Research Article, Biomater Med Appl Vol: 1 Issue: 2
Clinical Performance of Moldable Bioceramics and Resorbable Membrane for Bone and Mucosa Regeneration in Maxillofacial Surgery
Daculsi Guy1, Seris Elodie2,3, Christian Verner4,5 and Said Kimakhe4,5
1INSERM, UMR 1229, Regenerative Medicine and Skeleton (RMeS), Université de Nantes, ONIRIS, 44042 Nantes, France
2BIOMATLANTE SA, rue E. Belin, 44360, France
3CIC INSERM BioDiMI, CHU and Bordeaux University, France
4UFR Odontologie, Université de Nantes, 44042 Nantes, France
5CHU Nantes, PHU 4 OTONN, 44093 Nantes, France
*Corresponding Author : Daculsi Guy
INSERM, UMR 1229, Regenerative Medicine and Skeleton (RMeS), Université de Nantes, France
Tel : 33 (0)240 41 29 15
E-mail: guy.daculsi@univ-nantes.fr
Received: October 13, 2017 Accepted: November 15, 2017 Published: November 22, 2017
Citation: Guy D, Elodie S, Verner C, Kimakhe S (2017) Clinical Performance of Moldable Bioceramics and Resorbable Membrane for Bone and Mucosa Regeneration in Maxillofacial Surgery. Biomater Med Appl 1:2.
Abstract
The objectives are to present the novel concept of moldable injectable osteogenic CaP bioceramic, and the data issued from a non-interventional study to demonstrate the efficacy of an injectable bone substitute (bioceramic/hydrogel) in association with a collagen resorbable membrane and the performance to guide bone regeneration and soft tissue healing in maxillofacial surgery. Historically, dental implantology required both bone augmentation and reconstruction. However, with the aging of the population, it is also necessary to prevent the bone loss after dental extraction. To overcome the limitations associated with autografts, several bone substitutes have been developed. However, there are still numerous difficulties in the handling, reproducibility of the resorption, and bone regeneration associated with the biomaterials. Therefore, it is important to optimize new bone substitute technologies. Bioactive injectable bioceramics, particularly putties, are often used in these applications. In this preclinical and clinical study, we demonstrate the efficacy and the performance of a combined use of calcium phosphate microporous granules and hydrosoluble polymers for maxillofacial bone reconstruction and regeneration. The biomaterial was extruded into bone defects and recovered by a
resorbable porcine collagen membrane, after tooth extractions in 78 patients. The clinical follow-up revealed no incidents or complications. Furthermore, infections or foreign body reactions were not reported during the 17 months of follow-up. We analyzed the drilling waste taken from 11 patients during the dental implantation sequence (at 5-10 months). Resorption and bone ingrowth were assessed using microtomography, scanning electron microscopy, light microscopy, and image analysis. We found that the highly kinetic process of resorption/absorption and bone in growth was achieved within a few months. The bone ingrowth was architectured and well vascularized.
Keywords: Putty; Bioceramics; Resorbable membrane; Clinical cases; Bone Regeneration
Introduction
There are numerous clinical indications for bone grafts. The ideal graft material should favor bone apposition and growth while simultaneously being degraded by body fluids and cells. Ultimately, the material should be replaced by mature bone tissue within a healing period of weeks. Because autologous and allogeneic bone grafts fulfill some of these requirements, the clinicians, routinely use biological materials. However, biological materials have intrinsic limitations. Harvesting autologous bone requires a second surgical site, which can cause complications, the material is limited in quantity, and it may lead to immunogenic rejection or transfer certain pathogens and viruses [1-3]. For these reasons, researchers and clinicians have developed synthetic bone substitutes. Our approach has focused on composite biomaterials that combine bioceramics with hydrogels to replace and regenerate bone tissue in osseous defects.
