Research Article, J Nephrol Ren Dis Vol: 1 Issue: 1
Calcific Uremic Arteriolopathy: Mortality Outcomes with and without Sodium Thiosulfate Therapy
Chamberlain I Obialo1* and Alexander Quarshie2
1Renal Section, Department of Internal Medicine, Atlanta, Georgia, USA
2Clinical Research Center, Morehouse School of Medicine, Atlanta, GA, USA
*Corresponding author: Chamberlain I Obialo
Morehouse School of Medicine, Atlanta, Georgia, USA
Tel: (404)756-1320
Fax: (404)756-1313
E-mail: cobialo@msm.edu
Received: February 08, 2017 Accepted: February 10, 2017 Published: February 16, 2017
Citation: Obialo C, Quarshie A (2017) Calcific Uremic Arteriolopathy: Mortality Outcomes with and without Sodium Thiosulfate Therapy. J Nephrol Ren Dis 1:1.
Abstract
Calcific uremic arteriolopathy (CUA) is commonly seen in patients with end stage kidney disease (ESKD) and carries a high mortality risk. Treatment with intravenous sodium thiosulfate (STS) is beneficial but its mortality advantage over therapy without STS is unknown.
We retrospectively reviewed our ESKD patient's records over a 10-year period and identified 45 biopsy confirmed cases of CUA. Associations between patients who received STS therapy and those who did not and various categorical end points were assessed using the chi-squared tests while differences in continuous end points were examined using Mann-Whitney- U tests. Multivariate logistic regression (MLR) models were used to evaluate associations between mortality and several clinical variables. Survival analysis utilized Kaplan-Meier plots.
The mean age of the 45 cases was 63, 60% female, mean body mass index was 34 and mean dialysis vintage was 4yrs. Of the 45 cases, 23 (51%) received STS while 22 (49%) did not. The mean level of serum albumin, phosphorus and parathyroid hormone was 2.8 g/dl, 6.7 mg/dl and 989 pg/dl respectively. One –year mortality was higher in patients with proximal than those with distal lesions (48% vs. 20%, p = 0.05). Overall mortality was 26% in patients who received STS vs. 59% in those who could not receive STS (p = 0.03). Patients who did not receive STS were also more likely to have major surgeries than those who did, 86% vs. 52%, p = 0.01. Adjusted MLR analysis showed a significant association between mortality and: serum phosphorus, Odds Ratio (OR) 3.4, p = 0.03; proximal skin lesions, OR 8.4, p = 0.05; and severe lesions, OR 17.0, p =0.03.
Sodium thiosulfate therapy may confer a survival advantage over no STS. We encourage hospitals and dialysis companies to procure and make this agent more available to physicians.
Keywords: Sodium thiosulfate; Mortality; Calcific Uremic Arteriolopathy;Hemodialysis
Introduction
Calcific Uremic Arteriolopathy (CUA) also known as Calciphylaxis is an uncommon disorder seen mainly in patients with end stage kidney disease (ESKD) or post kidney transplantation [1-3]. It frequently develops as an extremely painful skin nodule or plaque that often progresses to cutaneous ulceration and necrosis.
The pathobiology of this condition is poorly understood but is thought to be related to vascular and soft tissue calcifications seen in ESKD [4]. The injury to vascular endothelium results in cutaneous arteriolar narrowing and hypercoagulable state that ultimately results in tissue infarction which is clinically observed in CUA [5].
Untreated, the mortality associated with this condition could range from 50 -80% [2,4,5] and is often a result of overwhelming local or systemic infection. There is no definitive therapy for CUA although earlier studies had advocated parathyroidectomy as the only curative intervention [6,7]. In a meta-analysis by Hafner et al [6] parathyroidectomy was associated with 65% survival compared to 35% in patients who did not undergo parathyroidectomy. However, a multi-interventional therapeutic approach rather than a single therapy has been found to be currently more effective [8,9].
Recently, promising results have been observed in patients treated with intravenous sodium thiosulfate (STS) [9-15]. The beneficial effects include pain control and wound healing, however there is no randomized trial on the survival advantage of therapy with STS. This agent is often unavailable at the hospitals affiliated with our institution hence some of our patients were able to receive STS while others could not. In this paper, we explored the survival advantage of therapy with STS in our ESKD patients with CUA. Patients who did not receive STS served as control. In addition, we also examined the relationships between mortality and various clinical variables.
