Research Article, Biomater Med Appl Vol: 1 Issue: 2
Bioactivity Assessment of Ag-HA
Flavia M Fonseca1, Andrea M Costa1, Jose B Campos2, Rubens LSB Marçal1, Daniel Navarro da Rocha1 and Marcelo H Prado da Silva1*
1Instituto Militar de Engenharia - IME, Pça. Gen. Tibúrcio, 80, P. Vermelha, Urca, Rio de Janeiro, R.J, Brazil
2Universidade do Estado do Rio de Janeiro - UERJ, Rua Fonseca Teles, São Cristovão, Rio de Janeiro, RJ, Brazil
*Corresponding Author : Marcelo H Prado da Silva
Instituto Militar de Engenharia – IME, Pça. Gen. Tibúrcio, 80, P. Vermelha, Urca, Rio de Janeiro, R.J, Brazil
E-mail: marceloprado@ime.eb.br
Received: September 13, 2017 Accepted: October 11, 2017 Published: October 17, 2017
Citation: Fonseca FM, Costa AM, Campos JB, Marçal RLSB, Rocha DN, et al. (2017) Bioactivity Assessment of Ag-HA. Biomater Med Appl 1:2.
Abstract
Cell culture medium has been used as an alternative to Kokubo’s solution in order to approach in vitro to in vivo bioactivity assessment. Moreover, the production of biomaterials with silver ions (Ag) has shown an antimicrobial effect and these are potential graft materials to prevent or reduce bacterial infection. In the present study, synthesis of hydroxyapatite (HA) and silverdoped hydroxyapatite (Ag-HA) with 0.13 mol% Ag (mol Ca:Ag) were performed and the bioactivity assessment was analyzed by scanning electron microscopy with field emission gun (FEG-SEM). The Ag-HA samples showed discrete bone-like apatite formation, indicating bioactivity behavior after in vitro incubation in McCoy cell culture medium after 7 days. Silver introduction into hydroxyapatite structure showed to be effective to produce a bioceramic with potential antibacterial effect and bioactivity.
Keywords: Bioactivity; Hydroxyapatite; Silver; McCoy; Whitlockite
Introduction
Bioactivity is a positive response when a material has an interaction with living tissue, leading to the formation of calcium phosphate deposits on the bioactive surfaces. The formation of apatite-like layer on the porous material surface, characteristic of bioactive scaffolds can also be reproduced on in vitro experiments [1]. The mechanism of bioactivity is well-stablished by the proportional effect of ion-exchange and dissolution-precipitation reaction on the biomaterial surface [1,2].
SBF medium is a solution similar in ion concentration to human blood plasma, used as in vitro test to predict biomaterials bioactivity, by surface formation of bone-like apatite [2]. Parameters such as pressure, temperature, pH and ions concentration are mimicked in this solution, to reproduce human body plasma in vitro. The SBF solutions can be prepared by mixing stable concentrated solutions, which increase the reproducibility of in vitro tests due to negligible changes of pH during preparation. The high stability of thus prepared SBF enables the evaluation of hydroxyapatite formation on the surface of bioactive materials without the negative effect of spontaneous precipitation. Solutions similar to SBF are prepared by mixing stable concentrated salt solutions, which increase the stability and reproducibility of in vitro experiments, or by the presence of proteins, using culture medium [3-5]. Nowadays, cell culture medium has been used as an alternative to SBF, for bioactivity assessment, in order to approximate the in vivo behavior. The choice of cell medium and the routine to refresh the cell culture medium during the experiments can minimize secondary effects; despite sometimes the hyperoxia condition promote cell proliferation [6].
In addition to bioactivity properties, studies have indicated that the silver nanoparticles can reduce bacterial biofilm formation, further enhancing bone tissue regeneration of bioactive materials [7]. The present study aims to observe the bioactivity behavior of silvercontaining hydroxyapatite (Ag-HA). Ag-HA synthesis had been prepared by an acidic route, with the least amount of silver, to promote bioactivity, bactericidal activity and less toxicity for organism.
