Research Article, J Pharm Drug Deliv Res Vol: 11 Issue: 1
Assessment of Subacute Toxicity of Combined Exposure to Methyl morphine and Flunitrazepam Using a Rat Model
Esther Omugha Abam*, Dorcas I Oniagba and Tumininu Ijabadeniyi
Department of Chemical and Food Sciences, Bells University of Technology, Ogun State, Nigeria
*Corresponding Author:Esther Omugha Abam
Department of Chemical and Food Sciences, Bells University of Technology, Ogun State, Nigeria
Tel: +234-8036743380;
E-mail: eoabam@bellsuniversity.edu.ng
Received date: 07 December, 2021, Manuscript No. JPDDR-21-39532;
Editor assigned date: 09 December, 2021, Pre QC No. JPDDR-21-39532 (PQ);
Reviewed date: 23 December, 2021, QC No. JPDDR-21-39532;
Revised date: 28 December, 2021, Manuscript No. JPDDR-21-39532 (R);
Published date: 07 January, 2022, DOI:10.4172/2325-9604.1000200
Citation: Abam EO, et al (2022) Assessment of Sub-Acute Toxicity of Combined Exposure to Methyl Morphine and Flunitrazepam using a Rat Model. J Pharm Drug Deliv Res 11:1.
Abstract
The aim of this study is to assess the toxic effects of combined abuse of these drugs. Methods: Thirty-five (35) male Wistar rats were divided into five (5) groups of seven animals each. Group 1 served as control and was administered distilled water only, groups 2 and 3 received a daily dose of 3.25 mg kg-1 bw of codeine and`0.03 mg kg-1 bw rohypnol respectively. Groups 4 and 5 both received combined doses of 3.25 mg kg-1 bw of codeine and 0.03mg kg-1 bw of rohypnol daily. All groups were exposed for 28 days except group 5 which was sacrificed after one week of withdrawal of treatments (on day 35). At the end of the exposure, some liver and brain function biomarkers were studied spectrophotometrically.
Keywords: Methylmorphine; flunitrazepam; ATPases; hyperbili rubinemia; hypercholesterolemia; transaminases; opiate; benzodiazepine; cholesterogenesis
Introduction
Methyl morphine (codeine) is a derivative of the seeds of the opium poppy, Papaver somniferous var. album’ [1]. It is one of the natural opiates derived from the plant, the other being morphine. Codeine is commonly administered as an analgesic, antitussive and anti-diarrheal agent although its analgesic effect is much less potent than morphine [2]. The mechanism of action of codeine in
The brain is same as other opioids. They interact with the three opioid receptor subtypes: μ, κ, and δ, of which the μ-opioid receptor has received a lot of research focus and hence is the most well- known. On activation of the G protein-coupled μ receptor, acute changes in neuronal excitability ensue and this is believed to be the underlying mechanism through which they relieve pain and carry out all other effects including producing feelings of euphoria and contentment [3-6]. All opioids have demonstrated significant abuse potential in rodents, primates and humans [7-10]. Codeine is metabolized in the liver to produce morphine, a ten times more potent agonist of the μ receptor and the reaction is catalyzed by CYP2D6, a cytochrome P450 enzyme [11]. Subsequent conjugation with glucuronide yields glucuronide morphine conjugates [12]. Norcodeine is produced from codeine by the action of CYP3A4 while 3- and 6- glucuronides are produced by the action of UGT2B7 on codeine, nor codeine, and morphine.
Codeine abuse in both pill and syrup forms among youths in Nigeria have become very worrisome. Reports show that as much as 3 million bottles of codeine containing cough syrups were consumed daily in Kano and Jigawa States alone [13] before the intervention. This led the Nigerian government to place a ban on the production and import of codeine containing cough syrup in May, 2018 after a BBC investigation into its role in a looming addiction epidemic [14]. Consequently codeine containing syrups were no longer supposed to be freely sold as Over-the-Counter drugs; nevertheless, they have continued to get into the hands of youths through unscrupulous pharmacists and Patent medicine dealers.
