Research Article, Endocrinol Diabetes Res Vol: 11 Issue: 1
Comparative Investigations Using a Type II Diabetes Model to Examine the Expression and Functionality of Ca2+ Sensing Receptors in Rat Mesenteric Arteries
Hari Prasad Sonwani*
Department of Pharmacy, Apollo College of Pharmacy, Chhattisgarh, India
*Corresponding Author: Hari Prasad Sonwani
Department of Pharmacy, Apollo College of Pharmacy, Chhattisgarh, India
E-mail: harisonwani10@gmail.com
Received date: 11 November, 2023, Manuscript No. ECDR-23-119877;
Editor assigned date: 14 November, 2023, PreQC No. ECDR-23-119877 (PQ);
Reviewed date: 28 November, 2023, QC No. ECDR-23-119877;
Revised date: 22 January, 2025, Manuscript No. ECDR-23-119877 (R);
Published date: 29 January, 2025, DOI: 10.35248/2470-7570.1000425
Citation:Sonwani HP (2025) Comparative Investigations Using a Type II Diabetes Model to Examine the Expression and Functionality of Ca2+ Sensing Receptors in Rat Mesenteric Arteries. Endocrinol Diabetes Res 11:1.
Abstract
Myocyte hyperpolarization is indirectly caused by the activation of endothelial intermediate-conductance, calcium-sensitive K+ channels (IKCa) by the extracellular Calcium-sensing Receptor (CaR) in vascular endothelial cells. We investigated whether a rat model of type II diabetes affected the expression and function of CaR. The functional expression of the CaR and IKCa in rat mesenteric arteries was examined using pressure myography, Western blotting, sharp microelectrode, and K+ selective electrode recordings. Calhex 231 reduced myocyte hyperpolarization to the CaR activator calindol. The extracellular (K+) surrounding the myocytes was increased by U46619-induced vascular contraction; iberiotoxin-induced suppression of this "K+ cloud" was required to disclose calindol-induced vasodilatations. These were considerably smaller in Zucker Diabetic Fatty rat (ZDF) vessels than in Zucker Lean (ZL) controls, and they were triggered by Calhex 231. Hyperpolarizations of myocytes ZDF arteries had lower responses to calindol than ZL arteries did. ZDF arteries showed decreased expression of CaR protein in endothelial cells and decreased expression of IKCa; nevertheless, the hyperpolarizations caused by IKCa and mediated by 1-EBIO remained unaltered. Reduced CaR expression rather than a change in IKCa channels is the cause of the decreased CaR mediated hyperpolarizing and vasodilator responses in ZDF arteries. Iberiotoxin was needed to detect CaR-mediated vasodilatation, indicating that CaR contributes to vascular diameter, which is negatively correlated with the degree of vasoconstriction. A disruption in the CaR pathway could potentially lead to the long-term elevation of basal vascular tone and vascular problems linked to type II diabetes.
Keywords: Calcium-sensing receptor; Type II diabetes; ZDF rat; Endothelium; K+ cloud; Calcium-activated potassium channels; Vasodilatation
Abbreviations
BKCa: Large conductance calcium-sensitive K + channel; CaR: Calcium-sensing Receptor; 1-EBIO: 1-ethyl-2- benzimidazolinone; EDH: Endothelium-Dependent smooth muscle Hyperpolarization; IKCa: Intermediate conductance calcium-sensitive K+ channel; KATP: ATP-sensitive K+ channel; NS309: 6,7-dichloro-1H-indole-2,3-dione-3-oxime; SKCa: Small conductance calcium-sensitive K+ channel; ZDF: Zucker Diabetic Fatty; ZL: Zucker Lean; U46619: 11a,9a-epoxymethano-PGH2
Introduction
It has long been known that the extracellular Calcium-sensing Receptor (CaR) plays a crucial role in maintaining calcium homeostasis in general [1,2]. According to a recent study, calindol, a positive allosteric modulator of the CaR, activates the endothelial cells in both rat and porcine coronary arteries, causing the vascular myocytes to become hyperpolarized [3,4]. TRAM-34 (1-((2- chlorophenyl)-diphenyl-methyl)-1H-pyrazole)) exposure eliminated this effect, so active inhibitor of calcium-sensitive, intermediateconductance K+ channels, or IKCa; or by removing the endothelium, it was determined that CaR activation causes the IKCa channels in endothelial cells to selectively open [5,6]. In addition, Calhex 231. In addition to counteracting the effects of calindol, (a negative allosteric modulator of CaR; caused a slight depolarization of rat mesenteric artery myocytes, suggesting that the CaR may be partially activated at basal circumstances [7]. According to Edwards et al. ligands like acetylcholine cause endothelial