Materials and Methods
Synthesis of Ag-HA
Hydroxyapatite (HA) and Ag-HA powders were synthesized by a chemical wet method as described elsewhere [8-10]. The synthesis was performed using a calcium phosphate aqueous solution, followed by the addition of silver nitrate with a theoretical silver content of 0.13 mol% of Ag (mol Ca:Ag). At the end, the solution showed a translucent aspect without precipitates and a pH level below 4. In order to obtain a hydroxyapatite precipitate, 0.1M potassium hydroxide (KOH) solution was added to the mixture and an adjustment of pH up to 12 was achieved. The precipitates were aged for 24 h and, after filtering and washing process, the obtained powders were dried at 70°C, then, the powders were de-agglomerated and sieved through a 75 μm sieve.
Additionally, HA and Ag-HA pellets (10×1 mm dimensions) were produced by uniaxial pressing at 250 MPa (hydraulic pressing SSP - 10A Shimadzu) for further FEG-SEM and bioactivity characterization. The pellets were heat treated at 1100°C, and then sterilized in an autoclave.
Characterization: HA and Ag-HA powders were analyzed by X-ray diffraction (XRD) using Panalytical X”PERT PRO diffractometer with CuKα radiation, 2θ from 10° to 80°, scanning step of 0.05° and a collecting time of 100 seconds per step θ-2θ. Rietveld refinement method was performed by the TOPAS-Academic program in order to determine the percentages of calcium phosphate phases.
Surface morphology was investigated by scanning electron microscopy with field emission gun (FEG-SEM - Quanta FEG 250, FEI) and the semi-quantitative chemical analysis was performed by energy dispersive X-ray spectroscopy (EDS).
The quantitative chemical analysis was performed by X-ray fluorescence spectroscopy (XRF) and X-ray photoelectron spectroscopy (XPS). XRF analysis was performed in the fluorescence spectrometer (AXIOS-MAX Panalytical) after the production of HA and Ag-HA pellets with lithium tetraborate and sintered at 1100°C. XPS spectrum was obtained at 55° (angle between the surface and axis) and the carbon 1s peak at 284.6 eV was used as a reference for spectrum calibration. The peak of Ag3d was analyzed by the software CASA XPS.
For bioactivity assessment, the HA and Ag-HA pellets were immersed in Falcon tubes with McCoy`s 5A medium in aseptic conditions using laminar flow hood. The samples were maintained at 37°C under 5% CO2 atmosphere, in an incubator (PANASONIC, COM-19AIC-PA) for 7 days.
