Research Article, J Pharm Drug Deliv Res Vol: 7 Issue: 1
Application of Acoustic- Vibratory Milling For Screening and Scale-Up of Micro/ Nanosuspensions in Early Drug Discovery and Development
Hoang D Lu*, J Alejandro D’Aquino, Joseph Caruso, Avijit Ghosh and Sachin Lohani
Janssen Research and Development, LLC
*Corresponding Author : Hoang D Lu
Janssen Research and Development, LLC, 1400 McKean Rd, Spring House, PA 19477, USA
E-mail: hlu39@its.jnj.com
Received: March 03, 2018 Accepted: March 29, 2018 Published: April 05, 2018
Citation: Lu HD, D’Aquino JA, Caruso J, Ghosh A, Lohani S (2018) Application of Acoustic-Vibratory Milling For Screening and Scale-Up of Micro/ Nanosuspensions in Early Drug Discovery and Development. J Pharm Drug Deliv Res 7:1. doi: 10.4172/2325-9604.1000175
Abstract
Current methods of milling an active pharmaceutical ingredient (API) often suffer from limited control over particle size and solid-state form or require large amounts of material for optimization, rendering them unsuitable for use in early discovery efforts. We here develop operating workflows of an acoustic-vibratory milling technique, which enables both materials-sparing screens and scaled-up production of micro/nanosuspensions with well-defined and tunable particle size distributions. Workflows are based on the generation of guidance maps for API insensitive outputs (temperature) and API dependent outputs (particle size), with various operational parameters for milling such as container headspace, container geometry, milling media load & size, milling time, and excipient compositions. The
experimental conditions for low and high intensity milling were identified and implemented for six model compounds with a wide range of physicochemical properties, to conduct materials-sparing screens (10 mg) at high throughput (>36 conditions per run) and short milling times (2 hours) to successfully produce suspensions for all compounds tested. The scale-up capability of the approach is demonstrated for model API, mebendazole, by producing more than 20 g of suspensions per run with predefined size and solidstate form that corresponds to small-scale screens by two orders of magnitude. This work develops new tools that help enable micro/ nano-suspension development in early-drug discovery settings.
Keywords: Active pharmaceutical ingredient; Early-drug discovery settings
Introduction
The develop ability assessment of Active Pharmaceutical Ingredients (APIs) during discovery stage serves a critical function of ensuring that the properties of the selected drug-candidate meet the desired profile. The efficacy, toxicity, and biopharmaceutical assessment of an early drug candidate is often challenging, because of its poor physicochemical properties and limited availability of chemical matter.
Although conventional suspension formulation approaches are preferred for in vivo evaluation, unoptimized suspensions often yield inadequate systemic exposure for drug candidates with poor physicochemical properties. Manipulating the particle size of a drugsubstance is a well-established approach for tuning its dissolutionrate, bio-availability, uptake/bio-distribution, flow, processability, and surface area to volume ratio [1-3]. Furthermore, as a drug candidate transitions from early discovery to development it becomes critical to know whether size-manipulation steps may be required for reducing the particle size of the drug substance batch intended for First-In-Human (FIH) and/or FIH enabling studies, because of significant associated costs in terms of time and material loss. Therefore, it is important to understand the role of API particle size on bioperformance in early discovery.
APIs can be processed into specific sizes through top-down and bottom-up approaches, whereupon bulk API is milled or discrete API is controllably-assembled respectively. Of these, suspensions formed by milling has been amongst the most common methodology for pharmaceutical development.[4] Conventional suspensions obtained from commonly used approaches for particle size reduction such as, probe sonication, high shear homogenization, dump-and-stir, can show significant batch to batch variability that can confound interpretation of in vivo results [5]. Although various recent publications have described small-scale screening of optimized nanosuspensions [6,7], the use of these approaches in early drug discovery is still limited, primarily because of challenges with translating operating conditions used in small-scale screening experiments to larger-scale production. To enable rapid evaluation of drug candidates in the discovery space, it is necessary to not only rapidly screen a variety of conditions using minimal API, but also to produce larger batches for pharmacokinetic, pharmacodynamic and toxicological evaluation within tight timescales.
On the small scale-spectrum of milling APIs, acoustic mixing has recently been identified as a methodology capable of mixing APIs in material-sparing screens [6-8]. In this process, acoustic energy is transferred as mechanical energy to vessels containing API [9,10]. The addition of hard media such as zirconium beads in vessels result in the mechanical breakdown of APIs into smaller particles. This approach has been previously used for excipient screening to narrow down formulation compositions by milling in 96-well plates, whereupon scaled-up production was subsequently performed using traditional ball-mills [6]. However, to mill APIs to the comparable size and solid-state form identified with small scale-screens, new milling conditions must be empirically determined through a whole new set of experiments. In contrast, the potential use of acousticvibratory milling for both screening and larger scale production can expedite timelines in drug discovery. This approach is traditionally not performed since the operating conditions of acoustic-vibratory milling are currently not well understood. The existing knowledge gap limits larger scale production of micro/nano suspensions based on results of small scale screening using this milling technology.
To address this gap, we studied the operating conditions of acoustic-vibratory milling for particle size reduction in both small scale and large scale experiments. Herein, we describe an experimental methodology to screen for optimized suspensions on a 10 mg scale and to directly scale-up by 2 orders of magnitude to > 20 g batches, and to produce suspensions with desired particle size and solid-state form. We discuss the application of this methodology to six model compounds with a wide range of physicochemical properties. We also determine relationships between operational parameters (container headspace, container geometry, milling media load & size, milling time) and milling outputs (particle size and milling temperature) to generate “operational parameter guidance maps” that assist in milling parameter selection and directing workflows for suspension development. The proposed methodology addresses the limitations with the existing approaches for particle size reduction and enables a streamlined application of micro/nano-suspension formulations to early drug discovery.
Materials and Methods
Materials
Mebendazole (MBZ), cyclosporine A, itraconazole, phthalocyanine, beta-carotene, and clofazimine, were obtained from TCI America, and tween 80 was obtained from Sigma-Aldrich. High density polypropylene containers with 2 mL, 8 mL, and 16 mL (marketed as 2 mL, 7 mL, and 15 mL containers respectively) were obtained from Bertin Instruments. Zirconium beads 5 mm, 2.8 mm, 1.4 mm, and 0.1 mm in size was obtained from Bertin Instruments, and 0.5 mm zirconium beads were obtained from OPS diagnostics.
Acoustic-vibratory milling: Zirconium bead milling media (density 5.5 g/ml) was weighed and transferred to high-density polypropylene tubes at the desired bead loading (weight of beads in grams per container volume in mL expressed as a percentage; w/v%). API and excipient were subsequently added to tubes, to which varying amounts of water was included to produce tubes with defined headspace volume. Headspace fill volume is defined as one minus the fraction of air in milling vessel (1 headspace fill volume is completely filled with liquid and no air). For milling experiments not containing API, 0.5 % w/v tween 80 was used as the filling liquid. For API milling, API to tween 80 was kept at a constant 4:1 mass ratio. For small scale experiments 1.4 mm beads at 25% w/v load in 2 mL tubes containing 10 mg API was used. For scale-up experiments, 1.4 mm beads at 25% w/v load in 8 mL tubes, with 1440 mg API was used. Bead loadings, bead size, and excipient concentrations were adjusted accordingly in guidance-map generation experiments and described below.
Samples were milled with the resonant acoustic mixing setup (LabRam II, Resodyn Acoustic Mixers). In brief, tubes containing milling media, API, excipients, and liquid were secured and clamped in between the LabRam II mixing chamber, the mixed at various acceleration parameters and time. High density polypropylene containers were used, as other plastic and glass containers used deformed or shattered in high milling accelerations.
Sample characterization: The physical form of milled and neat samples was determined with X-ray diffraction (X’Pert Pro, PANalytical). Suspension size was assessed with laser diffraction (Mastersizer 2,000 + Hydro 2000S, Malvern Instruments). The morphology and size of suspensions and neat material was characterized with optical microscopy at 40x magnification (Eclipse LV-1000U, Nikon).