In 1920, Albee reported the first successful use of a calcium phosphate (CaP) reagent for the repair of a bone defect in a human [4]. Then, largely through the separate efforts of Jarcho, de Groot, Aoki, and LeGeros [5-8], synthetic hydroxyapatite (HA) and β-tricalcium phosphate (TCP) became commercially available as bone substitute materials for dental and orthopedic applications. From these initial studies on bioceramics, controversy developed concerning the resorption and clinical efficiency of these materials from the pre-clinical and first clinical reports. This controversy arose from the initial characterization of bone deficiency: often only the Ca / P ratio was considered, and a complete crystallographic analysis was not performed. Crystallographic analyses are essential to identify the ionic content of bone substitute materials, such as the carbonates, magnesium, and other molecules, which can strongly influence their resorption, interaction with cells, and biocompatibility.
More than 50 years after Albee’s report, the clinical use of a TCP preparation in surgically created periodontal defects in animals was reported by Nery et al. [9]. The term BCP (biphasic calcium phosphate) was first used by Ellinger et al. [10] to describe the bioceramic previously known as TCP by Nery et al. in 1975 [11]. However, it was later shown that the bioceramic consisted of a mixture of 80% HA and 20% β-TCP using chemical and crystallographic analyses [12].
Despite performing less well than autologous bone graft materials, the development of BCP and other related bioceramics was supported by a considerable clinical need for synthetic bone substitutes. While biological bone grafts exhibit osteoinduction, osteogenesis, osteoconduction, and resorbability, they still have the afore mentioned drawbacks. By contrast, synthetic bioceramics are available in large quantities and are safe materials. However, the most attractive feature of bioactive bone graft materials such as BCP ceramic is their ability to form a direct bond with the host bone, resulting in a strong interface compared to bioinert or biotolerant materials that form a fibrous interface [13].
Bioceramics have open macropores that range in size from 100– 600 μm and micropores from 0.1–10 μm, which allow the penetration of body fluids, cells, and tissues and promote vascularization. BCP ceramics are based on an optimum balance between the more stable phase (HA) and the more soluble phase (β-TCP), this close association of HA and TCP phase are not just a mechanical mixture, is a nano scale combination at the molecular level obtained during the synthesis. These bioceramics are soluble and gradually dissolve in vivo, seeding new bone formation as they release calcium and phosphate ions into the biological medium. The formation of a dynamic interface between the bioactive ceramic and host bone is believed to result from a sequence of events involving interactions between the cells and the formation of carbonate HA (similar to bone mineral) through dissolution/precipitation processes [14], these processes is part of the new generation of self-healing bone substitutes.
Minimally invasive surgery (MIS) relies on the generation of injectable bone substitutes. BCP ceramics have been used in the development of these new injectable, moldable bone substitutes [15]. In’Oss™ is a substitute that consists of a combination of CaP microporous granules and hemisynthetic hydrosoluble polymer. This unique and innovative composite material is non-self-hardening, injectable, and moldable. It has previously been shown that injectable putty is well adapted for the filling of osseous defects [16,17].
The purpose of this non-interventional study was to assess the safety and performance of the bioceramic/hydrogel bone substitute in regenerative therapy following maxillofacial surgery. A clinical and X-ray follow-up was performed in the healed extraction sockets that were treated with the putty in combination with a resorbable collagen membrane to protect the implantation site after the surgery, and acts as a barrier for epithelial cells deep colonization. The histological analysis was conducted in 11 patients from the bone collected during the implantation or prosthetic preoperative step.
Materials and Methods
Device description
In’OssTM (Biomatlante SA; Vigneux-de-Bretagne, France) is a synthetic, absorbable CaP-based bone graft substitute with chemical properties similar to natural bone. The material is a biphasic ceramic composed of 60% HA and 40% β-TCP, combined with hydrogel (Hydroxy Propyl Methyl Cellulose). The putty was prepared with microporous BCP granules mixed with hydro soluble polymer and the suspension was placed in a syringe ready to use. The putty acts as a non-hardening injectable biomaterial (Figure 1), presenting moldable and rheological properties capable of ensuring in situ bonding of the mineral phase and high permeability. In’Oss™ has been CE marked and FDA approved. The characterization of the material was performed using X-ray Diffraction (XRD), Fourier Transformed Infrared spectroscopy (FTIR), and scanning electron microscopy (SEM). The three-dimensional (3D) structure of the putty was determined using microtomography (micro CT) (Skyscan 1072).