Results and Discussion
XRD characterization
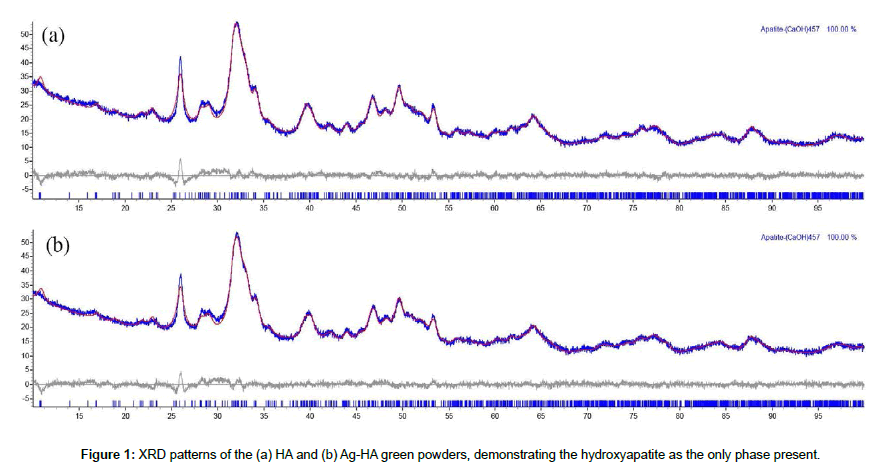
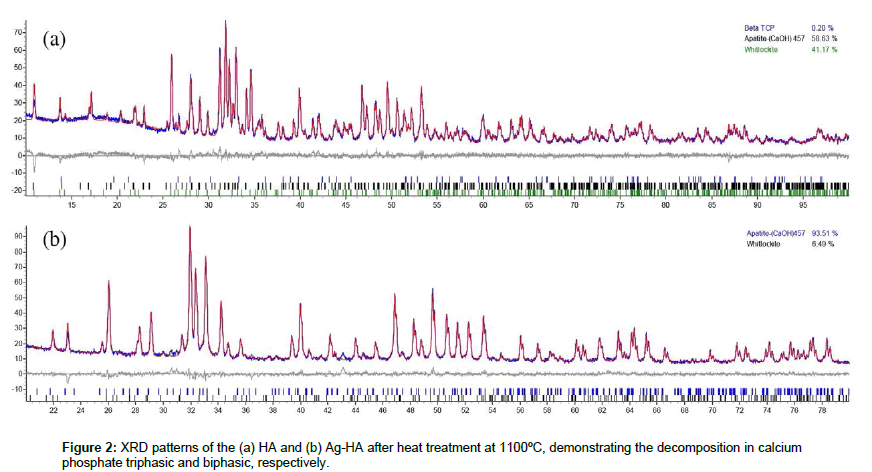
Figures 1 and 2 show XRD analysis of HA and Ag-HA samples before and after heat treatment at 1100°C. Green powders of HA and Ag-HA revealed the presence of hydroxyapatite as the only phase present. HA powders showed phase transformation after heat treatment at 1100°C, exhibiting 3 phases (58.63% HA, 41.17% whitlockite and 0.3% β-TCP), while the Ag-HA decomposition at 1100°C showed a calcium phosphate biphasic (93.51% HA and 6.49% whitlockite), thus suggesting a higher thermal stability of Ag-HA when compared to HA. Both HA and Ag-HA powders demonstrated an increase of their crystallinity, proportional to the increase of the heat treatment temperature, which can be supported by the higher intensity of the diffraction patterns at 1100°C.
The HA and Ag-HA diffraction patterns were refined by Rietveld refinement method for crystallite size, crystal density and lattice parameter Table 1. In the present study, the ionic substitution of Ca2+ by Ag+ in the HA crystalline structure was confirmed by an alteration in the HA lattice parameter. Previous studies [11,12] reported that the addition of 0.1% Ag+ lead to the thermal stability of material, in accordance with the XRD results of the present study. In addition, with the increase in the temperature to 1100°C, a crystallite growth was verified in Ag-HA. However, no change in the density of the HA structure was observed.
| HA | HA 1100ºC | Ag-HA | Ag-HA 1100ºC | |
|---|---|---|---|---|
| a | 9,43306 Å | 9,41728Å | 9,50382 Å | 9,42350 Å |
| b | 18,73321 Å | 18,85308Å | 18,72082 Å | 18,84116Å |
| c | 6,87299 Å | 6,88682 Å | 6,88035 Å | 6,88758 Å |
| ρ | 3,14 g/cm3 | 3,15g/cm3 | 3,14g/cm3 | 3,16g/cm3 |
| Crystallite size | 179,09 nm | 227,38 nm |
Table 1: The lattice parameters, crystal density and crystallite size of HA and Ag-HA.
FEG-SEM and chemical characterization
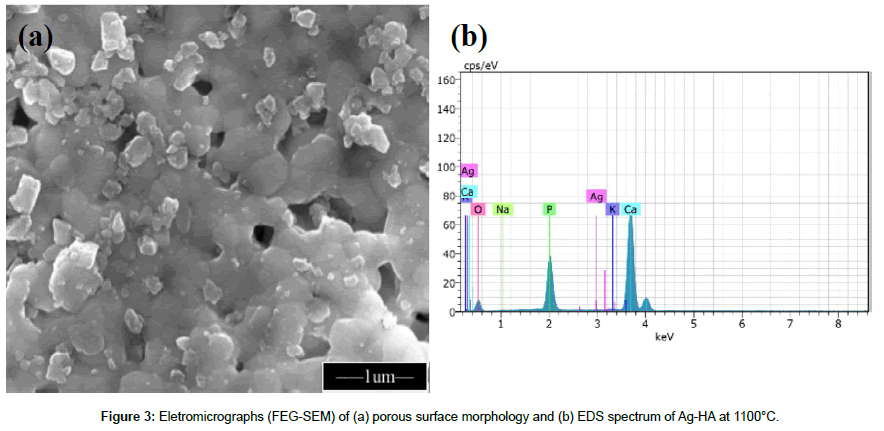
Figure 3 shows surface morphology of HA and Ag-HA sintered ceramic pellets with microporosity, heat-treated at 1100°C. Due to small amount of Ag in the synthesis [0.13 mol% of Ag (mol Ca: Ag)], the EDS results did not show the presence of Ag ions.