Flunitrazepam (Rohypnol®) is another commonly prescribed and abused psychoactive drug belonging to a class of drugs called benzodiazepines. Structurally, these drugs consist of the fusion of a benzene and diazepine ring and they act as positive allosteric modulators of the alpha Gamma Amino Butyric Acid (GABAα) receptor complex. Benzodiazepines are widely prescribed as sedatives and hypnotics in the treatment of anxiety, insomnia and seizure disorders and may also be used as skeletal-muscle relaxants, pre-anesthetic agents and for the treatment of alcoholic withdrawal [15,16]. Rohypnol is prescribed for use in Europe, Latin America, and elsewhere, however it has never been approved for use or sale in the United States of America [17]. Much of the concern over the abuse of Flunitrazepam stems from its use in facilitating "date rape" [18]. Date rape involves the use of rohypnol illicitly to aid rape. Due to its ability to mentally and physically paralyze the victims, they are unable to clearly recall the assault. When the drug is used in combination with alcohol, effects can result in anterograde amnesia [19]. The abuse of benzodiazepines has remained very common among youths. Evidence implies that the abuse of benzodiazepines may not only be recreational but may also be an off-shoot of medical use. Some evidence has emerged linking benzodiazepine abuse to its therapeutic use. Reports of abusive patterns of use begin to emerge soon after the widespread clinical use of GABAα agonists and allosteric modulators [20].The purpose of this work was to investigate the effect of combined abuse of both drugs on brain and other organ functions using a rat model.
Materials and Methods
Drugs
Parkalin® cough syrup with codeine and Swipha flunitrazepam tablets were obtained under permission from the medical director of Bells university of technology clinic. Na+- ATP is a product of Sigma Aldrich, Missousi, USA. All other chemicals used were of the purest analytical grade.
Experimental design
Thirty-five male albino rats weighing between 100-120 g (8-10 weeks old) were obtained from the animal house of the department of physiology, University of Ibadan, Nigeria. These animals were housed in the animal house of Bells University of technology, Ota, and Ogun State and were allowed to acclimatize for two weeks before the start of the experiment [21]. After acclimatization, the rats were weighed and randomly distributed into five groups of seven animals each. In addition to a standard rat pellet diet and distilled water which was administered to all the groups, Group 2 was administered 3.25 mg kg-1 body weight of codeine in the cough syrup by gastric intubation in addition, Group 3 was administered 0.03 mg kg-1 body weight of rohypnol by gastric intubation. Groups 4 and 5 were administered a combination of 3.25 mg kg-1 body weight of codeine in the cough syrup and 0.03 mg kg-1 body weight of rohypnol by gastric intubation, however, all the groups were treated for thirty days and sacrificed while Group 5 rats were sacrificed after one week of withdrawal of treatment; this served as the withdrawal group. Doses administered were chosen with reference to adult human therapeutic dosage. A summary of the experimental design is depicted in (Table 1). At the end of the exposure, blood was collected into heparinized tubes by cardiac puncture under light anesthesia. Liver, kidney and brain were harvested and homogenized in 150 mmol L-1 ice cold KCl to obtain a 10% homogenate which was then centrifuged at 15,000 g for 15 minutes at 4°C and the supernatant was stored at -20°C till analysis. Protein was determined in the tissue homogenates. The experiment was carried out in accordance with the Bells University of technology, chemical and food sciences’ (Biochemistry Unit) guidelines for the care and use of laboratory animals.
| Groups | Treatment |
|---|---|
| 1 (control) | Distilled water only |
| 2 | 3.25 mg kg-1 bw codeine |
| 3 | 0.03 mg kg-1 bw rohypnol |
| 4 | 3.25 mg kg-1 bw codeine and 0.03 mg kg-1 bw rohypnol |
| 5 (withdrawal) | 3.25 mg kg-1 bw codeine and 0.03 mg kg-1 bw rohypnol |
Table 1: Summary of experimental protocol and treatments.