Materials and Methods
This is a retrospective review of our cases of biopsy confirmed CUA over a 10 year period, 2003-2013. We started by reviewing the electronic health records (EHR) of our entire ESKD population during the study period and collated all cases with upper and lower extremity wounds, abdominal or gluteal lesions, peripheral vessel disease, skin ulcerations, skin or genital necrosis. A total of 80 cases were initially identified, but only 45 of these had biopsy confirmed CUA. In depth review of the hospital EHR of these 45 cases were then performed.
All our ESKD patients are often hospitalized at three different hospitals in the metropolitan Atlanta, Georgia area. Sodium thiosulfate was infrequently available at these hospitals; hence some patients were able to receive STS while others could not. Even when available, the duration of therapy was variable. Most patients received 25g of intravenous STS for between 8 to 32 weeks depending on its availability. Lesions located on the trunks, buttocks, above the elbows and knees were classified as proximal while genital and lesions located below the knees and elbows were classified as distal. The severity of skin lesions was classified according to the staging methods reported by Zitt et al [14]: Mild lesions were characterized by pain, erythema, induration, plaques or livido reticularis; moderate lesions had bullae, small ulcerations, or circumscribed necrosis; severe lesions had extensive or deep penetrating ulcerations, large necrotic skin, eschars or infected lesions. Patients who had extensive or repetitive wound debridement with application of wound vacuums, limb amputations or revascularizations were considered to have had major surgeries. Minor debridement did not constitute major surgery.
Statistical Analysis
Continuous data are summarized as mean ± SD where applicable and categorical data as percentages. Relationships between continuous data and STS therapy status were assessed using Students t-test or Mann-Whitney-U tests where applicable while associations between patients who received STS therapy and those who did not and various categorical end points were assessed using Pearson Chi-squared tests. Multivariate logistic regression models were fitted to examine mortality associations, adjusted for multiple confounders. Survival analysis utilized with Kaplan-Meier methods with censoring at 24 months. Software SAS version 9.2 (SAS Institute, Cary, NC, USA) was used for all analyses. The study was approved by our institutional IRB and conducted in accordance with the Helsinki Declaration 1975 (as revised in 1983).
Results
Table 1 shows that most of the patients were female and obese with a mean body mass index (BMI) of 34. They have also been on HD for about 4 years. They all had low serum albumin, hyperphosphatemia and hyperparathyroidism. The serum phosphate levels were significantly higher in patients who did not receive STS. Major surgical interventions were significantly more common in patients who could not receive STS while one year mortality was also higher in patients who did not receive STS. The duration of STS therapy ranged from 8 to 32 weeks. However, the duration of therapy had no impact on survival. The mean duration of survival was greater in patients who received STS for 8 weeks than in those treated for over 8 weeks (21 ± 8 months vs. 18 ± 10 months, respectively, p = 0.5).
| Variables | All Patients | STS | No STS | P Value |
|---|---|---|---|---|
| N | 45 | 23 | 22 | |
| Age (yr.) | 63 ± 10 | 62 ± 10 | 65 ± 10 | 0.3 |
| Female (%) | 60 | 61 | 59 | 0.9 |
| BMI | 34 ± 5 | 35 ± 5 | 33 ± 5 | 0.3 |
| Diabetes Mellitus (%) | 64 | 74 | 55 | 0.2 |
| HD Vintage (yr.) | 4.4 ± 2.5 | 4.7± 2.8 | 4.2± 2.2 | 0.1 |
| Warfarin Rx (%) | 49 | 57 | 41 | 0.4 |
| Serum Albumin (g/dL) | 2.8 ± 0.7 | 2.9 ± 0.7 | 2.7 ± 0.7 | 0.2 |
| Serum Calcium (mg/dl) | 9.2 ± 0.9 | 9.3 ± 0.9 | 9.0 ± 0.9 | 0.2 |
| Serum Phosphorus (mg/dl) | 6.7 ± 1.2 | 6.4 ± 1.1 | 7.1± 1.2 | 0.05 |
| Serum PTH (pg/dl)* | 820 | 900 | 640 | 0.7 |
| Major Surgery (%) | 69 | 52 | 86 | 0.01 |
| One Year Mortality (%) | 36 | 22 | 50 | 0.05 |
Note: STS: Sodium Thiosulfate Therapy; HD: Hemodialysis; BMI: Body Mass Index
*Represents median value
Table 1: Demography and other characteristics.