cells in rat mesenteric arteries to open their small-conductance, calcium-sensitive K+ channels, or IKCa and SKCa, resulting in membrane hyperpolarization [8]. The surrounding myocytes then experience Endothelium-Dependent Hyperpolarization (EDH) and relaxation as a result of this transmission. The resistance arteries of diabetic rats exhibit a reduction in EDH, as reported by Fukao et al. [9]. The exact cause of this is unknown, but the resulting decreased vasodilationreaction could be a factor in the hypertension that many diabetes individuals experience [10]. It was recently demonstrated by Ward et al. that an animal model of type I diabetes had decreased CaR expression [11]. We therefore questioned whether a similar decrease in CaR expression in the vascular endothelium of diabetic resistance arteries might be a factor in the previously reported reduced EDH response in such vessels, since activation of the vascular CaR is linked to the opening of endothelial IKCa channels. In this work, using Zucker Diabetic Fatty (ZDF) rats as an animal model of type II diabetes, we examined the expression of CaR in the mesenteric arteries. Diabetes that is not insulin-dependent. We have shown for the first time that CaR activation can result in vasorelaxation using pressure myography. Moreover, we demonstrated that the vascular CaR is downregulated in the ZDF rat by combining electrophysiology, molecular biology, and myography. This could be one of the reasons behind the vascular problems observed in type II diabetes individuals as well as the reason for the decreased EDH response in the arterioles from such animals.
Materials and Methods
Animals
Second and third-order mesenteric artery branches (about 150-250 mm) were used for the experiments. Diameter was separated from the age-matched lean controls (ZL; fa+/fa-; mean body weight 326 ± 9 g, n=12) and male Zucker fat (ZDF; fa+/fa+) diabetic rats (12-14 weeks old; mean body weight 318 ± 7 g, n=12). InsteadIn certain Wistar rats (body weight 250–300 g) used in early tests were previously put to death by CO2 asphyxiation in accordance with schedule 1 of the UK animals (scientific procedures) act 1986. Every animal was kept in a 12 hour light-dark cycle with unlimited access to food and drink. The ZDF animals' urine had a glucose content of 4100 mM, while the control animals' urine had no detectable glucose. The ZDF rats had a substantially higher mean serum glucose concentration than the ZL rats, according to blood samples (P=0.0001). After the death of each ZL or ZDF animal on a separate day, the artery segments were either processed right away for Western blot examination or used freshly for myography and electrophysiology. Myography under pressure secondorder arterial segments were placed on glass cannulae in the heated chamber of a pressure myograph. The Krebs solution (which contained 10 mM indomethacin and 300 mM NG-nitro-L-arginine) was gassed with 5% CO2 in air and the intraluminal pressure was maintained at 70 mm Hg by a pressure-servo unit. The Krebs solution composition was slightly modified. The diameter of the artery was measured continuously using a video dimension analyzer (living systems, Burlington, Vermont, USA) and recorded with a Mac minicomputer (Apple UK and Ireland, Cork, Ireland) and PowerLab/45P recorder in combination with Chart v5.2 software (ADInstruments, Chalgrove, UK). The washing process involved continuous superfusion of the chamber. Under static bath conditions, but the flow was stopped and responses to medicines were monitored with Krebs solution at 25 mL min-1. Iberiotoxin (100 nM) was applied to arteries for 30 minutes prior to and during drug treatment in order to inhibit the large-conductance, calcium-sensitive K+ channel, or BKCa. Both the bath solution and the artery lumen were bathed with Calhex 231 and TRAM-34 when they were utilized; control and test responses were established in separate artery segments. The recordings were made both before and during the recordings. Using U46619 (11a,9aepoxymethano- PGH2; 2-30 nM), arteries were precontracted for each segment of an artery in order to reduce vessel diameter by roughly 50%), and relaxations were reported in relation to the diameter both in the fully relaxed vessel and immediately before to the injection of the relaxant medication (defined as 0%). relaxed artery when 100 mM (or 100%) of papaverine is present.