Therefore, quantitative chemical analysis by XRF and XPS confirmed the presence of Ag element in the pellets. In the Table 2, XRF analysis revealed that the concentration of Ag in the Ag-HA samples was lower than that added in the synthesis, 0.13%. In the present study, the calculated Ca/P molar ratio was 1.57, exhibiting a different Ca/P molar ratio from that of the stoichiometric value of HA (1.67). This obtained Ca-deficient HA indicates the influence of Ag ions into HA structure, supporting the Rietveld refinement results.
| Elements | Percentage (%) |
|---|---|
| P | 19.20 |
| Ca | 39.10 |
| Ag | 0.064 |
Table 2: Chemical analysis by X-ray fluorescence of Ag-HA.
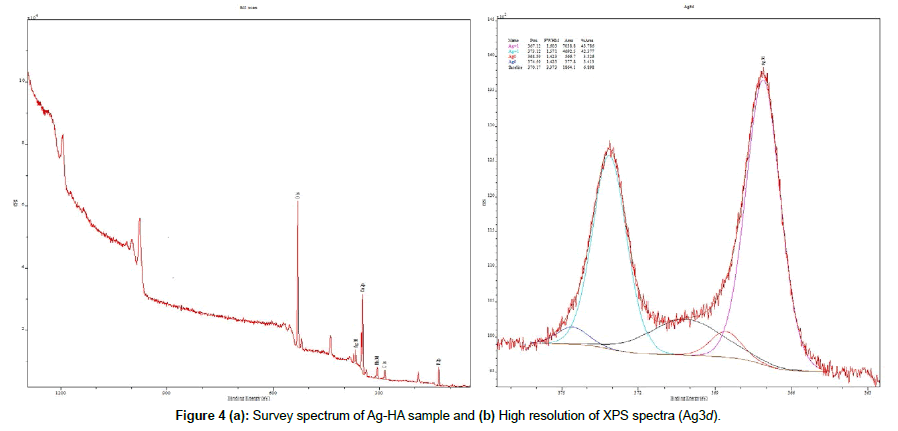
Figure 4 shows the XPS spectrum of Ag-HA sample, where the Ag, Ca, P and O elements were detected through the peaks: Ca2p (347.3 eV), P2p (133.09 eV), O1s (532.1 eV), and the doublet Ag3d5/2 (367.12 eV) and Ag3d3/2 (373.12 eV). Based on the deconvolution analysis, Figure 4(b), it is possible to observe the ejected-electrons spectrum from Ag d orbitals, which a percentage area of 86.2% in Ag1+ (Ag3d5/2=367.12 eV and Ag3d3/2=373.12 eV, Δ=6 eV) and 6.9% Ag0 (Ag3d5/2=368.59 eV and Ag3d3/2=374.59 eV, Δ=6 eV). Thus, the presence of Ag ion in two oxidation states in the Ag-HA powder composition was determined.
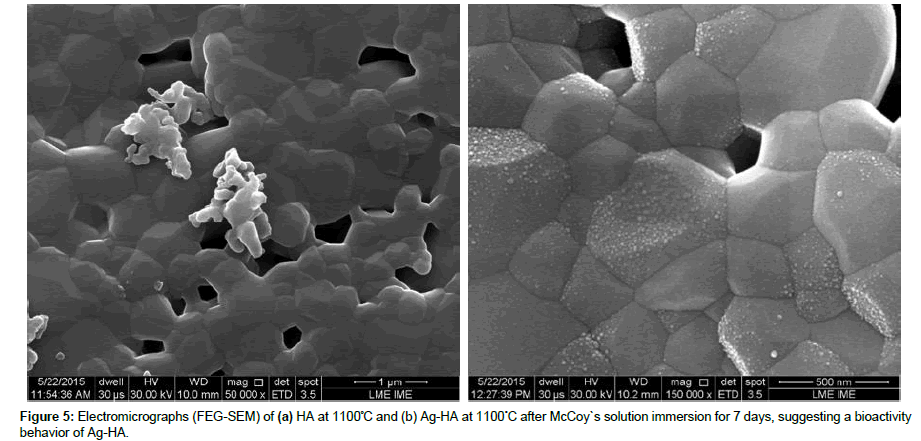
FEG-SEM analysis of samples after 7 days immersed in McCoy culture indicated a nanoprecipitation on the surface of the Ag-HA samples heat treated at 1100°C, Figure 5(b), suggesting a bioactivity behavior of Ag-HA. In this study, the heat treatment temperature of HA at 1100°C lead to a decrease in the apatite precipitation, which is related to the decrease of dissolution rate for a biomaterial in a simulate body fluid, even with the HA phase decomposition observed by XRD analysis. HA pellets did not show bone-like apatite, characteristic of bioactivity assays; however, an indication of incipient surface dissolution can be verified, Figure 5(a).