Results and Discussion
Exploration of operational parameter design space
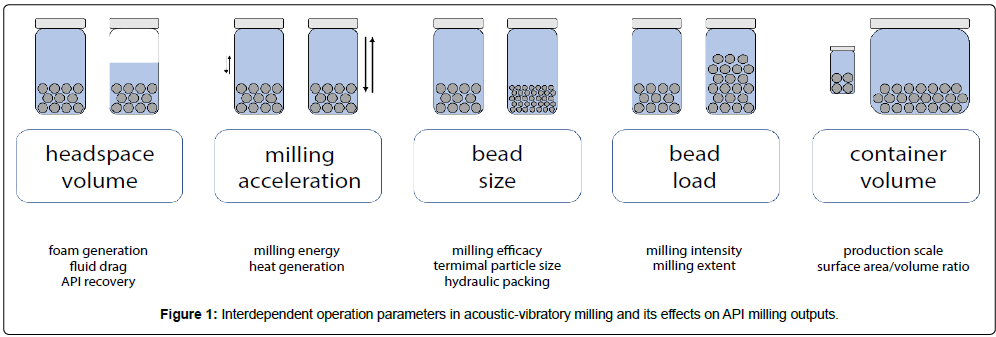
There are a variety of interdependent acoustic-vibratory milling operational parameters that can be simultaneously tuned to affect milling efficacy, such as vessel headspace volume, container geometry, milling acceleration, milling media size, milling media load, milling time, and temperature (Figure 1). To provide insight on how these parameters can be optimized, and what operating space is suitable for formulating micro/nanosuspensions with defined properties, the relationships between these parameters were thoroughly investigated (Figure 1).
In brief, complete headspace volume fill was identified as a suitable operational parameter (Supplementary Information: Milling Headspace Volume, Figure S1-2). While complete headspace volume fill increases drag, vessel temperature, and the milling time required for size reduction (~10x fold increase in time, when compared to a vessel containing only 12.5% headspace volume fill), partial headspace volume fill results in air entrapment and foam generation. Foam results in excessive API loss in the liquid phase (~90% vessel volume as foam when using 12.5% headspace volume fill) and should be critically avoided, since API material quantity is often a major limiting factor in drug discovery. Important to note, this complete headspace milling setup contrasts with that of traditional ball mill setups that are commonly optimized to have gas-filled headspace and as such suffer from foam generation and subsequent API loss [11].
Milling accelerations between 25-100g were identified as suitable operating parameters (Supplementary Information: Milling Acceleration, Figure S3). At low accelerations, however, milling media was more prone to hydraulic packing effects, resulting in incomplete fluidization and inhomogeneous milling. In contrast, milling media could be completely fluidized at high acceleration (100 g) and can generally result in more homogenous milling runs.
In the complete headspace volume fill and 100 g acceleration setup, the ability to operate with milling media of various sizes was assessed. Milling with zirconium beads of size 5 mm, 2.8 mm, 1.4 mm, 0.5 mm, and 0.1 mm normalized to a constant total bead weight per vessel and at otherwise identical operational conditions resulted in vessel temperatures between 30ºC to 60°C (Supplementary Information: Bead size, Figure S4). Smaller 0.1 mm beads and 5 mm beads generally resulted in lower temperatures due to the lower energy of 0.1 mm beads per bead, and lower number of 5 mm beads at the same bead weight respectively, while 1.4 mm beads resulted in the highest temperature output. These results highlight how bead size can have significant impact on vessel operating conditions and is an important consideration, especially when working with thermolabile APIs. Milling accelerations can be varied to modulate operational temperatures (Figure S4) with all bead sizes tested, such that milling can be performed <30°C for all sizes tested. This enables milling with small beads to produce nanosuspensions, larger beads to produce microsuspensions, or intermediately sized beads for the capability to efficiently create either nano/microsuspensions for even thermolabile APIs.
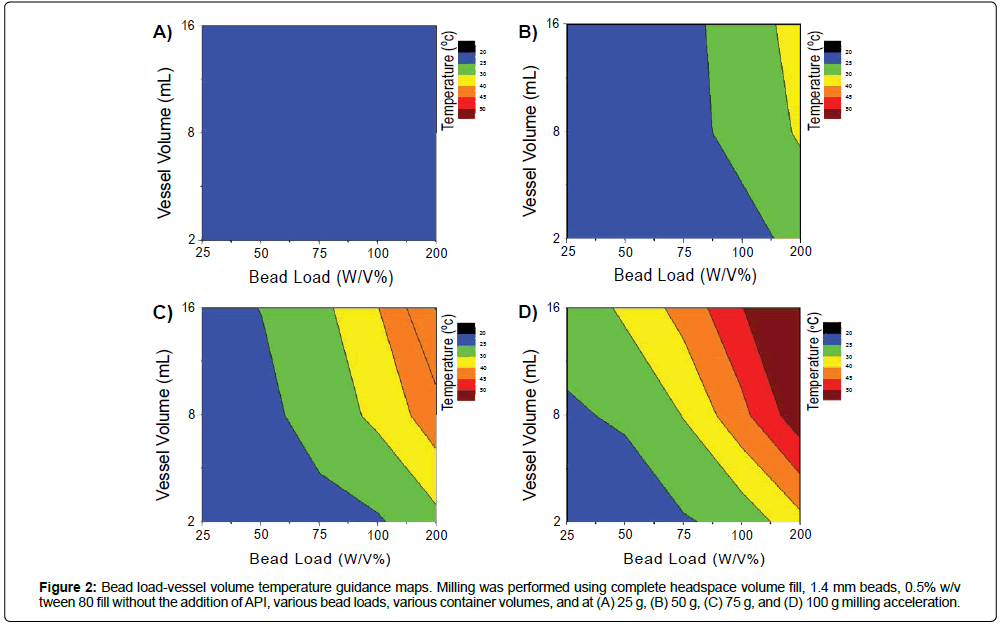
Milling at different bead loads and vessel sizes was additionally assessed, since these parameters directly affect milling intensity, batch size, and output temperature. (Supplementary Information: Bead Load and Vessel Size, Figures S5-S6). Increasing the bead load results in increased bead collision and heat generation rates, while increased tube volume decreases surface area to volume ratios and heat dissipation rates. The cross-relation between these parameters and temperature were used to create “operational guidance maps” (Figure 2), for bead loading-vessel volume selection in milling situations. For example, to constrain milling temperatures below 40˚C at 100 g acceleration, milling in 2 mL vessels must be below 100% w/v bead load, or milling in 8 mL vessels must be below 75% w/v bead load. The temperature guidance maps provide contours for milling designs, and offer parameter constraints on how to interdependently tune scalability and milling intensity when milling APIs that possess different temperature sensitivities. Important to note, the temperature output remains the same when milled with or without API (Figure S6); these temperature maps are insensitive to the API assessed, and can be applied to for general milling conditions for a variety of programs and applications. Thus, increasing vessel number is an attractive alternative to scaling production amount that preserves vessel surface area to volume ratios, in contrast to scaling vessel size as in most traditional milling applications. The acoustic-vibratory milling setup is well suited for this methodology of scaling, and can accommodate up to >36 8 mL vessels as a given time. In conjunction with increases in API concentration, this strategy can be used to scale production levels to support development work.
Figure 2: Bead load-vessel volume temperature guidance maps. Milling was performed using complete headspace volume fill, 1.4 mm beads, 0.5% w/v tween 80 fill without the addition of API, various bead loads, various container volumes, and at (A) 25 g, (B) 50 g, (C) 75 g, and (D) 100 g milling acceleration.