Electrophysiology and measurement of extracellular K+
A Krebs solution containing indomethacin and NG-nitro-L-arginine (the above composition) was superfused (3 mL min-1) with small segments of artery (second or third order; length 2-3 mm) that were pinned to the Sylgard base of a thermostatically controlled bath. The Krebs solution was bubbled with 95% O2/5% CO2 (pH 7.5; 37 1C). As previously mentioned, myocytes were impaled via the adventitial surface using microelectrodes loaded with 3 M KCl (resistance 40–80 MO) in order to capture membrane potentials [12]. In certain trials, de-ionized water was briefly injected into the artery segments to eliminate the endothelium light bulb, Calindol, 6,7-dichloro-1Hindole- 2,3-dione, 1-ethyl,2-benzimidazolinone (1-EBIO), NS309 3- oxime with lev-cromakalim were all added to the bath as bolus injections in amounts determined to produce (temporarily) the indicated final concentrations. To the Krebs solution reservoir, Calhex 231 and TRAM-34 were introduced, superfusing the bath. The preparation of Calhex 231 and Calindol followed the earlier instructions. K+ selective microelectrodes were prepared using a K+ ionophore as previously described, but without the shielding pipette, in order to measure changes in the extracellular K+ concentration of myocytes. In order to generate a calibration curve, the electrode was first calibrated using Krebs solution, which had a range of K+ concentrations. Electrodes that were unable to produce a repeatable output within the measured concentration range were disposed out. Selected electrodes were carefully advanced into the adventitia until a stable output was obtained, indicating a K+ concentration ((K+)) that was similar to the Krebs solution. The electrodes had a typical 90% response time of 5 s acquired. The impact of U46619 in the absence of iberiotoxin and its subsequent presence was ascertained by the medications' inclusion to the bath's superfusing solution. WPI, Stevenage, UK is a high impedance amplifier that was used for all of the recordings. A MacLab system (ADInstruments) was used to digitize and analyze the signals, and an active processing circuit (Humbug; Digitimer, Hertfordshire, UK) was used to selectively eliminate 50 Hz interference.
Western blotting
The procedure for Western blotting was followed as previously mentioned [13]. In a nutshell, endothelium-intact rat mesenteric artery segments were homogenized in newly made extraction buffer that contained 20 mM Trizma base, one vial containing 100 mL of extraction buffer, together with 2.5 mM sucrose, 5 mM EDTA, 1 mM EGTA, 1 mM dithiothreitol, 1 mM phenylmethylsulphonylfluoride, and a protease inhibitor cocktail. Using a Bradford reagent (Bio-Rad, Hertfordshire, UK), protein concentrations were measured for each lane. Equal protein loading and after staining for five minutes with a 0.1% Ponceau S solution (Sigma-Aldrich, Dorset, UK), the transfers were evaluated visually. Actin was used as a loading control in a western blot to further confirm the consistency of protein loading for some of the samples. Following sample mixing and separation on polyacrylamide gels (6–10%, w/v, as specified; about 90 min at 120 V), samples were transferred to nitrocellulose membranes (about 90 min at 80 V) using Laemmli buffer (containing the detergent SDS) [14]. The membranes were first blocked with 2% BSA (overnight at 41C), then incubated for 1 hour at room temperature with either 1:2000 anti-b-actin (mouse), 1:5000 anti-CaR (mouse monoclonal), or 1:500 anti-IK1 (M20; a rabbit anti-hIK1 antibody, raised against the human N-terminal IK1 peptide (GGDLVLGLGALRRRKC) [15]. monoclonal; AC15 and with secondary antibodies (1:20,000 horseradish peroxidase-conjugated) against goat anti-rabbit (IK1) or goat anti-mouse (CaR, b-actin). A chemiluminescence detection system (ECL+; GE Healthcare, Buckinghamshire, UK) was used to achieve detection.
Contents
The sources of the pressure myograph, pressure-servo unit, and video dimension analyzer were living systems (Burlington, Vermont, USA); WPI (Stevenage, UK) provided the high impedance amplifier; ADInstruments (Chalgrove, Oxfordshire, UK) provided the MacLab system; and Digitimer (Welwyn Garden City, Hertfordshire, UK) provided the Humbug active processing circuit. U46619 from Calbiochem (Nottingham, UK); Calhex 231 and TRAM-34 from Toronto Research Chemicals (North York, Ontario, Canada). We gratefully received supplies of Calindol, 1-EBIO, and NS309 (6,7-dichloro- 1H-indole-2,3-dione 3-oxime) from Dr. P. Christophersen (Neurosearch A/S, Ballerup, Denmark). Protease inhibitor Fluka Feinchemikalien (Neu-Ulm, Germany) provided the K+ ionophore (number 60398).
Ponceau S and drink analysis of data: Every value is expressed as mean ± S.E. mean. The quantity of arteries from distinct animals are indicated by n. GraphPad Prism version 4 for Macintosh (GraphPad Software, San Diego, CA, USA) was used for statistical analysis. ANOVA was used first, followed by a Bonferroni multiple comparison test or a Student's unpaired t-test, as applicable; a value of P=0.05 was deemed significant.