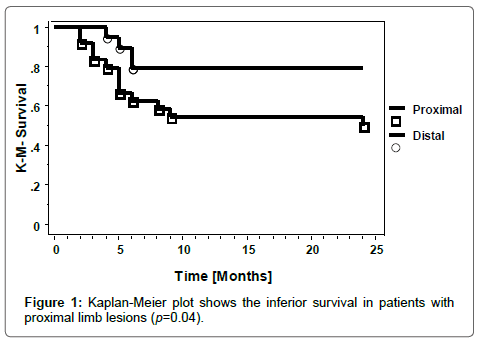
Differences between patients with proximal and distal lesions are shown in Table 2. Patients with proximal lesions were more likely to be bigger, had worse outcomes and a tendency to have more major surgical interventions. The survival plot in relation to site of lesion is shown in Figure 1.
| Variables | All Patients | Proximal | Distal | P Value |
|---|---|---|---|---|
| N | 45 | 25 | 20 | |
| Age (year) | 64± 10 | 64± 10 | 63 ± 9 | 0.5 |
| Female (%) | 60 | 60 | 60 | 0.9 |
| BMI | 34 ± 5 | 35 ± 5 | 34 ± 6 | 0.6 |
| HD Vintage (year) | 4.4 ± 2.5 | 4.2 ± 2 | 4.6 ± 3 | 0.7 |
| Major Surgery (%) | 69 | 76 | 60 | 0.3 |
| One Year Mortality (%) | 36 | 48 | 20 | 0.05 |
Table 2: Relationship between site of lesions and other characteristics.
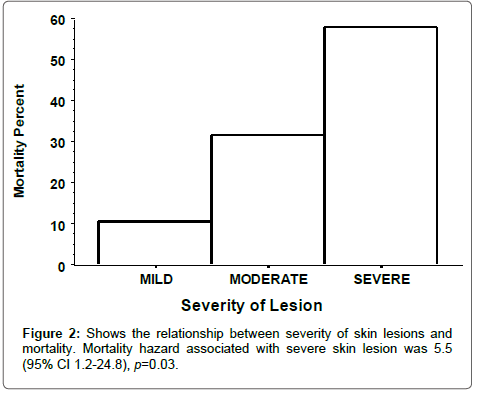
The severity of the skin lesions was a major determinant of mortality (Figure 2). Of the deceased patients, 11 of 19 (58%) had severe lesions, 6 of 19 (32%) had moderate lesions while only 2 of 19 (10%) had mild skin lesions, p = 0.003. The hazard risk associated with severe skin lesions was 5.5 (95% CI 1.2-24.8), p = 0.03. About one third of the deceased patients 6 of 19 (32%) were receiving Cinacalcet at the time of diagnosis while 6 of 26 (23%) patients who survived were also on Cinacalcet. Hence, there was no association between Cinacalcet use and survival, OR 1.3 (95% CI 0.09-17.0), p = 0.7
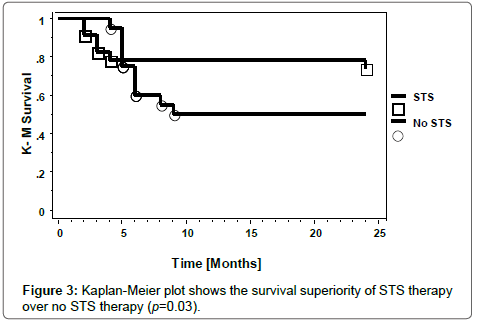
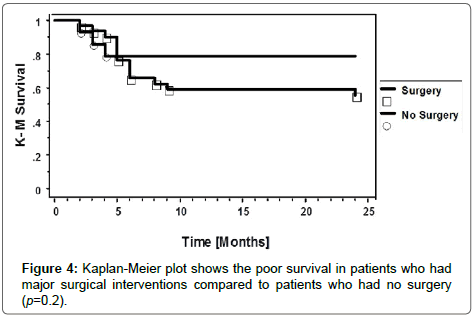
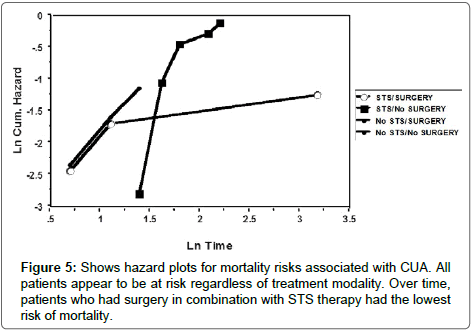
The overall survival of the patients over the 2- year censor period was significantly superior in the recipients of STS than in those who did not receive STS. (Figure 3; p = 0.03). Univariate regression model showed a significant association between mortality and STS treatment, OR 0.2 (95% CI 0.1-0.8), p = 0.02. However, in a multivariate logistic regression model with adjustment for multiple confounders, only serum phosphate, site and severity of skin lesions showed a significant association with a 2-yr mortality. Age, gender, presence of diabetes, BMI, STS treatment, serum albumin, calcium, and PTH levels had no association with mortality at 2 years (Table 3). Patients who had extensive or major surgery had a worse long term outcome than those who did not. (Figure 4; p = 0.2). The high mortality risks associated with CUA is depicted in Figure 5. It shows the inherent high mortality risk present in all patients regardless of therapy with or without STS in combination with or without major surgery. However, the mortality risk was greatest in patients who had surgery but did not receive STS.
| Variables | OR (95% CI) | P value |
|---|---|---|
| Serum Phosphorus | 3.4 (1.1-10.5) | 0.03 |
| Proximal Skin Lesions | 8.4 (1.0-71.5) | 0.05 |
| Severe Skin Lesions | 17.0 (1.3-221.9) | 0.03 |
Table 3: Adjusted multivariate logistic regression showing the association between mortality and select variables.
Discussion