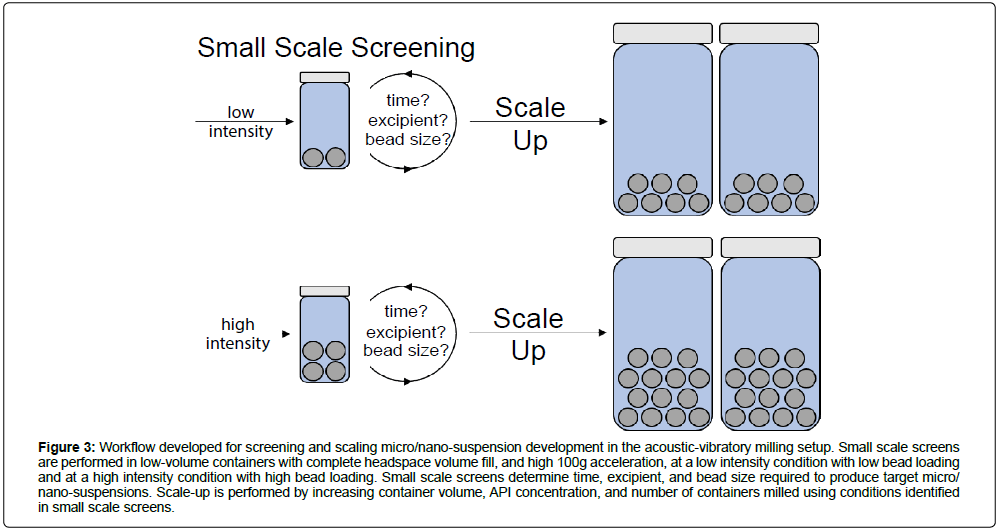
Together, the headspace volume, milling acceleration, bead size, bead load, and vessel volume-temperature guidance maps narrow down on specific operational parameter design space that should be used for screening and scale-up production in acoustic-vibratory milling (Figure 2, Figures S1-S6). Vessel headspace volume should be filled, to minimize foam generation and API loss. Operation temperatures are highly sensitive to milling acceleration. In this work, accelerations between 25g and 100g were tested. For standardization, 100g is selected as a suitable acceleration for high-energy milling and for rapid particle size reduction. Milling media (bead size) had small effects on operation temperature, and 0.1 mm to 1.4 mm to 5 mm can be chosen and used for the intended milling application (small beads for nanosuspension and large beads for microsuspension production). Bead loading greatly affects operation temperatures, and can be varied to tune milling intensity (25% w/v for low intensity milling, and 100% w/v for high intensity milling applications). Vessel size should also be constrained to prevent excessive heat buildup, as in this at or below 8 mL to constrain excessive heat buildup. Scaling up the batch size by increasing vessel numbers milled per run is an attractive method to scale production volumes. Thus, based on these considerations the suggested milling workflow (Figure 3) for small scale screen is:
Figure 3: Workflow developed for screening and scaling micro/nano-suspension development in the acoustic-vibratory milling setup. Small scale screens are performed in low-volume containers with complete headspace volume fill, and high 100g acceleration, at a low intensity condition with low bead loading and at a high intensity condition with high bead loading. Small scale screens determine time, excipient, and bead size required to produce target micro/ nano-suspensions. Scale-up is performed by increasing container volume, API concentration, and number of containers milled using conditions identified in small scale screens.
100% headspace volumes fill
100g acceleration
Beads between 0.1 to 5 mm in size
25% w/v bead load for low intensity milling
100% w/v bead load for high intensity milling
2 mL at 5 mg/mL - low concentration and low volume
Milling time with two hour increments
After assessing resultant suspension properties in the small screen, parameters may be additionally changed for subsequent tests to hone down on formulation target properties until the desired formulation properties are achieved. Afterward, scaled up production can be performed (Figure 3) with the following workflow:
100% headspace volume fill
100g acceleration
Bead size dependent on small scale screen
Bead load dependent on small scale screen
2-8 mL at 5-180 mg/mL for higher volumes and higher concentrations
1-36x tubes per run
Milling time based on small scale screen
Small scale screening
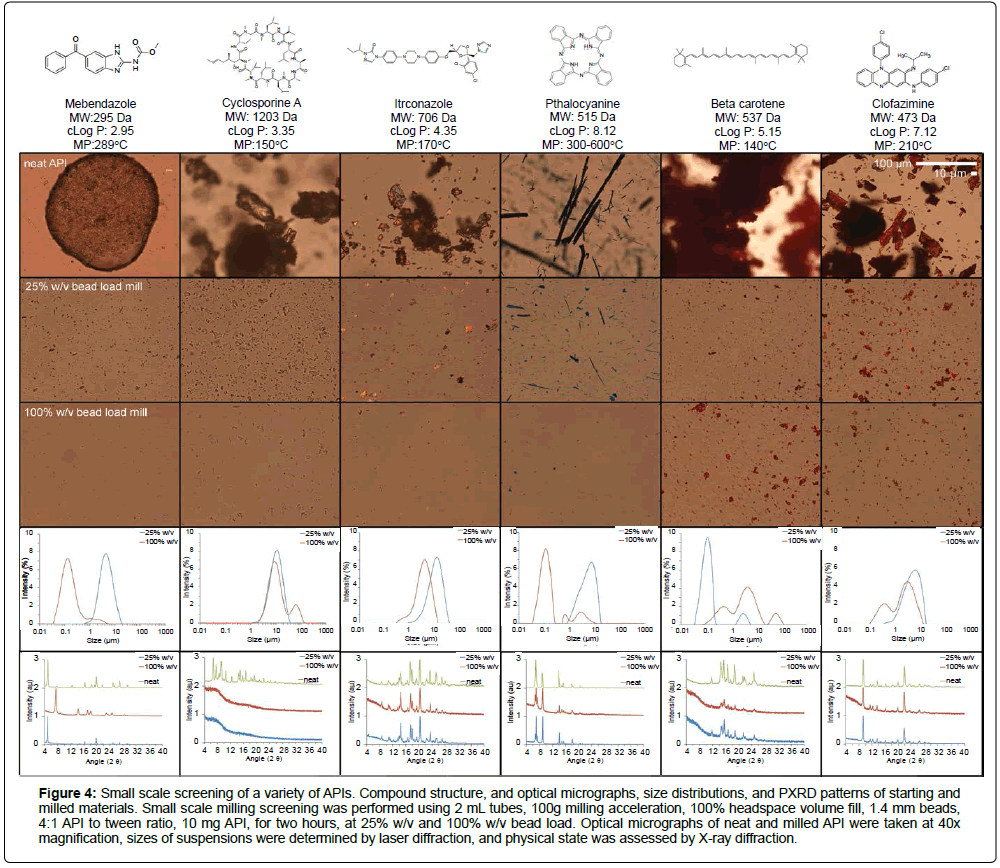
After exploring operational parameter design spaces, we sought to determine if the preselected ‘mild’ and ‘intense’ milling condition workflows can be used to appropriately screen micro/ nano suspensions production (Figure 4). For this purpose, six model compounds with a variety of physicochemical properties, including those with predominately aromatic, predominately aliphatic, and polypeptide structures with MW ranging between 295 to 1203 Da, cLogP between 2.95 to 15.5, and melting point between 150°C to >300°C were chosen, to explore the range of attributes that APIs may possess in pharmaceutical development. When milled in a ‘small scale’ (10 mg, 2 mL) condition, micronization of all APIs could be observed (Figure 4). Neat API (starting material) that consist of agglomerated or large crystals > ~100 μm in size could be reduced to micro/nanosuspensions with median sizes near or below 10 μm, depending on the milling conditions used (Table 1). The use of intense milling conditions (100% w/v bead load) generally resulted in further particle size reduction compared to mild milling conditions (25% w/v bead load). However, this scenario is not universal, as in one of the six APIs tested, intense milling of beta-carotene led to larger particle sizes than in the mild milling condition. These results are consistent with previous observations that certain compounds can aggregate into larger particle diameters if overmilled [12]. The results also underscore the importance of including both a mild and intense milling condition during screens to gain insights into whether API was excessively milled and highlight the API dependent nature of the particle-size obtained from a given milling condition.