Resorbable collagen membranes were mainly used for guided tissue and bone regeneration in dental and maxillofaciale surgery. Porcine origin resorbable collagen membrane (EZCure™, Biomatlante SA) classically used in periodontal/dental surgery procedures (periodontal, dental implant, bone defect, or ridge reconstruction) to aid in wound healing post-surgery was used.
Clinical study
The clinical study was performed according to the classification “Case Analysis”, non-interventional study. The clinical centers involved in the study were from South Korea (two clinicians), France (seven clinicians), Germany (two clinicians), and Italy (nine clinicians).
The following variables were assessed during the study:
Before filling: imaging evaluation (panoramic dental and retroalveolar radiographies)
During surgery: evaluation of product stability and complications
Immediately post-filling: imaging evaluation with dental radiography (measure of the bone filling stage and bone/filling material interface)
One and three months after filling:
Clinical follow-up: safety evaluation
Imaging evaluation with dental radiography (measure of the radio-dense area at the bone/filling
material interface, defect filling, and post-filling height and volume)
Before implantation or prosthetic consultation:
Imaging evaluation (panoramic and retro alveolar radiographies)
Site valuation: implant set-up (look of the operative site/drilling feel/bone type)
Primary implant stability
The inclusion criteria for patients were: aged 18–80 years old, only a single tooth extraction required and would benefit from prosthetic reconstruction with a dental implant, in good general health as assessed by the clinician, and willingness and ability to comply with the pre- and post-operative diagnostic and clinical evaluations required by the study.
The exclusion criteria were: pregnancy, medical conditions requiring long-term use of steroids, leukocyte dysfunction or deficiency, history of bleeding disorder, and history of neoplastic disease requiring chemotherapy and/or radiation therapy to the head and neck. Patients with severe uncontrolled metabolic bone disorders, HIV, or hepatitis were excluded.
Surgical procedure
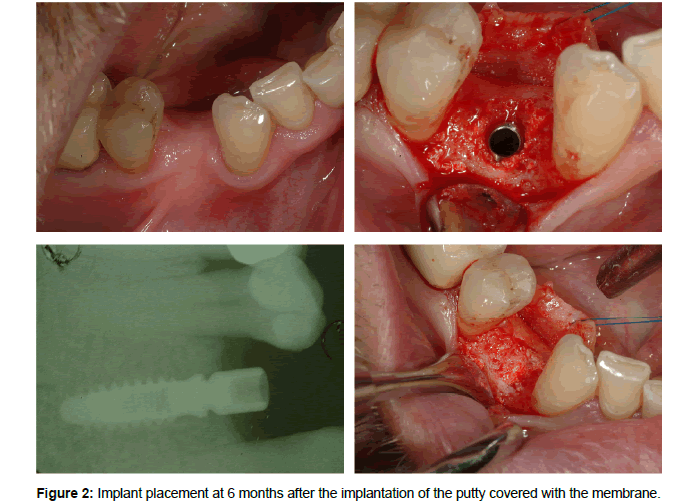
Each tooth was extracted using a traumatic conservative approach to avoid damage to adjacent structures. Tooth sockets were then filled with putty, and the surgical site was closed using the collagen membrane to preserve the alveolar ridge (Figure 2). Five to six months after the filling, a dental implant was placed (Figure 3). The patients were followed for an additional six weeks. A follow-up was performed at one and three months, recording clinical and radiological features.
Study plan
After screening and enrollment, patients underwent a single tooth extraction, followed by placement of the putty and the membrane for preservation of the alveolar ridge. After 4–9 months, a dental implant was placed. The patients were then followed for approximately six additional weeks (Table 1).
| Evaluations received | T0 | T1 (J30) | T3 (J90) |
Implantation consultation |
|---|---|---|---|---|
| Radiography | X | X | X | X |
| Product stability in the operative site | X | |||
| Clinical follow-up | X | X | ||
| Radio-dense area at bone filling material interface | X | X | X | |
| Defect filling | X | X | X | |
| Look of the operative site | X | X | ||
| Primary implant stability | X | |||
| Drilling feel of the site filled | X |
Table 1: Screening and Evaluation.