Biochemical analyses
Determination of cholesterol and triglycerides in plasma and brain tissues Plasma cholesterol and triacylglycerol assays were carried out using Redox cholesterol and triacylglycerol determination kits, Rx Monza, according to the methods outlined in the kits. Brain lipids were extracted as described by which involve the use of a chloroform-methanol mixture (2:1 v/v) for extraction. A solution of 0.05 M KCl was used in washing the extract and subsequently, aliquots of the lipid extracts were then used for the determination of cholesterol and triacylglycerol. Details of this procedure are well documented.
Assay for brain total, Na+K+, Ca2+ and Mg2+ ATPase activities: The activities of total ATPase (E.C.3.6.1.3), Na+/K+ ATPase (E.C.3.6.1.37), Ca2+ ATPase (E.C.3.6.1.38) and Mg2+ ATPase (E.C.3.6.1.39) in the brain homogenate were assayed using the methods respectively. Details of the procedure are well documented.
Assay for plasma and liver amino transferees: Aspartate Aminotransferase (AST) activity, Alanine Aminotransferase (ALT), Alkaline Phosphatase (ALP) were determined in the plasma and liver homogenates using Randox assay kits, Rx Monza. Protein concentration was also determined in the brain homogenate using the Randox protein determination kit.
Assay for plasma and liver creatinine and bilirubin concentration: Bilirubin and creatinine levels were determined using Randox analytical kits, Rx Monza, according to the methods outlined in the kits.
Statistical analysis
Results are expressed as mean ± SEM. The statistical significance was evaluated by one-way Analysis of Variance (ANOVA) followed by Turkey’s test using Statistical Package for the Social Sciences (SPSS) version 15.00 for Windows (SPSS Inc.; CA, USA). A value of p<0.05 was considered statistically significant between groups.
Results
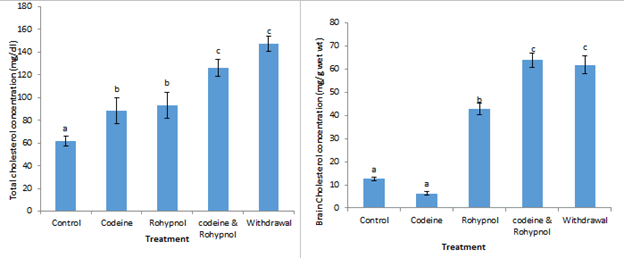
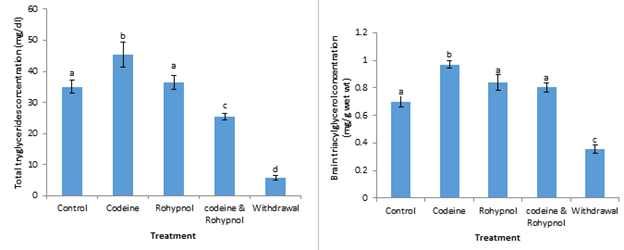
Cholesterol concentration in plasma and brain of the animals is depicted in Figure 3. Animals treated with codeine and rohypnol showed a 44.00% and 51.06% increase respectively in plasma cholesterol concentration compared with control while the codeine and rohypnol co-treated group exhibited about 2-fold increase in total cholesterol concentration compared with control indicating an additive effect of the toxicants. One-week withdrawal from combined treatment maintained a 2.4 fold increase in plasma cholesterol. The reduction in brain cholesterol induced by codeine was not statistically significant. Rohypnol treatment on the other hand induced a 240% increase in brain cholesterol while co-treatment with both drugs displayed a codeine-induced potentiation of rohypnol effect from 240% to 400% increase. This high cholesterol was maintained after one week withdrawal of co-treatment. Total and brain triglyceride concentration is displayed in Figure 4. Codeine treatment resulted in a significant increase (p<0.05) in triglyceride concentration in the plasma compared with control, however, rohypnol treatment had no effect on plasma triglyceride. Co-treatment with both codeine and rohypnol depressed plasma triglyceride by 27% compared with control while one week of withdrawal of treatment further depressed plasma triglyceride from 27% to 86%. Codeine induced a significant increase of 39% in brain triglyceride concentration compared to control. Rohypnol on the other hand had no effect on brain triglycerides. Similarly, co-treatment with codeine and rohypnol had no effect on brain triglyceride compared with control. However, a significantly lowered brain triglyceride was observed after one week of withdrawal of co-treatment.