Figure 4: Small scale screening of a variety of APIs. Compound structure, and optical micrographs, size distributions, and PXRD patterns of starting and milled materials. Small scale milling screening was performed using 2 mL tubes, 100g milling acceleration, 100% headspace volume fill, 1.4 mm beads, 4:1 API to tween ratio, 10 mg API, for two hours, at 25% w/v and 100% w/v bead load. Optical micrographs of neat and milled API were taken at 40x magnification, sizes of suspensions were determined by laser diffraction, and physical state was assessed by X-ray diffraction.
| Suspension Passing Size (µm)-25% w/v beadload | ||||||
|---|---|---|---|---|---|---|
| Mebendazole | Cyclosporine A | Itraconazole | Phthalocyanine | Beta carotene | Clofazimine | |
| d(0.1) | 2.23 | 5.07 | 4.22 | 2.06 | 0.07 | 2.48 |
| d(0.5) | 4.67 | 11.03 | 11.01 | 5.83 | 0.12 | 7.28 |
| d(0.9) | 9.78 | 21.52 | 22.36 | 12.10 | 0.23 | 16.41 |
| Suspension Passing Size (µm)-100% w/v beadload | ||||||
| Mebendazole | Cyclosporine A | Itraconazole | Phthalocyanine | Betacarotene | Clofazimine | |
| d(0.1) | 0.07 | 4.96 | 1.34 | 0.07 | 0.38 | 0.42 |
| d(0.5) | 0.15 | 10.93 | 3.70 | 0.13 | 3.68 | 3.26 |
| d(0.9) | 0.48 | 59.70 | 7.83 | 224 | 17.90 | 9.59 |
Table 1: Suspension sizes of a variety of APIs in small scale screening. Small scale milling screening was performed using 2 mL tubes, 100g milling acceleration, 100% headspace volume fill, 1.4 mm beads, 4:1 API to tween ratio, 10 mg API, for two hours, at 25% w/v and 100% w/v bead load.
For four of the six compounds screened, the Powder X-ray Diffraction Pattern (PXRD) patterns of neat API and milled samples remained consistent with that of the starting material, and attests that the initial crystalline forms were preserved after milling (Figure 4). However, milled cyclosporine A does not display the same characteristic PXRD patterns of the starting material, and instead exhibits a broad peak observed with amorphous materials, suggesting that cyclosporine A was completely amorphized after milling. In this work, we have used Tween 80 as an illustrative example of a nanosuspension stabilizing excipient. In practice, the screening can be performed using a variety of other stabilizing excipients and can be implemented within our proposed methodology, for example, in case of cyclosporine A, the stabilizing excipient poloxamer 407 can be added to the screened compositions, as it has been reported elsewhere to result in a stable nanosuspension [13].
Also, while the PXRD patterns of MBZ milled under mild conditions matched that of the starting material, the PXRD patterns of MBZ milled under intense conditions changed to that of a new polymorph. These results highlight that APIs can change their solidstate form when milled under the acoustic-vibratory setup, and that the extent of the change is dependent on the milling operational parameters and the nature of the API. Our results are consistent with previous reports that milling can induce form conversion and amorphization of the starting APIs, and highlights the necessity to monitor the physical state of materials during screening [14,15]. The phase change of MBZ under intense milling conditions is consistent with conversion of the initial metastable phase, Form C, into a thermodynamically stable phase, Form A, that has been documented previously [16,17]. This form conversion is likely accelerated by the increased temperature and impact events from elevated bead load. These results again highlight the value of including a mild and intense loading condition during screening step to assess how excessive milling can drive form conversion into a potentially more stable (but also less soluble form) in drug discovery. The early identification of the most stable polymorphs can provide crucial insight on drug developability and candidate selection. Altogether, these small-scale screening experiments demonstrate that the mild/intense operational parameter design space can be utilized to generically produce micro/ nano suspensions for all six of the model compounds used in this work. Furthermore, the ease and extent of API size reduction as well as the nature of the solid-sate form obtained under a given milling condition was dependent on the nature of the starting material and the API.
Conditions to form microsuspensions for all six model APIs were identified, while conditions to form nanosuspensions were identified for three of the six APIs in the first test. For the goal of producing nanosuspensions, this generic first-pass screen still guides how operational parameters should be tuned for the intended purpose in the three cases in which nanosuspensions were not formed - milling for longer times at high bead loading for both itraconazole and clofazimine, and varying excipient composition for cyclosporine (Figure 4). The inclusion of both a mild and intense milling condition described above provides additionally provides insight on the risk of aggregation and/or physical form conversion during milling (Table 1).
Scale up production
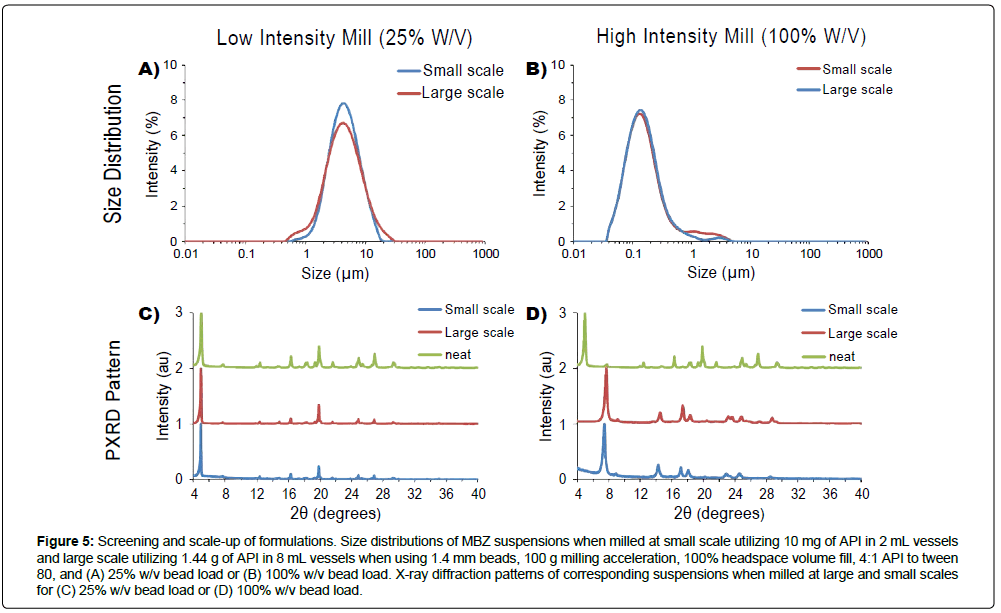
While the small-scale screening of compounds enables the selection of excipient compositions and milling conditions, scaled-up production is necessary to support toxicology and tolerability studies in pharmaceutical development where significantly higher doses in larger animals are required. To assess if the micro/nanosuspensions developed in small-scale screens (10 mg) can be also produced in larger scales (20 g) with the identical operating conditions, API was milled at higher concentrations and limited to 8 mL volume containers, as larger 16 mL containers resulted in excessive heat buildup (Figure 2). Subsequent work was performed on MBZ, as MBZ exhibited the largest size and physical form sensitivities to milling conditions, compared with the other APIs assessed (Figure 4). MBZ was milled at 25% w/v and 100% bead load in 8 mL containers, and at 180 mg/mL API, resulting in the production of 1.44g of suspension per tube. Large-scale milling of MBZ at 25% w/v bead load resulted in the formation of microparticles with d(0.1), d(0.5), and d(0.9) passing sizes of 1.9 μm, 4.6 μm, and 11 μm respectively (Figure 5a). These sizes are similar to that formed in 25% w/v bead load in small scale screens, which formed microparticles with d(0.1), d(0.5), and d(0.9) passing sizes of 2.3 μm, 4.7 μm, and 9.8 μm respectively. Large-scale milling of MBZ at 100% w/v resulted in the formation of nanoparticles with a mean diameter of with d(0.1), d(0.5), and d(0.9) passing sizes of 72 nm, 150 nm, and 380 nm respectively, which is similar to that in the small scale screen which formed nanoparticles corresponding passing sizes of 72 nm, 150 nm, and 480 nm respectively at the same 100% w/v bead load (Figure 5b). Repeat runs of the experiments indicate high reproducibility and <10% variability among runs (Figure S7).