Study duration:
Period of inclusion: 12 months
Period of patient participation in the study: 7–12 months
Global period of the research: 19–24 months
The efficacy of mucosa healing using the periodontal resorbable collagen membrane for closing the site of implantation was clinically assessed at one and three months, taking into account any inflammation of the mucosa, and evaluation of the soft tissue healing (uneventful healing, wound dehiscence, membrane exposure, reddens, signs of infection, swelling).
Harvested implant analysis
The collected samples during the drilling for dental implantation were processed for micro CT and 3D qualitative imaging; quantitative image analyses of the bone ingrowth were also performed. After micro CT acquisition, the implants were embedded in polymethyl methacrylate resin, sectioned and polished, coated with gold palladium, and examined with SEM using backscattered electrons (BSE) (LEO 1450VP). Two-dimensional image analyses were performed using an image analysis system (Leica Quantimeter 5501W; Leica; Cambridge, UK). The sections were prepared with a diamond saw microtome and observed with light microscopy using polarized light. Thin sections of 7 μm were stained with Movat’s pentachrome stain and imaged using light microscopy. We focused on any residual membrane debris; normally, the resorption of the membrane is achieved within 24 weeks after surgery.
Statistical analysis
All data are expressed as averages and standard errors. Differences were evaluated using analysis of variance with Fisher’s probability least significant difference post-hoc test. Differences were considered significant when p<0.05.
Results
Before implantation, the density of BCP granules in the putty was 49% ± 2, calculated using micro CT 3D analysis. This value was used as a reference for the calculation of the percentages and putty resorption.
Human clinical cases
Seventy-eight subjects were included in this study, and 11 had bone samples collected before dental implantation. The population had an average age of 51 years [range: 23-77], and 78% were nonsmokers.
Our results verified the safety of using a putty and collagen membrane for implantation site closure in more than 93% of the cases. The patients also showed no major complications in 93% of the cases. During the first few days (maximum eight days), hematoma, swelling, or pain was recorded in only 7% of the patients. One failure was listed, which was due to gingivitis, and required reopening of the site. The post-filling follow-up of patients at one and three months indicated no inflammation or infection in more than 89% and 98% of the patients, respectively. The resorbable collagen membrane was efficient when used in combination with the putty, preventing exposure and expulsion of the granules before total healing and bone ingrowth had occurred.
At the immediate postoperative imaging follow-up, the filling rates obtained from the 52 patients with usable data indicated that 79% had 75-100% filling, 13% had 50–75% filling, 2% had 25–50% filling, and 6% had 0–25% filling. Therefore, a filling rate of >50% was found in 92% of the clinical cases.
The post-filling follow-up of patients at one and three months indicated a completely radio-dense area at the bone–biomaterial interface in 61% and 77% of the cases, respectively (for the 40 patients with usable data).
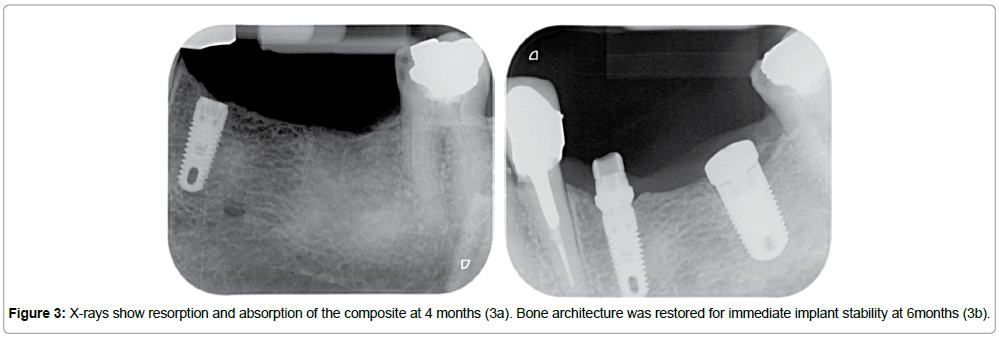
The clinical follow-up and X-ray examinations revealed resorption and a change in the radio-opacity of the material, which increased over time from bone regeneration and bone mineralization at the expense of the putty. The X-rays showed a large amount of resorption and absorption of the composite after only four months. Interconnected bone trabeculae were progressively found to replace the biomaterials (Figures 3a, 3b). No residue of the porcine collagene membrane can be observed, soft tissue regeneration was observed with normal mucosa.