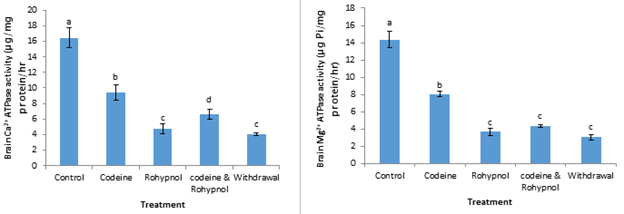
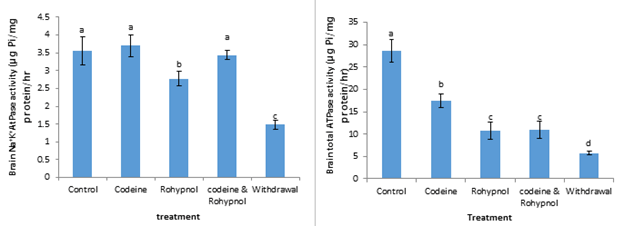
Brain Ca2+ and Mg2+ ATPase activities are expressed in Figure 5. Ca2+ ATPase activities were down-regulated in all the treatment groups. This depression in activity amounted to 43%, 71%, 60% and 75% in the codeine, rohypnol, codeine and rohypnol and withdrawal groups respectively. Codeine significantly inhibited brain Mg2+ ATPase activity to the tune of 44% compared with control while rohypnol, codeine and rohypnol co-treatment and one week withdrawal group all displayed a similar inhibition of 75%, 70% and 79% inhibitions respectively. Na+/K+ ATPase and Total ATPase activities in the brain of the rats are represented in Figure 6. Codeine treatment as well as codeine and rohypnol co-treatment had no effects on brain Na+/K+ ATPase activity, however, rohypnol and withdrawal induced a significantly decreased (p<0.05) enzyme activity. This reduction in enzyme activity increased from 22% to 58% from the rohypnol-treated to the one week withdrawal group. Total ATPase activities in the brain of the rats were decreased by 39 % by codeine treatment after twenty-eight days of treatment. Rohypnol and the codeine and rohypnol co-treated groups displayed similar inhibitions (62%) of total ATPase in the brain. This inhibition was further increased to 80% after one week of withdrawal of treatment.