Figure 5: Screening and scale-up of formulations. Size distributions of MBZ suspensions when milled at small scale utilizing 10 mg of API in 2 mL vessels and large scale utilizing 1.44 g of API in 8 mL vessels when using 1.4 mm beads, 100 g milling acceleration, 100% headspace volume fill, 4:1 API to tween 80, and (A) 25% w/v bead load or (B) 100% w/v bead load. X-ray diffraction patterns of corresponding suspensions when milled at large and small scales for (C) 25% w/v bead load or (D) 100% w/v bead load.
In addition, the PXRD patterns of suspensions in the large and small scale formulations are similar among those in the 25% w/v case, and those in the 100% w/v case respectively (Figure 5c-5d). These results demonstrate that, at least under the conditions tested and for MBZ, suspensions identified in small-scale screens can be scaled-up for production using identical operating parameters. In the LabRam setup, >36 tubes can be simultaneously milled at once, allowing to scale up volume of production by increasing the number of tubes milled to produce ~50g of suspension per run and a scale-up factor of ~3 orders magnitude. Scaling by increasing vessel number, in contrast to only scaling by increasing vessel size, helps limit heat buildup by preserving vessel surface area to volume ratios. These scales of production can cover amounts of material needed in typical early-discovery settings such as tolerability assessments (20 g), and attest that the acoustic-vibratory milling setup alone can in certain scenarios be used to support the milling requirements in earlydiscovery projects.
Guidance maps for size and form selection
In the proposed acoustic-vibratory milling workflows, “mild” and “high” milling intensities for defined periods of time were used for screening and scaling experiments to explore the feasibility of creating micro/nano-suspensions. If micro/nano-suspensions are indeed considered feasible after initial screening tests, it is often desirable to mill samples to a target size distribution and polymorphic form for the intended therapeutic purpose. Towards understanding how to finely control suspension sizes between 100 nm to 10 μm, we additionally characterized the milling design space of MBZ, and assessed the relationship between bead load, milling time, bead size, and additional of excipients on MBZ particle size and solid-state form.
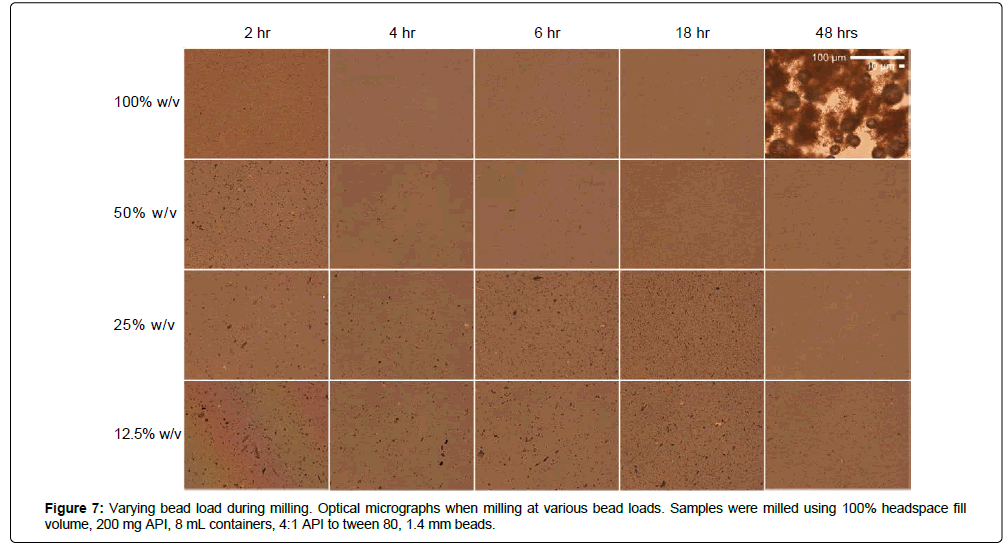
To determine the effect of bead load on milling efficacy in the acoustic-vibratory milling setup, MBZ was milled at using 1.4 mm beads and at various bead loads over time, and resultant suspensions were characterized by laser diffraction, X-ray diffraction, and optical microscopy (Figures 6-8). Increased bead load resulted in more rapid and efficient milling; milling with 100% w/v load for two hours yielded suspensions with a median diameter of 152 nm, while milling with a 25% w/v load for two hours yielded suspensions with a median diameter of 4.6 μm (Figure 6). By increasing bead load, the frequency of collision increase due to the increased concentrations of beads. In addition, milling at higher bead loads results in higher operational temperatures, which can in some scenarios lead to more efficient particle size reduction. However, operation at higher temperatures and excessively harsh milling conditions can also result in particle aggregation, which was observed with milling at 100% w/v load for 48 hours resulting in particles with d(0.5) of 22 μm, while no aggregation was observed for the other milling conditions. Optical microscopy also reveals the general trend that there is more efficient milling of MBZ when using higher bead load, and overmilling at high temperature/high bead load conditions resulted in particle agglomeration (Figure 7). In addition, higher bead load resulted in more rapid conversion of MBZ Form C into Form A. For example, MBZ converted all into form A within 2 hours of milling at 100% w/v bead load, while MBZ retained in form C for the other bead loads tested after 2 hours of milling. Interestingly, the form A conversion coincides with the particle size reduction to a nanodistribution, and highlights the relationship between nanosuspensions and instability that is oftentimes observed. Overall, these results highlight the large dependence of size reduction rates and physical form on bead loading during milling.
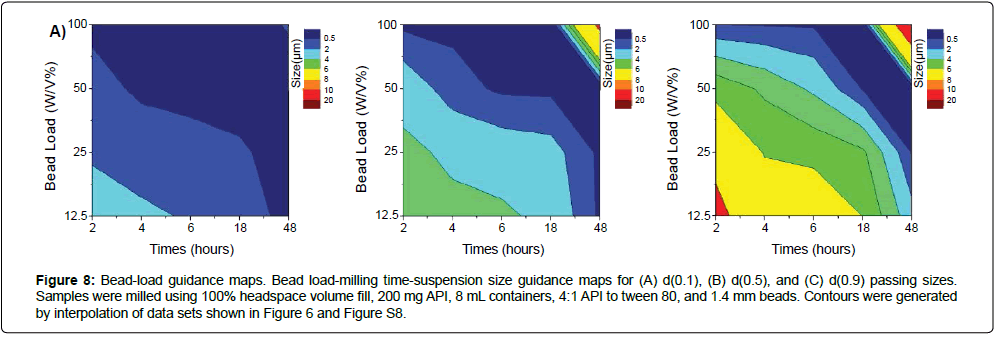
Figure 8: Bead-load guidance maps. Bead load-milling time-suspension size guidance maps for (A) d(0.1), (B) d(0.5), and (C) d(0.9) passing sizes. Samples were milled using 100% headspace volume fill, 200 mg API, 8 mL containers, 4:1 API to tween 80, and 1.4 mm beads. Contours were generated by interpolation of data sets shown in Figure 6 and Figure S8.
Importantly, by systematically determining the relationships between bead load and milling time on particle size at each time point (Figure S8), these trends can be cohesively utilized to generate operational guidance maps (Figure 8) for operational parameter selection. The use of these relationships can be informative for a priori guidance of milling conditions for APIs in pharmaceutical development. For example, to target nanosuspensions with d(0.5) less than 1 μm, milling can be performed at either 100% w/v bead load for two hours, or 12.5% w/v bead load for 48 hours (Figure 8b). Cross-reference of temperature guidance maps (Figure 2), indicates that these milling conditions would be at 57°C or 29°C respectively. Together, these maps guide that suspensions with the same properties can be formed by high intensity milling at high temperatures for two hours, or low-intensity milling at low temperatures for 48 hours. While the precise boundaries and size contours of each size guidance map would be dependent on the nature of the API and starting material, these maps provide important generic insight on the tradeoffs between different milling parameters (such as between milling intensity-time-temperature) to help select milling parameters to for API micronization.