Some of the reported risk factors for CUA include: female gender [1,2,12]; long vintage on hemodialysis [16]; obesity with large BMI over 30 [1,17]; and hypoalbuminemia [13,18]. In concordance with these studies, our study confirms these associations. Although bone mineral disorders, hyperparathyroidism, increasing use of calcium based phosphate binders and vitamin D or their analogues have been blamed for the rising incidence of CUA [2], we could not show an association with the use of either statins or calcitriol as reported in one study [16], (data not shown).
In agreement with other published reports, we also observed that our patients with proximal limb lesions were more obese and had worse outcomes than those with distal limb lesions [4-6,13]. It has been suggested that larger proportion of adipose tissue present in the proximal limbs may predispose to relative hypo perfusion of the skin and subcutaneous tissue [4]. Relative tissue adiposity could result in increased tensile stress on the connective tissue septa from skin to the deep fascia and ultimately on the arterioles with resultant cutaneous hypo perfusion and ischemic necrosis [19]. Other reported mortality risk factors include: older age, CRP, and severity of the skin lesions [14]. We observed a significant association between mortality and severity of the skin lesions and in addition, also noted a significant association between 2-year mortality and serum phosphorus level. Elevated phosphorus level is a known independent risk factor cardiovascular disease and mortality [20,21]. The association between serum phosphate level and mortality in both ESKD [20] and pre-dialysis chronic kidney disease subjects [21] has been well documented.
The parathyroid hormone (PTH) levels were very high in all of our patients with CUA. Some reports have indicated that for PTH levels over 300, parathyroidectomy may be associated with rapid resolution of CUA and improved survival [6,7]. None of our cases had parathyroidectomy as most were placed a calcimimetic agent “Cinacalcet”. Some authors successfully used Cinacalcet in one case and suggested that this agent could serve as an alternative to parathyroidectomy [22]. We were unable to show an association between Cinacalcet use and survival. However, only one quarter of our patients was on this medication.
Over 40% of our CUA cases were on warfarin, but we were unable to show if this therapy contributed to the risk of CUA since there were no controls. It has been reported that warfarin may increase the risk of CUA through its effect on extracellular matrix gla-protein (MGP) [4,13,23]. This protein (MGP) is an inhibitor of extrinsic calcification whose activity depends on vitamin K-dependent carboxylation. Warfarin inhibits K-dependent carboxylation of MGP, hence may predispose to vascular calcification. Indeed, one report has described a case of CUA that resolved after cessation of warfarin [24]. A recent publication by Nigwekar et al noted that dialysis patients with CUA had a relatively lower carboxylated MGP (cMGP) concentration than those patients without CUA [25]. The authors concluded that vitamin
K deficiency mediated by reduction in relative cMGP concentration may have a role in the pathogenesis of calciphylaxis.