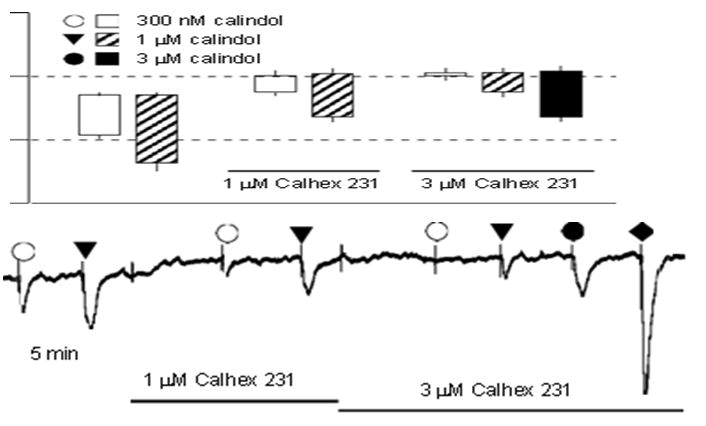
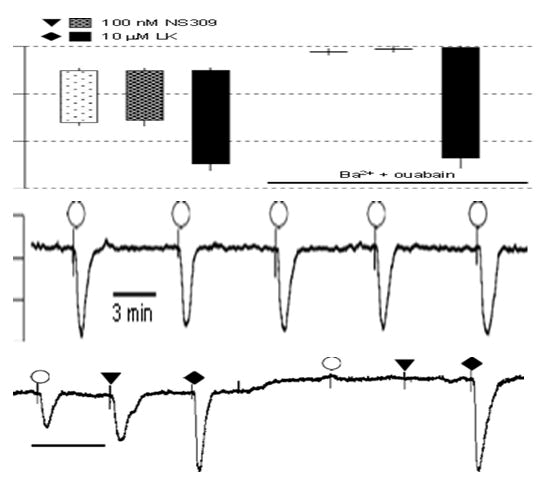
Outcomes: Prior to the trials utilizing ZDF animals and the corresponding ZL controls in this work, some preliminary research was conducted with Wistar rats. Features of Wistar vessels' CaRinduced hyperpolarization: Calindol inhibition by Calhex 231 and by Ouabain+Ba2 rat mesenteric artery myo-cytes had concentrationdependent hyperpolarization upon exposure to calindol, as depicted in Figure 1. When 1 mM Calhex 231 was exposed to, a tiny little depolarization with statistical significance (from -52.9 ± 0.2 to not further investigated -49.9 ± 0.8 mV, n=4; Po0.01) elevated by 3 mM Calhex 231 (potential for membranes) P40.05; -49.4 ± 0.8 mV, n=4. The effects of hyperpolarization of 0.3 and 1 mM calindol were suppressed in a concentration-dependent way (Po0.01, n=4) by 1 and 3 mM Calhex 231, respectively, but were not seen in arteries that had been denuded of endothelium (results not shown). The combination of 30 mM barium+500 nM ouabain, which inhibits the EDH response to acetylcholine eliminated both the hyperpolarizations caused by calindol and those to NS309, a direct opener of both IKCa and SKCa channels [16]. Conversely, hyper-polarizations to the KATP opener, levcromakalim, which is ATP-sensitive and employed as a positive barium+ouabain had no effect on control (Figure 2).
In order to rule out the possibility of changes in calindol: Generated hyperpolarizations were indicative of a time-varying phenomena, control experiments were carried out in which vessels were repeatedly (five times) exposed to 300 nM calindol at 5 min intervals during continuous impalements. There was no significant difference between the effects of calindol over this time period (repeated measures ANOVA, P=0.29, n=5).
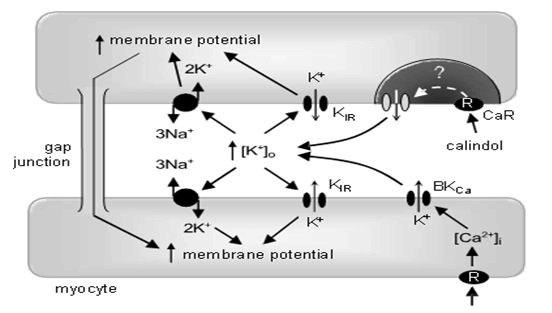
Collectively, these experiments suggest that myocyte: Hyperpolarizations triggered by the opening of endothelial Ca2+ sensitive K+ channels depend on increasing the (K+) in the myoendothelial space, with subsequent activation of inwardly rectifying K+ channels and Na+/K+ -ATPases sensitive to Ba2+ and ouabain, respectively (Figure 3). CaR-induced vasorelaxation and intravascular K+ clouds. Calindol-induced relaxations of precontracted mesenteric arteries were only rarely observed (in the absence or presence of L-NA and indomethacin; data not shown) despite the myocyte hyperpolarizations that were always seen in quiescent vessels when these were exposed to calindol (Figures 1 and 2). Previously, we and others obtained circumstantial evidence that agonist-generated K+ clouds emanating from BKCa channels on contracted vascular myocytes could reduce or abolish certain endothelium dependent vasorelaxations [17]. Experiments were therefore performed using K+ selective electrodes to determine whether the hypothetical K+ clouds existed when vessels were exposed to a pre-contracting agent such as the Thromboxane (TP) receptor agonist U46619.