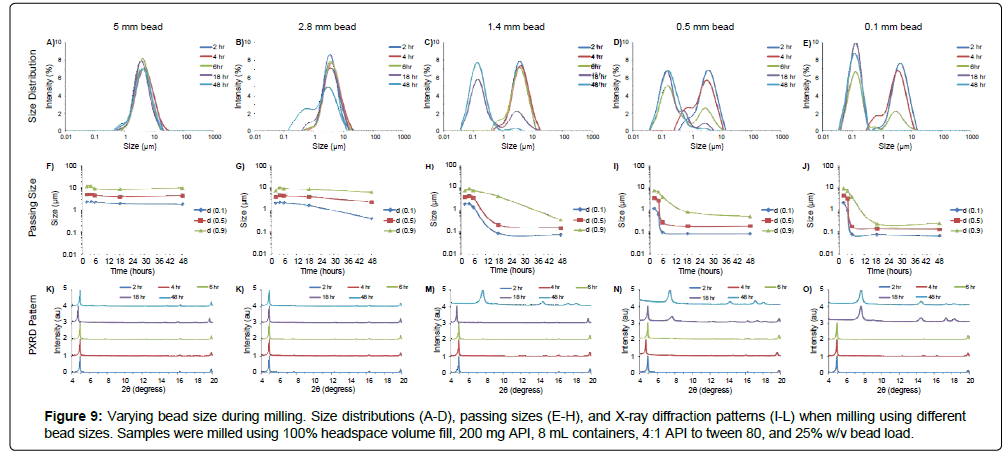
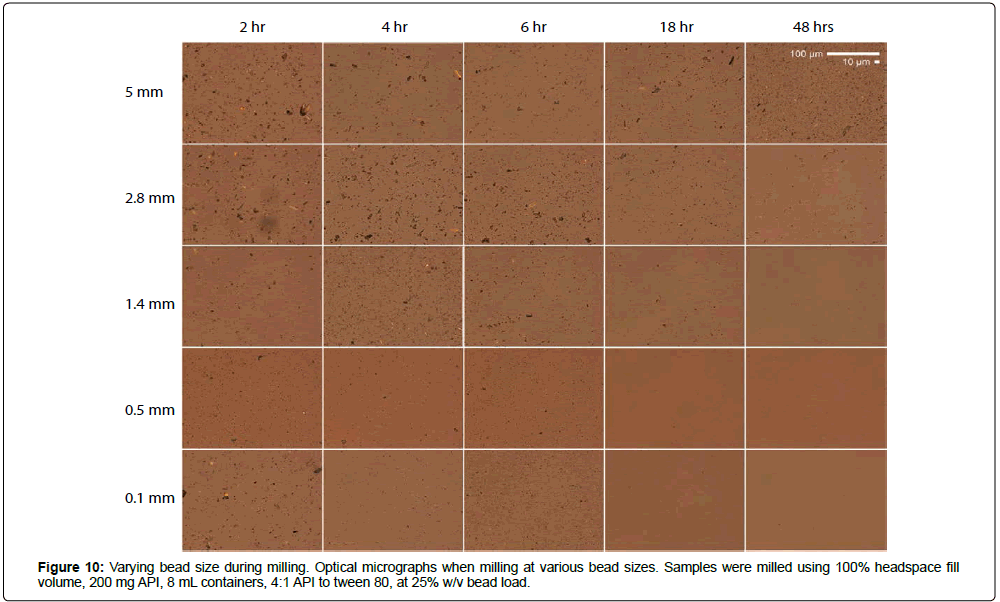
To better understand how bead size affects milling efficacy in the acoustic-vibratory milling setup, MBZ was milled at constant 25% w/v bead load using 5 mm, 2.8 mm, 1.4 mm, 0.5 mm, and 0.1 mm beads for various amounts of time, and characterized by laser diffraction, X-ray diffraction, and optical microscopy (Figures 9-11). Smaller beads were more efficient at milling the MBZ into smaller particles. For example, milling for 6 hours with 0.1 mm beads produced a suspension with a median diameter of 166 nm, while milling with 1.4 mm beads under the same conditions produced a suspension with a diameter of 3.9 μm (Figure 9). This is due to the increased bead-number density and collision frequencies that occur when milling with smaller and finer grinding media. However, MBZ converted into the thermodynamically more stable and less soluble Form A with excess milling that was also more apparent with the use of smaller media. For example, conversion was seen within 18 hrs of milling when using 0.1 mm beads, while MBZ did not undergo form conversion when milled with 1.4 mm beads for 18 hours. Thus, although smaller milling media might provide faster and more efficient particle size reduction, there is a higher chance of form conversion when utilizing smaller beads; this can desirable if the intention is to identify the suspensions with high stability for development, or undesirable if the intention is to identify suspensions with the highest solubility/bioavailability for development. Optical micrographs of the suspensions at various time points show that the suspension are polyhedral crystals with sizes that agree with those measured with laser-diffraction measurements (Figure 10).
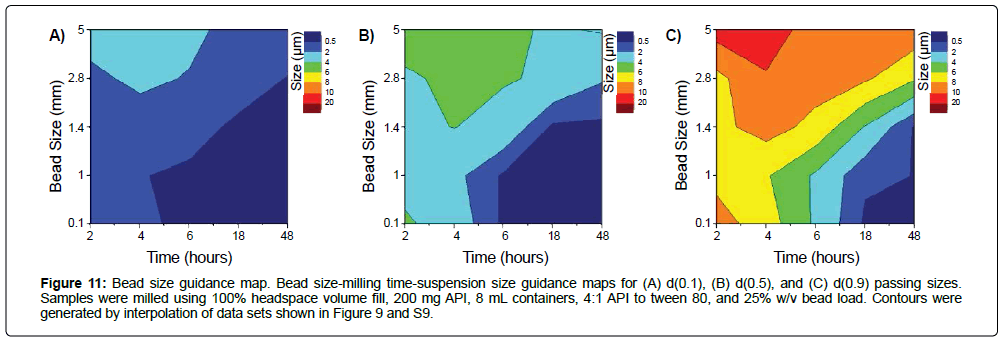
Figure 11: Bead size guidance map. Bead size-milling time-suspension size guidance maps for (A) d(0.1), (B) d(0.5), and (C) d(0.9) passing sizes. Samples were milled using 100% headspace volume fill, 200 mg API, 8 mL containers, 4:1 API to tween 80, and 25% w/v bead load. Contours were generated by interpolation of data sets shown in Figure 9 and S9.
In addition, although the use of large beads resulted in rapid breakup of agglomerated API, particle size was not significantly reduced even after prolonged milling. For example, the use of 5 mm beads was sufficient to break agglomerated API to form suspensions with d(0.5) of 4.8 μm after 2 hours of milling, or d(0.5) of 4.1 μm after 48 hours of milling. This result is due to the general poor efficiency of large beads in milling API below critical sizes. Thus, the use of large beads is not desirable for the formation of nanosuspensions. However, for the formulation of suspensions with micron size-diameters, the use of large beads endows the benefit of low-time sensitivity and minimizes the risk of overmilling.
Suspension sizes at each time point (Figure S9) were used utilized to generate additional guidance maps (Figure 11) for facilitating bead size selection. Larger beads produced suspensions with larger particle size, while smaller beads produced suspensions with smaller particle size. This also provides insight on the tradeoffs between operating at different bead sizes; to produce suspensions with d(0.5) less than 1 μm, either 0.1 mm or 0.5 mm beads can be used for milling at 6 hours, or 1.4 mm beads can be used for milling at 18 hours (Figure 10c). Thus, to produce nanosuspensions, beads of size 0.5 mm would be select selected, since this media mills more rapidly than the 1.4 mm beads, and are easier to handle and separate from suspensions compared to the 0.1 mm beads.