Local wound care with cautious debridement has been shown to improve survival in CUA [26,27]. In one study, one-year survival was 61.6% in patients who had surgical debridement compared to 27.4% in those who did not. However, aggressive debridement may expose subcutaneous tissue to increased risk of sepsis [27]. We observed that aggressive wound debridement, limb amputations and revascularization did not improve survival in our patients. Limb revascularization also failed to improve outcome in a recent case report [28]. There was a tendency towards more aggressive surgical interventions in our patients who could not receive STS.
The beneficial effects of STS therapy have been well documented [9-16] but we are unaware of any study that has demonstrated a survival advantage of therapy with STS versus no STS therapy for CUA. Mortality associated with CUA is high and could range from 50-80% [9,14,15,26]. A recent study noted a one -year mortality of 35% in treated patients, but could not show the mortality in untreated patients because of the design of the study [16]. Our overall mortality was 36% while the one-year mortality was 26% in those who received STS versus 48% in patients who did not receive STS. Thus, a beneficial effect on survival. A case report involving 14 patients reported improvement in pain and skin lesions by STS but no impact on mortality compared to historical patients [15]. However, that study only included patients who received STS with no control for untreated patients. Our study is unique in that we have been able to clearly show the survival advantage of STS therapy for CUA over major surgical interventions. We were able to perform the comparison of STS therapy versus no STS therapy as a result of the infrequent availability of STS at our admitting hospitals and inability to provide STS at our outpatient dialysis facilities.
Limitations of our study include our small sample size and its retrospective nature without proper randomization. Randomization would be difficult because of the inconsistent presentation of cases and availability of STS. We were also unable to control the duration of STS therapy which ranged from 8 weeks to over 32 weeks in some patients. However, our study could not show any advantage of prolonged therapy over 8 weeks duration. The selection of only subjects with biopsy confirmed CUA may have reduced our sample size as we could have missed mild cases that were not biopsied, however, it may have also resulted in oversampling as some of the biopsied cases turned out to be other non CUA conditions such as ischemic gangrene, cutaneous vasculitis and necrotizing fasciitis. Although, the role of STS alone may be called into question, all the patients had what could be considered good standard of care despite the no- availability of STS in some patients.
We are in total agreement with the concept of a multidisciplinary therapeutic approach to CUA which has been proposed by several investigators [8,9] and do not suggest abrogation of other proven supportive care for these patients. Such supportive care should include; intravenous STS, nutritional support, judicious wound care, adequate control of hyperphosphatemia and elevated PTH levels, and use of Cinacalcet and hyperbaric oxygen when indicated.
In conclusion, this study has shown that STS therapy may confer short and long term survival benefit for CUA and the inherent mortality risks associated with major surgical interventions in CUA. It also showed that serum phosphorus, the site and severity of the skin lesions are major determinants of long term survival. As a result of this survival advantage of STS therapy, we encourage hospitals and dialysis companies to acquire this agent and make it readily available to renal physicians. It is of note that over the last 12 months STS has not been available at any of the 3 hospitals involved in this study.
Conflict of Interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Acknowledgment
This study was supported in part by Grant # 8 U54 MDOO7588, NIMHD awarded to the Clinical Research Center at Morehouse School of Medicine. We thank Ms. Peggie Turner for her administrative support.
References
- Janigan DT, Hirsch DJ, Klassen GA, MacDonald AS(2000) Calcified subcutaneous arterioles with infarcts of the subcutis and skin (“Calciphylaxis”) in chronic renal failure. Am J Kidney Dis 35: 588-597.
- Fine A, Zacharias J (2002) Calciphylaxis is usually non-ulcerating: Risk factors, outcome and therapy. Kidney Int 61: 2210-2217.
- Perloff LJ, Spence RK, Grossman RA, Barker CF (1979) Lethal post-transplantation calcinosis.Transplant 27:21-25.
- Mazhar AR, Johnson RJ, Gillen D, Stivelman JC, Ryan MJ, et al. (2001) Risk factors and mortality associated with calciphylaxis in end stage renal disease. Kidney Int 60: 324-332.