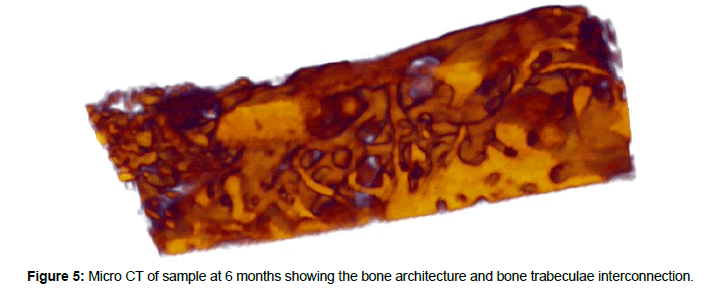
During the implantation drilling, the bone density was similar to natural bone trabeculae, and the implant stability was perfect in more than 90% of the cases. The density of this bone and its lamellar architecture was still present at six months using SEM. After six months, an increase in bone remodeling was observed, largely along the Haversian system (Figure 4). Using 3D micro CT reconstruction of the samples, the bone architecture between the few residual granules of bioceramic was revealed (Figure 5).
Bone histomorphometry illustrated that resorption and absorption of the putty and bioceramic granules had occurred at the implantation site. Indeed, at six months, 95% of the putty was resorbed and replaced by physiological bone. No residual bioceramic granules were observed on the surface; they could only be seen in the deep zone of the biopsy.
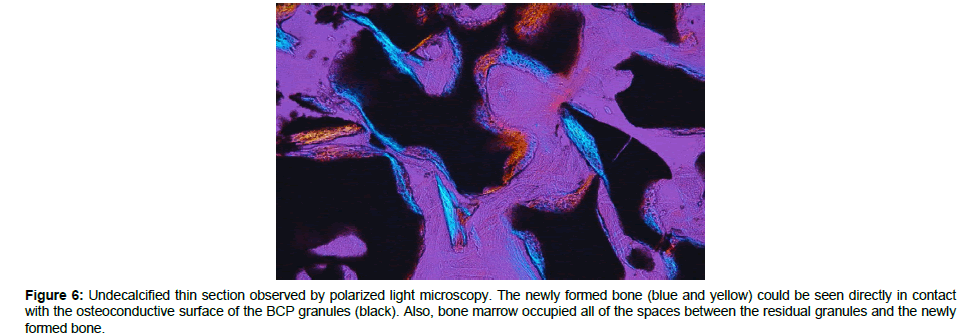
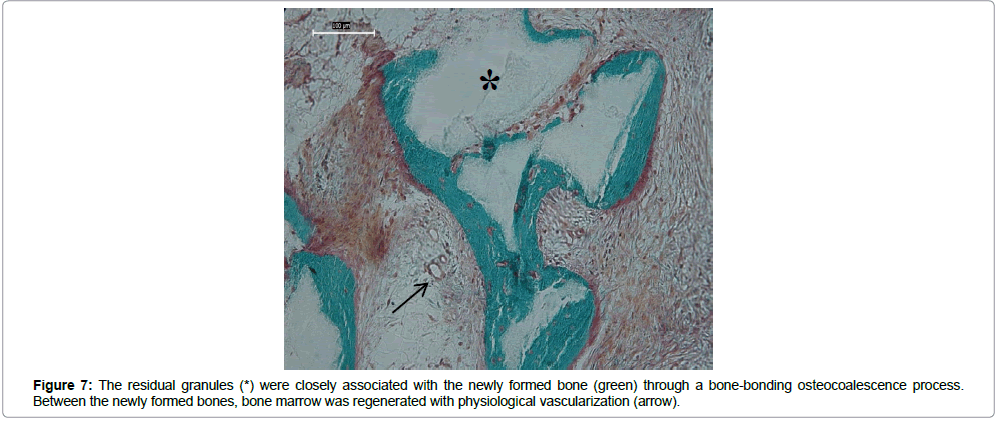
Finally, bone trabeculae were formed at the expense of the In’Oss™ composite, with bone marrow observed in the intergranular spaces and between the newly formed bone and residual granules (Figure 6). Moreover, the newly formed bone presented trabecular architecture, with interconnected and orientated bone trabeculae. The residual granules were closely associated with the freshly formed bone (Figure 7), and the bone marrow generated between the newly formed bone, displayed physiological vascularization. In all of the samples, we are unable to identify any residues from collagen membrane. After 4-6 months, total resorption had occurred, and the mucosa was regenerated.
Figure 6: Undecalcified thin section observed by polarized light microscopy. The newly formed bone (blue and yellow) could be seen directly in contact with the osteoconductive surface of the BCP granules (black). Also, bone marrow occupied all of the spaces between the residual granules and the newly formed bone.
Discussion
Although the osteoconductive efficacy of granulated CaP ceramics has been well illustrated, they are difficult to set up within exiguous sites. For this reason, the use of injectable or moldable composite materials allows for efficient filling of all osseous cavities, regardless of their shape. In this study, the composite proved to be biocompatible and resorbable. Owing to its initial plasticity, it easily assumes the shape of bone defects, eliminating the need to preshape materials to the implant site. The association of this composite with a resorbable collagen membrane