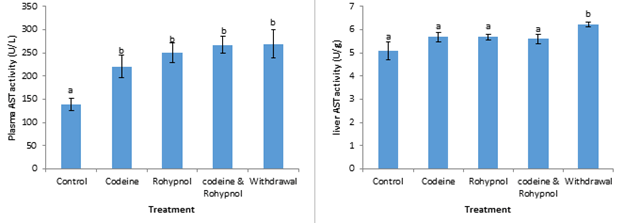
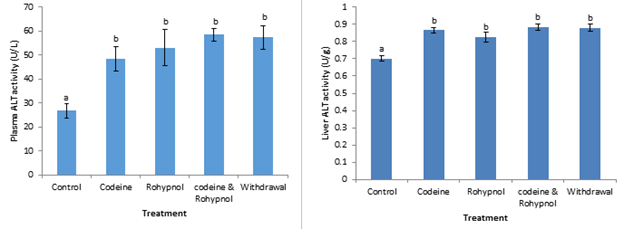
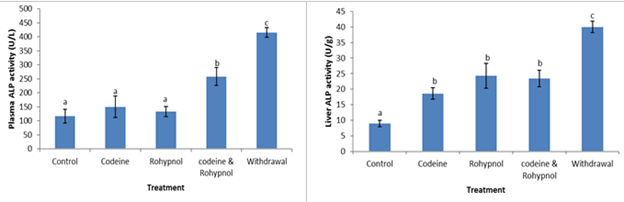
Activities of plasma and liver aminotransferases of the rats are depicted in Figures 7-9. The activities of plasma Aspartate Aminotransferase (AST) were significantly increased by 59%, 81%, 93% and 95% by codeine, rohypnol, codeine and rohypnol co-treatment and one week withdrawal of treatment respectively. Conversely in the liver, there was no significant difference in AST activities between all the treatments and control except in the withdrawal group which displayed a 22% increase in liver AST activity compared with control (Figure 7). Similarly, plasma and liver Alanine Aminotransferase (ALT) activities were also up-regulated by all the treatments (Figure 8). This increase was as much as 80, 98% and 118% in the plasma and 24%, 18% and 26% in the liver in the codeine, rohypnol and codeine and rohypnol treatments respectively. One week withdrawal of co-treatment had no effect on the observed increase in both plasma and liver. Alkaline Phosphatase (ALP) activity in the plasma was not influenced by codeine and rohypnol treatments but a 121% increase in activity was observed on combined treatment with codeine and rohypnol (Figure 9). This increase was further exacerbated during the one week withdrawal resulting in a 256% increase in ALP activity over control. Liver ALP activity followed the same trend with increases of 108%, 171%, 161% and 346% in the codeine, rohypnol, combined codeine and rohypnol and in the withdrawal groups respectively (Figure 9), implying an interaction that resulted in the activation of the enzyme.
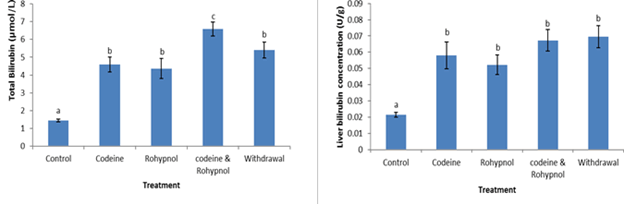
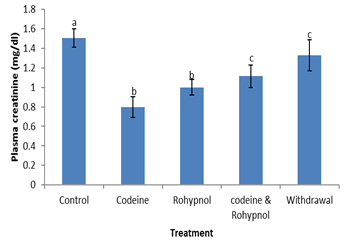
Total bilirubin concentrations are shown in Figure 10. Total bilirubin was up-regulated by individual codeine and rohypnol treatments (219% and 203% respectively), while combined treatment of codeine and rohypnol showed an additive interaction with an increase of 358 % compared with control. One week withdrawal of co-treatment brought about a reduction in this (from 358% to 275%). Liver bilirubin followed a similar trend up-regulation by all the treatments (171%, 144% and 214% increases in the codeine, rohypnol and codeine and rohypnol co-treated groups respectively). One week withdrawal of combined treatment did not bring about any reversal of the observed increases. Plasma creatinine levels are depicted in Figure 11. Plasma creatinine decreased by all the treatments (47%, 34% and 26% in the codeine, rohypnol and codeine and rohypnol co-treated groups respectively) implying an antagonistic interaction of both toxicants on plasma creatinine. A reversal toward control was observed in the withdrawal group further reducing the decrease from 26% to 12%.
Discussion
Opioids and benzodiazepines have raised a lot of concern in recent times due to their high degree of co-abuse among youths. These drugs have a high potential to create dependence and addiction. They both produce central nervous system effects, and the penchant by youths to abuse both drugs concomitantly has stimulated the scientific interest in their combined exposure.