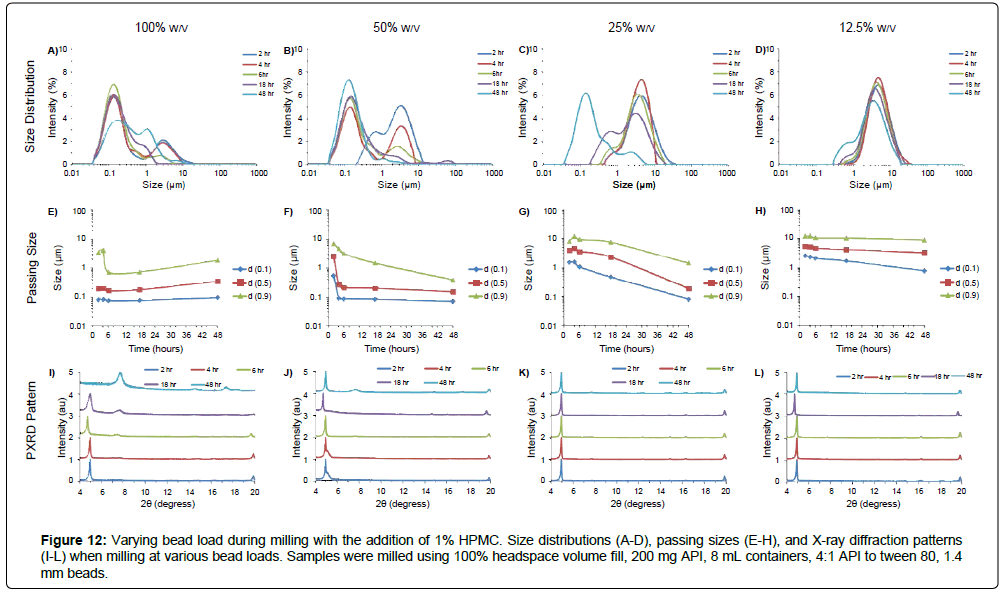
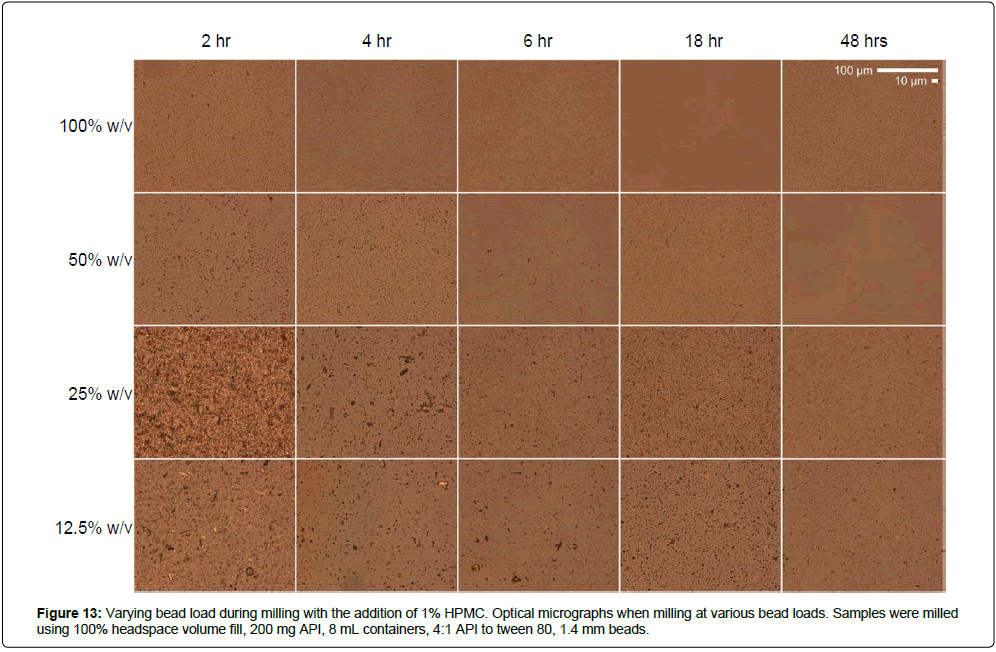
The addition of excipients during milling can inhibit form conversion and help stabilize the crystalline state of the API after size reduction. In the case of MBZ, milling particles into nanosize resulted in conversion of Form C to Form A during milling. To assess how the addition of an excipient affects particle size reduction rates and form conversion inhibition, MBZ was milled with 1.4 mm beads at various bead loadings in the presence of 1% hydroxypropyl methylcellulose K100 (HPMC) K100, which has been previously shown to stabilize Form C (internal data). The addition of HPMC generally decreases API size reduction rates, but also minimizes aggregation and form conversion (Figures 12-15, Supplementry Figure S10 and S11). For example, milling at 100% w/v load for 48 hrs resulted in the formation of 22 μm mean-diameter aggregates without the addition of HPMC, and the formation of 342 nm mean-diameter particles with the addition of HPMC. In addition, Form C to Form A conversion was observed even at 2 hrs of milling at 100% w/v load without HPMC, while conversion was not seen until after 48 hrs of milling with the addition of HPMC. Thus, while the addition of excipients reduces the API size reduction rate, the addition of excipients can also help reduce the risk overmilling and help preserve starting material crystalline state.
Figure 12: Varying bead load during milling with the addition of 1% HPMC. Size distributions (A-D), passing sizes (E-H), and X-ray diffraction patterns (I-L) when milling at various bead loads. Samples were milled using 100% headspace volume fill, 200 mg API, 8 mL containers, 4:1 API to tween 80, 1.4 mm beads.
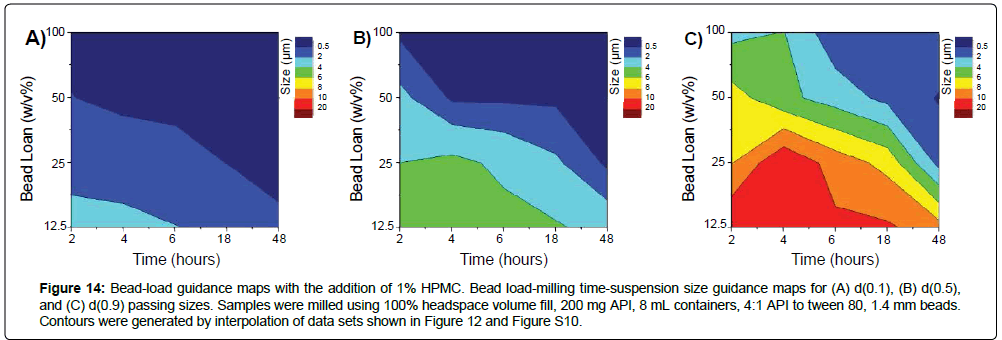
Figure 14: Bead-load guidance maps with the addition of 1% HPMC. Bead load-milling time-suspension size guidance maps for (A) d(0.1), (B) d(0.5), and (C) d(0.9) passing sizes. Samples were milled using 100% headspace volume fill, 200 mg API, 8 mL containers, 4:1 API to tween 80, 1.4 mm beads. Contours were generated by interpolation of data sets shown in Figure 12 and Figure S10.