protected the implantation site, and its synergistic effect on bone healing and regeneration of the soft tissue was demonstrated with clinical follow-ups and histology. The histology confirmed the total resorption of the membrane after four months. In’Oss putty does not have self-setting properties as a hydraulic bone cement; however, the putty has a larger capacity for cell colonization and resorption, promoting bone ingrowth at the expense of the material, and efficiency in regenerating the bone architecture [18]. As the polymer carrier disappears over time from the implant site, bone cells invade the spaces that emerge. Bone ingrowth then takes place around the granules as the BCP granules are resorbed. As a result, mechanical properties can be observed from the presence of the newly formed bone. Notably, the 3D structure of the network favorably influences cellular recruitment, mesenchymal STEM cells osteogenic differentiation, angiogenesis, and bone formation [4], events involved in the new generation of bioceramic for self-healing biomaterials. The SEM (using BSE) analysis of the bone samples confirmed that the regeneration of bone architecture and bone remodeling were consistent with lamellar bone Haversian system remodeling.
The clinical cases presented here have demonstrated excellent biofunctionality and safety, confirming previous pre-clinical data from human pilot studies in dentistry [16] and osteoarticular surgery [19]. The regeneration of bone architecture following the use of the putty was examined with histomorphometry on bone biopsies. The injectable putty proved to be biocompatible and resorbable. Also, because of its initial plasticity, the putty easily assumed the shape of the bone defects.
Conclusion
Taken together, the clinical, radiological, and histological data from this non-interventional clinical study have demonstrated the safety and efficacy of the injectable CaP bioceramic in bone regeneration during maxillofacial surgery. In addition, the study illustrated the safety and performance of the associated resorbable collagen membrane that guided tissue healing and bone regeneration, acting as a barrier to conjonctive and epithelial cells infiltration during the process of bone regeneration.
The histological analysis demonstrated that this matrix allowed bone regeneration, including both bone architecture and angiogenesis, and revealed the synergic effect of the association with the resorbable collagen membrane that protected the putty during the first weeks of healing and aided mucosa regeneration.