Drugs that produce central nervous system effects must be able to cross the blood brain barrier or alter the membrane integrity sufficiently enough to cause havoc. One of the vital constituents of cell membranes is cholesterol and it has been shown to account for a large proportion of the total cholesterol in the body. Cholesterol has been shown to play a vital role in synaptic transmission, and cholesterol metabolism defects have been linked with neurodegenerative disorders. Brain cholesterol derives mainly (over 95%) from in situ synthesis in glial cells since the Blood Brain Barrier (BBB) prevents uptake of lipoprotein-bound cholesterol from the blood. The high brain cholesterol observed by combined exposure to both drugs in this study can therefore be attributed to increased Cholesterogenesis through an up-regulation of the enzyme β-Hydroxyl-β-Methylglutaryl-Coenzyme A (HMG-CoA) reeducates, the rate limiting enzyme in the pathway. Other works have also identified toxicant-induced cholesterogenesis in the brain. In addition, the degradation and excretion of cholesterol from the brain is mainly driven by the neuron-specific cytochrome P450 oxidase CYP46A1, which hydroxylates cholesterol to 24S-Hydroxycholesterol (24-OHC), the most abundant oxysterol in the brain, which unlike cholesterol, can cross the BBB, entering the circulation for its disposal by the liver. An inhibition of this enzyme may also account for the accumulation of cholesterol in the brain of the codeine and rohypnol co-treated rats in this study. An experiment involving knockout of the cholesterol 24-hydroxylase gene in mice was found to result in accumulation of high cholesterol in the brain. This very high cholesterol content observed in the brain may also have caused changes in the membrane integrity of the cells by affecting the cholesterol/phospholipid ratio, a known index for measuring membrane permeability [21]. Once cell membranes are compromised, proteins such as the Adenosine Triphosphatases (ATPases), which are membrane-bound also, have their activities severely impaired. The central nervous system effects often observed with the use and abuse of these drugs could be mediated by the large increase in cholesterol concentration they induce in the brain.
The ATPases are a group of enzymes that catalyze the conversion of ATP to ADP and Pi while the energy produced is used to transport ions across membranes against-their concentration gradient. They are very crucial in maintaining ionic balance across membranes which is vital in supporting processes such as signal transduction, nerve transmission secondary active transport of macromolecules such as amino acids and sugars. Hence disruptions in the activities of any of this class of enzymes are often accompanied by far-reaching consequences especially in the brain. In this study, the activities of all the ATPases studied in the brain Ca2+, Mg2+, Na+K+ and total ATPases were down regulated by codeine and rohypnol while combined exposure to both toxicants displayed various interactions, mainly antagonistic. The inhibitory effect of cholesterol enrichment on all membrane ATPases studied and the stimulating effect of cholesterol enrichment on most other membrane transport proteins.
The activities of the ATPases have been shown to be modulated by the lipid milieu of the membrane–principally the cholesterol and phospholipid content [22]. Hence the disruptions in activities of the ATPases may be directly linked to the high brain cholesterol content observed in this study.