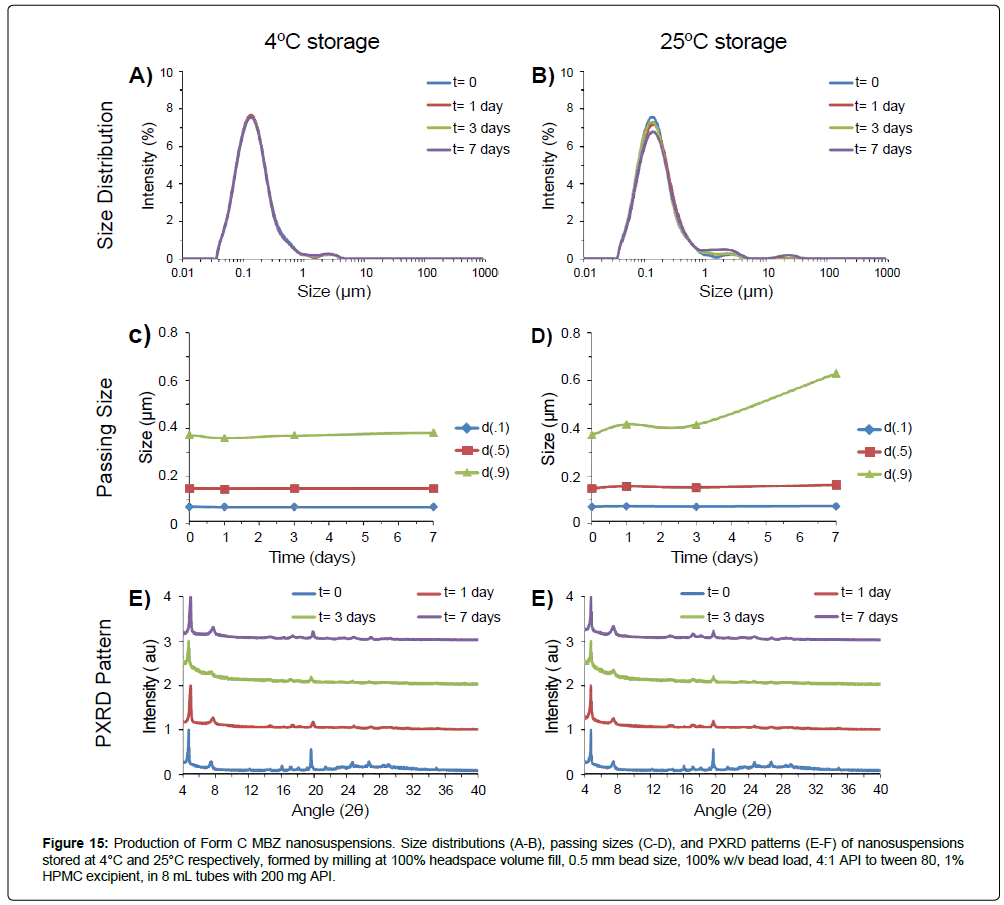
Figure 15: Production of Form C MBZ nanosuspensions. Size distributions (A-B), passing sizes (C-D), and PXRD patterns (E-F) of nanosuspensions stored at 4°C and 25°C respectively, formed by milling at 100% headspace volume fill, 0.5 mm bead size, 100% w/v bead load, 4:1 API to tween 80, 1% HPMC excipient, in 8 mL tubes with 200 mg API.
Form C mebendazole nanosuspensions
The development of operational guidance maps can be used to select for operational parameters to produce target formulations. For the rapid production of Form C MBZ nanosuspensions, for example, bead-size guidance maps indicate smaller beads would result in faster particle size reduction into the nanosuspension range (Figure 10), with 0.5 mm beads having similar milling efficacies but improved handling over 0.1 mm beads - leading to the selection of 0.5 mm size beads for this purpose. Bead-load micronization guidance maps indicate that higher bead loads result in more rapid micronization (Figure 7), but cross-references with bead-load te mperature guidance maps indicate that excessive bead loads can result in high temperatures and subsequently exacerbate API instability/form conversion - 100% w/v load was chosen as a tradeoff condition between increased milling efficacy and moderate operation temperature to rapidly create Form C MBZ nanosuspensions. Also, since the addition of HPMC aided in inhibiting form conversion, stabilizing HPMC excipient was included in the milling condition (Figure 13). With the use of these parameters, a Form C nanosuspension with d(0.5) of 148 nanometers was successfully formed after 6 hours of milling (Figure 15). MBZ suspensions remained physically stable at storage in 4˚C, and retained a d(0.5) of 148 nm upon storage after one week. At 25°C storage, sample sizes grew and suspensions exhibited d(0.5) of 164 nm. Form C crystallinity was preserved for at least one week in storage at 4°C or 25°C as assessed by PXRD. These results highlight the use of guidance maps to rapidly narrow down on operational parameters to produce target formulations with defined size and form.
Conclusion
Early pharmaceutical discovery can benefit from the development of a milling process that is capable of operating in both materialssparing screens and scale-up production capabilities (10 mg to 20 g). Acoustic-vibratory milling is a new methodology can that potentially operate as such, but the rules for milling efficiency and screening/ scale up have previously not been well understood. This contribution develops an experimental workflow for both the screening and production of micro/nanosuspensions. In our workflow, milling with complete headspace volume fill is chosen to minimize foam production, reduce heat generation, and maximize API recovery during milling. Screens are performed with both a mild and intense milling condition by varying bead loads and utilizing 10 mg sample, to provide insight on particle size-reduction extent and API susceptibility of form conversion into more stable/less soluble forms. Scale-up production of API is performed by increasing suspension concentration, strategically limiting tube size, and increasing tube number using the milling conditions identified in small-scale screening, to produce >20 g of materials per run. Operational parameters guidance maps were developed for temperature and particle size that provide insight on the tradeoffs and benefits/ disadvantages of certain milling conditions, and can be used to select for starting milling conditions to test to produce suspensions with defined properties. This work expands the understanding of acousticvibratory milling, and develops new tools for the streamlined process of developing milled formulation in pharmaceutical discovery.
Acknowledgments
Authors would like to acknowledge Sejal Patel, Agnes Kairer, Joy Fuerst, Sean Pu, David Breslin, Jason Riggs, Rose A. Dandridge, Erin Egan, Sarah Kaufhold, and Louis Loco for their contributions to this work.
References
- Kesisoglou F, Panmai S, Wu Y (2007) Nanosizing - Oral formulation development and biopharmaceutical evaluation. Adv Drug Deliv Rev 59: 631-644.
- Merisko-Liversidge E, Liversidge GG (2011) Nanosizing for oral and parenteral drug delivery: A perspective on formulating poorly-water soluble compounds using wet media milling technology. Adv Drug Deliv Rev 63: 427-440.
- Müller RH, Jacobs C, Kayser O (2001) Nanosuspensions as particulate drug formulations in therapy. Adv Drug Deliv Rev 47: 3-19.
- Leleux J, Williams RO (2014) Recent advancements in mechanical reduction methods: particulate systems. Drug Dev Ind Pharma 40: 289-300.
- Bhattachar SN (2015) Discovery Formulations: Approaches and Practices in Early Preclinical Development, in Discovering and Developing Molecules with Optimal Drug-Like Properties. Springer New York.
- Leung DH, Lamberto DJ, Liu L, Kwong E, Nelson T (2014) A new and improved method for the preparation of drug nanosuspension formulations using acoustic mixing technology. Int J Pharmace 473: 10-19.
- Li M, Zhang L, Davé RN, Bilgili E (2016) An Intensified Vibratory Milling Process for Enhancing the Breakage Kinetics during the Preparation of Drug Nanosuspensions. AAPS PharmSciTech 17: 389-399.
- Nagapudi K, Umanzor EY, Masui C (2017) High-throughput screening and scale-up of cocrystals using resonant acoustic mixing. Int J Pharmace 521: 337-345.
- Howe, H.W (2011) Method for resonant-vibratory mixing. Google Patents.
- Howe HW (2007) Apparatus and method for resonant-vibratory mixing. Google Patents.
- Mannheim V (2011) Empirical and scale-up modeling in stirred ball mills. Chem Eng Res Design 89: 405-409.
- Li M, Azad M, Davé R, Bilgili E (2016) Nanomilling of Drugs for Bioavailability Enhancement: A Holistic Formulation-Process Perspective. Pharmaceutics 8: 17.
- Mahendra N, Priyal P, Jayvadan P, Pankaj P, Rayasa SR (2010) Cyclosporine A-Nanosuspension: Formulation, Characterization and In Vivo Comparison with a Marketed Formulation. Scientia Pharmaceutica 78: 345-361.
- Weeber AW, Bakker H (1988) Amorphization by ball milling A review. Physica B: Condensed Matter 153: 93-135.
- Willart JF, Dujardin N, Dudognon E, Danède F, Descamps M (2010) Amorphization of sugar hydrates upon milling. Carbohydrate Res 345: 1613-1616.
- Rodriguez-Caabeiro F, Criado-Fornelio A, Jimenez-Gonzalez A, Guzman L, Igual A (1987) Experimental Chemotherapy and Toxicity in Mice of Three Mebendazole Polymorphic Forms. Chemotherapy 33: 266-271.
- Brusau EV, Camí GE, Narda GE, Cuffini S, Ayala AP (2008) Synthesis and Characterization of a New Mebendazole Salt: Mebendazole Hydrochloride. J Pharmac Sci 97: 542-552.
 Spanish
Spanish  Chinese
Chinese  Russian
Russian  German
German  French
French  Japanese
Japanese  Portuguese
Portuguese  Hindi
Hindi