Figure 1: Effect of Calhex 231 on smooth muscle hyperpolarizations to calindol in Wistar rat mesenteric arteries. (a) Typical trace showing concentration-dependent inhibition of hyperpolarizations to calindol by Calhex 231. At the end of the experiment, 10 mM Levcromakalim (LK) was added to confirm the integrity of the myocytes. (b) Graphical representation of mean results obtained from four experiments of the type shown in panel Each column represents the mean membrane potential (m.p.) before (+ S.E. mean) and after (-S.E. mean) addition of calindol in the absence or presence of Calhex 231 as indicated.
Figure 2: Effect of 30 mM Ba2++500 nM ouabain on smooth muscle hyperpolarizations to Calindol (Cal) and NS309 in Zucker Lean (ZL) rat mesenteric arteries. (a) Typical trace showing abolition of the hyperpolarization to 300 nM calindol or 100 mM NS309 (6,7- dichloro-1H-indole-2,3-dione 3-oxime) by 30 mM Ba2++500 nM ouabain in a segment of artery from a ZL rat. At the end of the experiment, 10 mM Levcromakalim (LK) was added to confirm the integrity of the preparation. (b) Graphical representation of mean results obtained from four experiments of the type shown in panel. Each column represents the mean membrane potential (m.p.) before (+ S.E. mean) and after (-S.E. mean) addition of calindol, 6,7- dichloro-1H-indole-2,3-dione 3-oxime (NS309) or levcromakalim in the absence or presence of Ba2++ouabain as indicated. (c) Typical time-control experiment to test for the reproducibility of calindol induced hyperpolarizations. After myocyte impalement, vessels were exposed to 300 nM calindol every 5 min during a 20 min impalement. Each number represents the maximum mean m.p. ± S.E. mean (n=5 vessels) recorded in the presence of 300 nM calindol. Over the 20 min duration of the experiments, there was no time-dependent change in the magnitude of calindol-induced hyperpolarizations (repeated measures ANOVA; P=0.29).
Following calibration, the smooth muscle layer immediately below the adventitia was probed with a K+ sensitive electrode until a stable reading was obtained. This suggested that the environment at the tip was probably extracellular and was not likely to be sampling a damaged myocyte. Exposure to U46619 (30 nM) resulted in a fast increase in (K+) of 11.2 ± 1.5 mM (n=3). This change was wellmaintained and frequently linked to rhythmic oscillations in K+ within the 1-2 mM range. When exposed to 100 nM iberiotoxin while U46619 was still present, the (K+) decreased to a steady level a somewhat greater quantity (5.3 ± 0.2 mM, n=3). Less than the 4.6 mM Krebs solution. Phenolphthalein (100 nM) generated K+ clouds of a magnitude similar to those obtained with U46619 (data not shown). These experiments showed that U46619-induced contractions did indeed generate a substantial, iberiotoxin-sensitive increase in (K+) (a K+ cloud) around the myocytes (Figure 3). Hypothetically, under such conditions of increased (K+), any further increase in (K+) after the opening of endothelial cell IKCa channels via CaR activation would generate minimal vasorelaxation. To test this possibility, myograph experiments were therefore conducted using the mesenteric vessels of normal Wistar rats with iberiotoxin (100 nM) in the Krebs solution. Under basal conditions, the mean diameter of Wistar pressurized arteries, bathed in Krebs solution containing iberiotoxin 100 nM, was 290 ± 29 mm (n=8). To generate tone, vessels were pre-contracted with U46619 (range 2-30 nM), the concentration of which was adjusted to obtain a contracted diameter of approximately 100 mm. Under these conditions, cumulative exposure to calindol (0.3-3 mM) from that of the ZL controls (331 ± 13 mm, n=5).
