy of omalizumab therapy in improving sinonasal outcomes in refractory AFRS patients with moderate or severe asthma [29]. It concluded that Omalizumab therapy can be considered as a potential adjunct for the treatment for patients with refractory AFRS with moderate or severe asthma [29].
Conclusion
Patients with AFRS represent a subgroup of chronic rhinosinusitis with nasal polyposis that are more difficult to manage, therefore identifying these individuals is important. The diagnosis needs to be considered in all patients with severe chronic rhinosinusitis with nasal polyposis. Patients often have asthma and complain of thick ‘wallpaper glue’ like nasal secretions that can also often be seen on nasal endoscopy. CT scanning often shows heterogeneity and MRI scanning may also help to identify cases. The diagnostic role of total and fungus-specific IgE in AFRS requires further evaluation. The role of genetic and immunological testing in AFRS requires developing as diagnostic tools. The diagnosis is therefore often a clinical one supported by investigations such as radiology (Table 1).
| Pre-operative | Intra-operative | Post-operative | ||
|---|---|---|---|---|
| Clinical suspicion of AFRS | Persistent or recurrent sinonasal disease including polyposis. | Functional endoscopic sinus surgery to clear all affected sinuses. Angled scope (e.g. 70 degree) to ensure all disease is cleared. Sinus specimens including secretions and debrided tissue sent for histo pathology, silver staining, immunological assessment, microscopy, culture and sensitivity. | Immunological testing | Fungal specific IgE stains in sinus mucin and mucosa. |
| Thick 'wallpaper' glue like secretions. | Immunofluorescence and PCR of sinus tissue to assess for increased levels of periostin. | |||
| History of atopy, asthma and/or Samter's triad. | Histopathological testing | H&E staining showing eosinophilic infiltrate without invasion and Charcot-Leyden crystals. | ||
| Immunocompetent Patient | Silver staining showing fungal hyphae. | |||
| Radiological investigations | CT-pansinus opacification and heterogeneity. | Microbiological testing | Fungal cultures should be interpreted with caution. | |
| MRI-Peripheral enhancement. T1 hypointensity, T2 signal void. | Fungal smear is more useful than cultures. | |||
| Staphylococcus aureus cultures may be positive. | ||||
| Immunological investigations | Increased total Ig E levels. | Clinical review | Consider 3 month course of post-operative oral corticosteroid treatment with tapering dose. | |
| Indefinite topical corticosteroid treatment. | ||||
| Prolonged follow up. | ||||
| No role for anti-fungal treatment. | ||||
| May be useful to monitor fungal specific blood IgE levels. | ||||
Table 1: Diagnostic and management protocol for AFRS.
References
- Ence BK, Gourley DS, Jorgensen NL, Shagets FW (1990) Allergic fungal sinusitis. Am J Rhinol 4: 169-178.
- Chaaban MR, Walsh EM, Woodworth BA (2013) Epidemiology and differential diagnosis of nasal polyps. Am J Rhinol Allergy 27: 473-478.
- DeShazo RD, Swain RE (1995) Diagnostic criteria for allergic fungal sinusitis. J Allergy Clin Immunol 96: 24–35.
- Ponikau JU, Sherris DA, Kern EB, Homburger HA, Frigas E et al. (1999) The diagnosis and incidence of allergic fungal sinusitis. Mayo Clin Proc 74: 877-884.
- Safirstein, BH (1976) Allergic Bronchopulmonary aspergillosis with obstruction of the upper respiratory tract. Chest 70: 788-790.
- Bent JP, Kuhn FA (1994) Diagnosis of allergic fungal sinusitis. Otolaryngol Head Neck Surg 111: 580-588.
- Chakrabarti A, Denning DW, Ferguson BJ, Ponikau J, Buzina W et al. (2009) Fungal rhinosinusitis: a categorization and definitional schema addressing current controversies. Laryngoscope 119: 1809-1810.
- Fokkens WJ, Lund VJ, Mullol J, Bachert C, Alobid I et al. (2012) European position paper on rhinosinusitis and nasal polyps 2012.: EPOS 2012: A summary for otorhinolaryngologists. Rhinology 50: 1-12.
- Manning SC, Holman M (1998) Further evidence for allergic patho-physiology in allergic fungal sinusitis. Laryngoscope 108: 1485-1496.
- Zinreich SJ, Kennedy DW, Malat J, Curtin HD, Epstein JI et al. (1988) Fungal sinusitis: diagnosis with CT and MR imaging. Radiology 169: 439-444.