The transaminases are enzymes present in some tissues and appear in the blood when those tissues have their cell membranes partially damaged or destroyed. ALT, AST and ALP are common and reliable biomarkers of hepatocellular injury or necrosis. A variety of hepatic disorders can lead to their elevation in the plasma. Of the three, ALT is thought to be more specific for hepatic injury because it is present mainly in the cytosol of the liver and in low concentrations in other organs unlike AST that has cytosolic and mitochondrial forms and is present in tissues of the liver, heart, skeletal muscle, kidneys, brain, pancreas, and lungs, and in white and red blood cells [23]. ALP is also abundant in the liver, bones, kidneys, gall bladder and intestines and abnormal ALP level is generally taken as an indication of a liver or bone injury. Drug-induced hepatic injury is very common in drug use and is the most frequently cited reason for withdrawal of an approved drug. In this study, all three transaminases studied were up-regulated by codeine and rohypnol treatment. ALP was however not affected by individual exposures to the toxicants but in co-treatment. Results of the transaminases as well as plasma and liver bilirubin indicate a drug-induced codeine and rohypnol hepatic injury which was mostly synergistic in co-treatment. The mechanism of drug-induced hepatic injury that have been described include; Disruption of the hepatocyte: Covalent binding of the drug to intracellular proteins can cause a decrease in ATP levels, leading to actin disruption. Disassembly of actin fibrils at the surface of the hepatocyte causes blebs and rupture of the membrane. Disruption of the transport proteins: Drugs that affect transport proteins at the canalicular membrane can interrupt bile flow. Loss of villous processes and interruption of transport pumps such as multidrug resistance–associated protein 3 prevent the excretion of bilirubin, causing cholestasis. Cytolytic T-cell activation: Covalent binding of a drug to the P-450 enzyme acts as an immunogenic, activating T cells and cytokines and stimulating a multifaceted immune response. Apoptosis of hepatocytes: Activation of the apoptotic pathways by the tumor necrosis factor-alpha receptor of Fas may trigger the cascade of intercellular caspases, which results in programmed cell death. Mitochondrial disruption: Certain drugs inhibit mitochondrial function by a dual effect on both beta-oxidation energy productions by inhibiting the synthesis of nicotinamide adenine dinucleotide and flavin adenine dinucleotide, resulting in decreased ATP production. Bile duct injury: Toxic metabolites excreted in bile may cause injury to the bile duct epithelium. From the results of this work, mechanisms numbers are cited as the most probable mechanisms by which codeine and rohypnol exert their hepatotoxicity, evidenced by the appearance of increased transaminases in the blood, hyperbilirubinemia and high liver bilirubin concentrations and low plasma creatinine levels. Bilirubin is another biomarker of liver function. Bilirubin is produced by the liver during breakdown of red blood cells. Other works have also observed a drug-induced Hyperbilirubinemia in humans.
Creatinine is a waste product of muscle activity produced from breakdown of creatine which is produced in the liver and transported to the muscles for muscle activity. High blood creatinine levels are usually indicative of an impaired kidney function because the kidneys are responsible for the removal of creatinine from the blood. Conversely, low blood creatinine may be attributable to an impaired liver function or reduced muscle mass since a reduced muscle mass will naturally produce reduced creatinine. CNS depressants are known to cause a lowering of brain action and muscle relaxation. Hence reduced creatinine observed in this study may be attributed to impaired liver function and the muscle relaxing effects of codeine and rohypnol.
Conclusion
Combined effects of both codeine and rohypnol treatments on most biomarkers studied were mostly additive, synergistic and potentiating, implying that co-exposure or abuse of both drugs simultaneously could have more devastating outcomes in the liver and brain than when used individually.