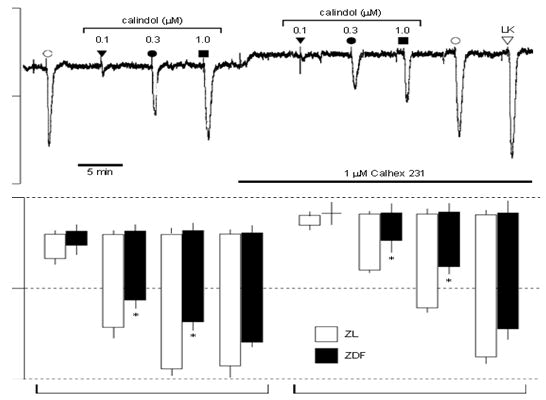
Student’s unpaired t-test). In the presence of U46619 (2-30 nM), the relaxations to 1 and 3 mM calindol (24.9 ± 4.1 and 72.9 ± 11.3%, respectively, n=5) in arteries from the ZDF rats were markedly smaller (two-way ANOVA, P=0.0007) than those in the ZL rats (79.0 ± 7.5 and 97.0 ± 1.2%, respectively, n=5). CaR-induced hyperpolarizations but not those to IKCa opening are reduced in ZDF arteries. The resting membrane potential of myocytes in endothelium-intact mesenteric arteries from ZDF rats (-54.6 ± 0.4 mV, n=12) was similar to that of control ZL rats (-53.9 ± 0.3 mV, n=12). Calindol produced concentration-dependent hyperpolarizations of mesenteric artery myocytes that were significantly smaller in artery segments from ZDF rats than those from ZL controls (two-way ANOVA; P=0.0002). In four out of four endothelium denuded segments from ZL rats, 1 mM calindol failed to induce any myocyte hyperpolarization (data not shown). Calhex 231, the allosteric inhibitor of CaR, produced small but significant myocyte depolarizations (of similar magnitude) in segments of mesenteric arteries from both ZL (2.1 ± 0.3 mV, n=4) and ZDF rats (1.9 ± 0.4 mV, n=4), and in the continuing presence of Calhex 231 hyperpolarizations to calindol were significantly reduced (two-way ANOVA; P=0.0005). In contrast, a submaximal concentration of the IKCa activator 1-EBIO (300 mM) produced hyperpolarizations (ZDF rats: 12.1 ± 1.1 mV; ZL rats: 14.5 ± 1.4 mV, each n=4) that were unaffected by the presence of Calhex 231 (ZDF rats: 12.9 ± 1.3 mV; ZL rats: 15.5 ± 1.1 mV, each n=4; P40.05). At the end of the experiment, vessels were exposed to levcromakalim (an opener of myocyte ATP-sensitive K+ channels) to confirm the integrity of the myocyte recordings. In the presence of 1 mM Calhex 231, hyperpolarizations to 10 mM levcromakalim were similar in both the ZL and ZDF arteries (ZDF rats: 23.6 ± 0.8 mV; ZL rats: 22.4 ± 0.3 mV, each n=4). In artery segments from four out of four ZDF rats, removal of the endothelium led to a complete loss of hyperpolarizations to 3 mM calindol (data not shown). The hyperpolarizations induced by calindol (which were reduced in the ZDF rats) result solely from the opening of endothelial IKCa channels. Thus, to determine whether the reduced effect of calindol resulted from some compromise in the IKCa channels (rather than in the CaR itself), responses to the IKCa activator 1-EBIO were also examined. In the experiments described in Figure 4, there was no significant difference between the hyperpolarizations generated by 300 mM 1-EBIO in ZDF and ZL vessels, although there was a trend towards a reduced 1-EBIO response in the ZDF vessels. To exclude the possibility that the lack of significance was due to the relatively small number of observations (four ZL and four ZDF vessels), the effects of 300 mM 1-EBIO along with those of 150 mM 1-EBIO were compared in artery segments from a further four ZL and four ZDF rats.
Hyperpolarizations induced by 150 and 300 mM 1-EBIO in artery segments from ZDF rats (5.5 ± 1.1 and 15.2 ± 0.6 mV, respectively, n=4) were still not significantly different from those in ZL vessels (4.9± 0.3 and 12.6 ± 0.5 mV, n=4; two-way ANOVA, P40.05). In a further series of experiments, we also examined the effects of 600 mM 1-EBIO (a saturating or maximally effective concentration). Hyperpolarizations induced by 600 mM 1-EBIO in artery segments from ZDF rats (15.8 ± 1.4 mV, n=9) were again not significantly different from those in ZL vessels (16.9 ± 0.6 mV, n=12; Student’st-test, P40.05).