- Pant H, Kette FE, Smith WB, Wormald PJ, Macardle PJ (2005) Fungal- specific humoral response in eosinophilic mucus chronic rhinosinusitis. Laryngoscope 115: 601-606.
- Collins M, Nair S, Smith W, Kette F, Gillis D, et al. (2004) Role of local immunoglobulin E production in the pathophysiology of noninvasive fungal sinusitis. Laryngoscope 114: 1242-1246.
- Wise SK, Ahn CN, Lathers DM, Mulligan RM (2008) Schlosser RJ: Antigen- specific IgE in sinus mucosa of allergic fungal rhinosinusitis patients. Am J Rhinol 22: 451-456.
- Chang YT, Fang SY (2008) Tissue-specific immunoglobulin E in maxillary sinus mucosa of allergic fungal sinusitis. Rhinology 46: 226-230.
- Clark DW, Wenaas A, Luong A, Citardi MJ, Fakhri S (2013) Staphylococcus aureus prevalence in allergic fungal rhinosinusitis vs other subsets of chronic rhinosinusitis with nasal polyps. Int Forum Allergy Rhinol 3: 89-93.
- Ebert CS Jr, McKinney KA, Urrutia G, Wu M, Rose AS et al. (2014) Expression of protease-activated receptors in allergic fungal rhinosinusitis. Int Forum Allergy Rhinol 4: 266-271.
- Ayers CM, Schlosser RJ, O’Connell BP, Atkinson C, Mulligan RM et al. (2011) Increased presence of dendritic cells and dendritic cell chemokines in the sinus mucosa of chronic rhinosinusitis with nasal polyps and allergic fungal rhinosinusitis. Int Forum Allergy Rhinol 1: 296-302.
- Laury AM, Hilgarth R, Nusrat A, Wise SK (2014) Periostin and receptor activator of nuclear factor κ-B ligand expression in allergic fungal rhinosinusitis. Int Forum Allergy Rhinol 4: 716-724.
- Montone KT, Livolsi VA, Feldman MD, Palmer J, Chiu AG et al. (2012) Fungal rhinosinusitis: A retrospective microbiologic and pathologic review of 400 patients at a single university medical center. Int J Otolaryngol 2012: 9.
- Marple BF (2001) Allergic fungal rhinosinusitis: current theories and management strategies. Laryngoscope 111: 1006-1019.
- Glass D, Amendee RG, Ochsner J (2011) Allergic Fungal Rhinosinusitis: A Review 11: 271-275.
- Dutre T, Dousary S, Zhang N, Bachert C (2013) Allergic fungal rhinosinusitis- more than a fungal disease? J Allergy Clin Immunol 487–489.
- Orlandi RR, Thibeault SL, Ferguson BJ (2007) Microarray analysis of allergic fungal sinusitis and eosinophilic mucin rhinosinusitis. Otolaryngol Head Neck Surg 136: 707-713.
- Schubert MS, Hutcheson PS, Gra RJ, Santiago L, Slavin RG (2004) HLA-DQB1 *03 in allergic fungal sinusitis and other chronic hypertrophic rhinosinusitis disorders. J Allergy Clin Immunol 114:1376-1383.
- Das S, Maeso PA, Becker AM, Prosser JD, Adam BL et al. (2008) Proteomics blood testing to distinguish chronic rhinosinusitis subtypes. Laryngoscope 118: 2231-2234.
- Loftus PA, Wise SK (2016) Allergic Fungal Rhinosinusitis: The latest in diagnosis and management. Adv Otorhinolaryngology 79: 13-20.
- Head K, Sharp S, Chong LY, Hopkins C, Philpott C (2018) Topical and systemic antifungal therapy for chronic rhinosinusitis. Cochrane Database Syst Rev 10: 9.
- Evans MO, Coop CA (2014) Novel treatment of allergic fungal sinusitis using omalizumab. Allergy Rhinol (Providence) 5: 172-4.
- Gan EC, Habib AR, Rajwani A, Javer AR (2015) Omalizumab therapy for refractory allergic fungal rhinosinusitis patients with moderate or severe asthma. Am J Otolaryngology 3: 672-677.
 Spanish
Spanish  Chinese
Chinese  Russian
Russian  German
German  French
French  Japanese
Japanese  Portuguese
Portuguese  Hindi
Hindi