- Weenig RH (2008) Pathogenesis of calciphylaxis: Hans Selye to Nuclear Factor Æ™-B. J Am Acad Dermatol 58:458-471.
- Hafner J, Keusch G, Wahl C, Sauter B, Hürlimann A, et al. (1995) Uremic small artery disease with medial calcification and intimal hyperplasia (so called calciphylaxis): A complication of chronic renal failure and benefit from parathyroidectomy. J Am Acad Dermatol 33: 954-962.
- Girotto JA, Harmon JW, Ratner LE, Nicol TL, Wong L, et al. (2001) Parathyroidectomy promotes wound healing and prolongs survival in patients with calciphylaxis from secondary hyperparathyroidism. Surg 130:645-651.
- Baldwin C, Farah M, Leung M, Taylor P, Werb R, et al. (2011) Multi-intervention management of calciphylaxis: A report of 7 cases. Am J Kidney Dis 58: 988-991.
- Cicone JS, Petronis JB, Embert CD, Spector DA (2004) Successful treatment of calciphylaxis with intravenous sodium thiosulfate. Am J Kidney Dis 43: 1104-1108.
- Brucculeri M, Cheigh J, Bauer G, Serur D (2005) Long-term intravenous sodium thiosulfate in the treatment of a patient with calciphylaxis. Semin Dial 18: 431-433.
- Araya CE, Fennell RS, Neiberger RE, Dharnidharka VR(2006) Sodium thiosulfate treatment for calcific uremic arteriolopathy in children and young adults. Clin J Am Soc Nephrol 1: 1161-1166.
- Nigwekar SU, Bhan I, Turchin A, Skentzos SC, Hajhosseiny R, et al. (2013) Statin use and calcific uremic arteriolopathy: A matched case – control study. Am J Nephrol 37: 325-332.
- Nigwekar SU, Brunelli SM, Meade D, Wang W, Hymes J, et al. (2013) Sodium thiosulfate therapy for calcific uremic arteriolopathy. Clin J Am Soc Nephrol 8: 1162-1170.
- Bleyer AJ, Choi M, Igwemezie B, de la Torre E, White WL (1998) A case control study of proximal calciphylaxis. Am J Kidney Dis 32: 376-383.
- Hayashi M, Takamatsu I, Kanno Y, Yoshida T, Abe T, et al. (2012) Japanese Calciphylaxis Study Group. A case control study of calciphylaxis in Japanese end-stage renal disease patients. Nephrol Dial Transplant 27:1580-1584.
- Oh DH, Eulau D, Tokugawa DA, McGuire JS, Kohler S (1999) Five cases of calciphylaxis and a review of the literature. J Am Acad Dermatol 40: 979-987.
- Velasco N, MacGregor MS, Innes A, MacKay IG (2006) Successful treatment of calciphylaxis with Cinacalcet – an alternative to parathyroidectomy. Nephrol Dial Transpl 21: 1999-2004.
- Coates T, Kirkland GS, Dymock RB, Murphy BF, Brealey JK, et al. (1998) Cutaneous necrosis from calcific uremic arteriolopathy. Am J Kidney Dis 32: 384-391.
- Banerjee C, Woller SC, Holm JR, Stevens SM, Lahey MJ (2010) Atypical calciphylaxis in a patient receiving warfarin then resolving with cessation of warfarin and application of hyperbaric oxygen therapy. Clin Appl Thromb Hemost 16: 345-350.
- Nigwekar SU, Bloch DB, Nazarian RM et al. (2017) Vitamin K – Dependent carboxylation of Matrix Gla Protein influences the risk of calciphylaxis. J Am Soc Nephrol 28.
- Kang AS, McCarthy JT, Rowland C, Farley DR, van Heerden JA (2000) Is calciphylaxis best treated surgically or medically? Surgery 128: 967-972.
- Weenig RH, Sewell LD, Davis MD, McCarthy JT, Pittelkow MR (2007) Calciphylaxis:Natural history, risk factor analysis and outcome. J Am Acad Dermatol 56: 569-579.
- Friedman SG (2002) Leg revascularization in patients with calciphylaxis. Am Surg 68:591-592.
 Spanish
Spanish  Chinese
Chinese  Russian
Russian  German
German  French
French  Japanese
Japanese  Portuguese
Portuguese  Hindi
Hindi