Collectively, the electrophysiological experiments show that an endothelial CaR was present in the mesenteric arteries from both ZL control and ZDF rats but that CaR-mediated myocyte hyperpolarizations were significantly reduced in the rat model of type II diabetes. In contrast, responses to three concentrations of the IKCa opener 1-EBIO were unaffected by the diabetic state.
Figure 3: Summary of the likely interrelationships between the calcium-sensing receptor (CaR), K+ channels and Na+/K+ ATPases in a mesenteric artery. The CaR and intermediate-conductance, calciumsensitive K+ channels (IKCa) proteins are colocalized within the endothelial cell, within a lipid-poor microdomain. In the presence of extracellular Ca2+, allosteric activation of the CaR by calindol stimulates the opening of endothelial cell IKCa channels by an unknown coupling mechanism. The resulting K+ efflux through IKCa increases myoendothelial (K+) and stimulates carried out in the presence of 100 nM iberiotoxin.
CaR-mediated relaxations are reduced in the vessels from ZDF rats. The basal diameter of pressurized mesenteric artery segments from ZDF rats in the presence of 1 mM Ca2+ and 100 nM ± inwardly rectifying K+ channels (KIR) and Na+/K+ ATPases (blocked iberiotoxin (35933 mm, n=5) was not significantly differ by Ba2+ +ouabain) to induce myocyte hyperpolarization. Exposure of vessels to 11a,9a-epoxymethano-PGH2 (U46619) (used to increase artery tone so that relaxant effects can be recorded in myograph experiments) elevates myocyte intracellular Ca2+ and indirectly activates iberiotoxin-sensitive large conductance calcium sensitive K+ channel (BKCa) channels. The K+ that exits the myocyte via this channel forms a ‘K+ cloud’, which increases the (K+) surrounding the cells by approximately 10 mM. In the presence of U46619, KIR channels and Na+/K+ ATPases are already activated; subsequent exposure to calindol is unable to relax mesenteric arterioles. However, selective inhibition of BKCa channels with iberiotoxin prevents the formation of the K+ cloud by 11a,9a-epoxymethano-PGH2 (U46619), allowing calindol to relax the vessel despite the presence of the spasmogen. Increased membrane potential (hyperpolarization) and increases in (Ca2+) and (K+) are indicated by upward-pointing arrows.
In ZDF arteries, CaR-induced hyperpolarizations are diminished but not those resulting from IKCa opening.
In endothelium-intact mesenteric arteries from ZDF rats, the resting membrane potential of myocytes was -54.6 ± 0.4 mV, n=12, comparable to that of control ZL rats (-53.9 ± 0.3 mV, n=12). In arterial segments from ZDF rats compared to ZL controls, calcineurin induced concentration-dependent hyperpolarizations of mesenteric artery myocytes that were considerably smaller (two-way ANOVA; P=0.0002). One milligram of calindol did not cause any myocyte hyperpolarization in any of the four endothelium-denuded segments from ZL animals (data not shown).
Segments of mesenteric arteries from both ZL showed little but considerable myocyte depolarizations (of comparable size) upon administration of Calhex 231, an allosteric inhibitor of CaR. hyperpolarizations to calindol were considerably decreased in ZDF rats (1.9 ± 0.4 mV, n=4) and 2.1 ± 0.3 mV, n=4, and in the continuous presence of Calhex 231 (two-way ANOVA; P=0.0005). On the other hand, the presence of Calhex 231 had no effect on the hyperpolarizations caused by a submaximal concentration of the IKCa activator 1-EBIO (300 mM) in rats (ZDF rats: 12.1 ± 1.1 mV; ZL rats: 14.5 ± 1.4 mV, each n=4; P40.05). Following the experiment, the vessels were subjected to verify the accuracy of the myocyte recordings, subject them to levcromakalim (an opener of ATPsensitive K+ channels in myocytes). When 1 mM Calhex 231 was present, the ZL and ZDF arteries showed comparable hyperpolarizations to 10 mM levcromakalim (ZDF rats: 23.6 ± 0.8 mV; ZL rats: 22.4 ± 0.3 mV, each n=4) in portions of four arteries from each ZDF. Rats that had their endothelium removed completely lost their ability to hyperpolarize in response to 3 mM calindol (data not shown). The current study indicates that the sole cause of the hyperpolarizations brought on by calindol (which were diminished in the ZDF rats) is the opening of endothelium IKCa channels. Thus, to ascertain whether a compromise in the IKCa channels was the cause of calindol's decreased effect (instead of in the CaR itself), the IKCa activator 1-EBIO responses were also investigated. The hyperpolarizations caused by 300 mM 1-EBIO in ZDF and ZL vasculature did not differ significantly in the experiments shown in Figure 4, however there was a tendency for the ZDF vessels to have a lower 1-EBIO response. The effects of 300 mM 1-EBIO and 150 mM 1-EBIO were compared in artery segments from four more ZL and four ZDF animals in order to rule out the possibility that the lack of significance was caused by the relatively limited number of observations (four ZL and four ZDF vessels). 150 and 300 mM 1- EBIO-induced hyperpolarizations in ZDF rat arterial segments (5.5 ± 1.1 and 15.2 ± 0.6 mV, correspondingly, remained essentially unchanged from those in ZL vessels (4.9 ± 0.3 and 12.6 ± 0.5 mV, n=4; two-test) (ANOVA method, P40.05). We also investigated the effects of 600 mM 1-EBIO (a saturating or maximally effective concentration) in a second set of tests. In hyperpolarizations produced by 600 mM 1-EBIO in ZDF rat artery segments (15.8 ± 1.4 mV, n=9) were not statistically different from ZL vascular segments (16.9 ± 0.6 mV, n=12; Student’s P40.05 in the t-test.