References
- Wisniak J (2013) Pierre-jean robiquet. Educación Química 24: 139–149. [Crossref], [Google Scholar]
- Achukwu PU, Omorodion NT, Tosan E, Aloh HE, Charles E, et al. (2019) Codeine and its histopathological effect on brain of albino rats: An experimental study. Acta Scientific Nutritional Health 3: 125-133. [Crossref], [Google Scholar]
- Jones DJ, Mogali S, Comer SD (2012) Polydrug abuse: A review of opioid and benzodiazepine combination use. Drug Alcohol Depend 125: 8–18. [Crossref], [Google Scholar], [Indexed]
- Papich MG (2016) "Codeine". Saunders Handbook of Veterinary Drugs (Fourth Edition). WB Saunders pp 183–184. [Crossref], [Google Scholar], [Indexed]
- Matthes HW, Maldonado R, Simonin F, Valverde O, Slowe S, et al. (1996) Loss of morphine-induced analgesia, reward effect and withdrawal symptoms in mice lacking the mu-opioid-receptor gene. Nature 383: 819–823. [Crossref], [Google Scholar], [Indexed]
- Comer SD, Sullivan MA, Whittington RA, Vosburg SK, Kowalczyk WJ, et al. (2008) Abuse liability of prescription opioids compared to heroin in morphine-maintained heroin abusers. Neuropsychopharmacol 33: 1179–1191. [Crossref], [Google Scholar], [Indexed]
- Haney M, Spealman R (2008) Controversies in translational research: Drug self-administration. Psychopharmacol (Berl) 199: 403–419. [Crossref], [Google Scholar], [Indexed]
- Kieffer BL, Gavériaux-Ruff C (2002) Exploring the opioid system by gene knockout. Prog Neurobiol 66: 285–306. [Crossref], [Google Scholar], [Indexed]
- Trigo JM, Martin-García E, Berrendero F, Robledo P, Maldonado R (2010) The endogenous opioid system: A common substrate in drug addiction. Drug Alcohol Depend 108:183–194. [Crossref], [Google Scholar], [Indexed]
- Strang J, Griffiths P, Abbey J, Gossop M (1994) Survey of use of injected benzodiazepines among drug users in Britain. Br Med J 308:1082. [Crossref], [Google Scholar], [Indexed]
- Kathiramalainathan K, Kaplan HL, Romach MK, Busto UE, Li NY, et al. (2000) Inhibition of cytochrome P450 2D6 modifies codeine abuse liability. J Clin Psychopharmacol 20: 435–444. [Crossref], [Google Scholar], [Indexed]
- NAFDAC online Newsletter (2018) "Codeine Syrup Crisis: NAFDAC Shuts Down Peace Standard Pharmaceutical Limited, Bioraj Pharmaceutical Limited Both In Ilorin, And Emzor Pharmaceuticals Ind. Ltd, Lagos.
- BBC News (2018) "Nigeria bans cough syrup with codeine after addiction outcry".
- Pierre-jean robiquet.
- Mizuno K, Katoh M, Okumura K, Nakagawa N, Negishi T, et al. (2009) Metabolic activation of benzodiazepines by CYP3A4. Drug Metab Dispos 37: 345-351. [Crossref], [Google Scholar], [Indexed]
- Lloyd J (2003) Executive Office of the President. Office of National Drug Control Policy (ONDCP): Drug Policy Information Clearinghouse Factsheet: Washington, DC.
- Druid H, Holmgren P, Ahlner J (2001) Flunitrazepam: An evaluation of use, abuse and toxicity. Forensic Sci Int 122: 136-141. [Crossref], [Google Scholar], [Indexed]
- [Crossref]
- Rosenbaum JF (2005) Attitudes toward benzodiazepines over the years. J Clin Psychiatry 66:4–8. [Crossref], [Google Scholar], [Indexed]
- Ator NA, Griffiths RR (1987) Self-administration of barbiturates and benzodiazepines: A review. Pharmacol Biochem Behav 27: 391–398. [Crossref], [Google Scholar], [Indexed]
- Olaniyan MF, Ozuruoke DFN, Fapohunda JS, Afolabi T (2017) Immunological effect of tramadol, codeine, flunitrazepam on plasma cortisol (anti-inflammatory agent), cortisol binding globulin (acute phase protein) and total bile acid in rabbits. J adv med 23: 1-8. [Crossref], [Google Scholar]
- Vaibhav D (2019) A simple model to solve complex drug toxicity problem. Toxicology Research 8: 157-171. [Crossref], [Google Scholar], [Indexed]
- Afolabi OK, Ugbaja RN, Ademuyiwa O (2016) Combined arsenic and di-(2-ethylhexyl) phthalate exposure elicits responses in brain ATPases different from hepatic and renal activities in rats. J Toxicol Environ Health Sci 8: 6-14. [Crossref], [Google Scholar]
 Spanish
Spanish  Chinese
Chinese  Russian
Russian  German
German  French
French  Japanese
Japanese  Portuguese
Portuguese  Hindi
Hindi