Acknowledgements
This research was funded by the European Union’s 7th Framework Program (grant number FP7-HEALTH-2009-241879; REBORNE) and promoted by Biomatlante.
The authors would like to thank Sophie Sourice (Inserm RMeS), Françoise Moreau, (Biomatlante) for their technical support in histology.
We particularly thank the following surgeon’s contribution to this study:
References
- Silber JS, Anderson DG, Daffner SD, Brislin BT, Leland JM, et al. (2003) Donor site morbidity after anterior iliac crest bone harvest for single-level anterior cervical discectomy and fusion. Spine 28: 134-139.
- Tomford WW (2000) Bone allografts: past, present and future. Cell Tissue Bank 1:105-109.
- Heary RF, Schlenk RP, Sacchieri TA, Barone D, Brotea C (2002) Persistent iliac crest donor site pain: independent outcome assessment. Neurosurgery 50: 510-517.
- Albee F (1920) Studies in bone growth: Triple calcium phosphate as a stimulus to osteogenesis. Ann Surg 71: 32-36
- Aoki H (1991) Science and medical applications of hydroxyapatite. Ishiyaku Euroamerica
- DeGroot K (1983) Ceramics of calcium phosphates: preparation and properties. Bioceramics of calcium phosphate 100-114.
- Jarcho M (1981) Calcium phosphate ceramics as hard tissue prosthetics. Clin Orthop Relat Res 157: 259-278.
- Legeros RZ (1993) Biodegradation and bioresorption of calcium phosphate ceramics. Clin Mater 14: 65-88.
- Nery EB, LeGeros RZ, Lynch KL, Lee K (1992) Tissue response to biphasic calcium phosphate ceramic with different ratios of HA/TCP in periodontal osseous defects. J Periodontol 63: 729-735.
- Ellinger RF, Nery E, Lynch K (1986) Histological assessment of periodontal osseous defects following implantation of hydroxyapatite and biphasic calcium phosphate ceramics: a case report. Int J Periodontics Restorative Dent 6: 22-33.
- Nery EB, Lynch KL, Hirthe WM, Mueller KH (1975) Bioceramic implants in surgically produced infrabony defects. J Periodontol 46: 328-347.
- Legeros RZ (1988) Calcium phosphate materials in restorative dentistry: a re- view. Adv Dent Res 2:164-180.
- Daculsi G, Laboux O, Malard O, Weiss P (2003) Current state of the art of biphasic calcium phosphate bioceramics. J Mater Sci Mater Med 14: 195-200.
- Daculsi G, LeGeros RZ, Heughebaert M, Barbieux I (1990) Formation of carbonate-apatite crystals after implantation of calcium phosphate ceramics. Calcif Tissue Int 46: 20-27.
- Daculsi G (2006) Biphasic calcium phosphate Granules concept for Injectable and Mouldable Bone Substitute. Adv Sci Technol Res 49: 9-13.
- Weiss P, Layrolle P, Clergeau LP, Enckel B, Daculsi G, et al. (2007) The safety and efficacy of an injectable bone substitute in dental sockets demonstrated in a human clinical trial. Biomaterials 28: 3295-3305.
- Daculsi G, Durand M, Fabre T, Vogt dF, Uzel AP, et al. (2012) Development and clinical cases of injectable bone void filler used in orthopaedic. IRBM 33: 254-262.
- Gauthier O (2001) In vivo comparison of two injectable calcium phosphate biomaterials: ionic cement and polymer-associated particulate ceramic. Key Eng Mater 192-195: 801-804.
- Fabre T, Dominique C, MaryseM, ClaudeM, G. Daculsi, et al. (2008) Pilot study of safety and performance of a mixture of calcium phosphate granules combined with cellulosic derived gel after tunnel filling created during surgical treatment of femoral head aseptic osteonecrosis. Key Eng Mater 361-363: 1295-1298
 Spanish
Spanish  Chinese
Chinese  Russian
Russian  German
German  French
French  Japanese
Japanese  Portuguese
Portuguese  Hindi
Hindi