Although the heat treatment of Ag-HA at 1100°C lead to a biphasic decomposition and higher thermal stability of HA, the presence of Ag ions in the HA surface showed a positive response into McCoy culture medium, suggesting a bioactivity behavior of hydroxyapatite doped with silver [0.13 mol% of Ag (mol Ca: Ag)].
Conclusion
Hydroxyapatite doped with silver (Ag-HA) was successfully synthetized by a chemical wet method and the ionic substitution of Ca2+ by Ag+ into HA crystalline structure was confirmed by an alteration in the HA lattice parameter.
XRF analysis confirmed the presence of Ag element into Ag-HA, supporting the XRD results.
McCoy’s 5A medium showed to be effective as a SBF medium for bioactivity tests on HA and Ag-HA. Ag-HA immersed in McCoy cell culture medium after 7 days showed an early bone-like apatite precipitation, in comparison with an incipient dissolution process of HA samples.
Acknowledgements
The authors declare no conflict of interest and would like to thank IME, CBPF, CAPES and R-Crio S.A. for the support and collaboration.
References
- Hench LL (1991) Bioceramics: From Concept to Clinic. J Am Ceram Soc 74: 1487-1510
- Mavropoulos E, Costa AM ,Costa LT, Achete CA, Mello A, et al. (2011) Colloids and Surfaces B: Biointerfaces. 83: 1-9.
- Navarro da Rocha D, Cruz LRO, Campos JBD, Marçal LRSB, Mijares DQ, et al. (2017) Mg substituted apatite coating from alkali conversion of acidic calcium phosphate. Materials Science and Engineering: C. 70: 408-417.
- Kokubo T, Matsushita T, Takadama H, Kizuki T (2009) J Eur Ceram Soc. 29: 1267-1274.
- Kokubo T, Takadama H (2006) How useful is SBF in predicting in vivo bone bioactivity? Biomaterials 27: 2907–2915.
- Halliwell B (2008) Review Are polyphenols antioxidants or pro-oxidants? What do we learn from cell culture and in vivo studies? Arch Biochem Biophys 476: 107-112.
- Saravanan S, Nethala S, Pattnaik S, Tripathi A, Moorthi A, et al. (2011) Preparation, characterization and antimicrobial activity of a bio-composite scaffold containing chitosan/nano-hydroxyapatite/nano-silver for bone tissue engineering. Int J Biol Macromol 49: 188-193.
- Marques da Silva H, Mateescu M, Ponche A, Damia C, Champion E, et al. (2010) Surface transformation of silicon-doped hydroxyapatite immersed in culture medium under dynamic and static conditions. Colloids Surf B: Biointerfaces 75: 349-355.
- Rohanová D, Horkavcová D, Helebrant A, Boccaccini AR (2016) Assessment of in vitro testing approaches for bioactive inorganic materials. J Non-Cryst Solids 432: 53-59.
- Navarro da Rocha D, Gobbo LA, Prado da Silva MH (2013) Production and characterization of niobate apatite. J Mater Res Technol 2: 24-29.
- Stanic V, Janackovic D, Dimtrisevic S, Tanaskovic SB, Mitric M, et al. (2011) Synthesis of antimicrobial monophase- silver doped hydroxyapatite nanopowders for bone tissue engeneering. Appl Surf Sci 257: 4510-4518.
- Santos IMG, Santos SC, Santos EA, Cunha FG, Rezende CX (2011) Zinc and Silver containing hydroxyapatite for applying as antibacterial bone grafts. X Encontro Anual da Sociedade Brasileira de Pesquisa em Materiais (SBPmat).
 Spanish
Spanish  Chinese
Chinese  Russian
Russian  German
German  French
French  Japanese
Japanese  Portuguese
Portuguese  Hindi
Hindi