Overall, the electrophysiological studies demonstrate that although the rat model of type II diabetes greatly decreased CaR-mediated myocyte hyperpolarizations, endothelial CaR was found in the mesenteric arteries of both ZL normal and ZDF rats. On the other hand, reactions to three IKCa concentrations Figure 1: The diabetes condition had no effect on EBIO.
TRAM-34's effects on CaR-induced hyperpolarizations in ZDF arteries 10 mM TRAM-34, a specific IKCa inhibitor, eliminated hyperpolarizations to 1 mM calindol in endothelium-intact mesenteric artery segments from both the ZDF and ZL rats. This drug alone resulted in modest but statistically significant myocyte depolarizations (Po0.03, one-tailed paired t-test) in the ZDF and ZL arteries (1.4 ± 0.5 mV, n=4).
Figure 4: Comparison of effects of Calhex 231 on endotheliumdependent smooth muscle hyperpolarizations to calindol and 1- ethyl-2-benzimidazolinone (1-EBIO) in endothelium-intact mesenteric artery segments from control (ZL) and diabetic (ZDF) rats. (a) Typical trace obtained showing inhibition by 1 mM Calhex 231 of the responses to transient exposure to calindol (0.1-1 mM), but not to 1- EBIO (300 mM), in a segment of artery from a ZL rat. Levcromakalim (LK; 10 mM) was added at the end of the experiment to ascertain the myocyte viability. The tissue was exposed to Calhex 231 for the period indicated by the horizontal bar. (b) Graphical representation of data from four separate experiments of the type shown in panel. Each column represents the mean membrane potential (m.p.) before (+ S.E. mean) and after (-S.E. mean) addition of calindol or 1-EBIO in the absence or presence of Calhex 231. *indicates ZDF response significantly different from corresponding ZL response. ZDL: Zucker diabetic fatty rat; ZL: Zucker Lean.
Results and Discussion
Identifying the inhibitory function of intravascular K+ clouds and the vasodilator effects of CaR activation. Through the opening of TRAM-34-sensitive endothelial IKCa channels, calindol consistently caused vascular myocytes in quiescent mesenteric arteries to release EDHs. These hyperpolarizations of the myocytes were completely eliminated in the presence of Ba2++ouabain, demonstrating the significance of the linkage between K+ efflux from endothelial cell IKCa and KIR channels and nearby Na+/K+ ATPases. Consequently, by comparison with the hyperpolarizing and vasodilator effects of acetylcholine and substance P, which are both dependent on the endothelium and entail the opening of IKCa, Calindol-induced vasodilatation should also have been a result of (and SKCa) channels regular happening.
Contrarily, calindol was rarely able to relax precontracted mesenteric arterioles in quiescent arteries over its hyperpolarizing concentration range (0.3-3 mM). We postulated that the K+ depletion linked to constricted blood arteries could generate a K+ rich milieu inside the vessel wall, so successfully obstructing the essential coupling step between calindol-induced IKCa and hyperpolarization of the myocyte is caused by activation and the (Ba2++ouabainsensitive) activation of KIR channels and Na+/K+ ATPases [18]. Significant inferential evidence supporting the existence of intravascular K+ clouds has been found in recent years produced by the doses of contractile agonists that are frequently used to produce tone in organ bath experiments. Moreover, local extracellular (K+) increases in vivo can produce physiologically meaningful blood flow enhancement [19]. According to Armstrong et al. and Kirby and Carlson, the most current studies on skeletal muscle have further demonstrated the significance of myocyte-derived K+ in producing swift increases in blood flow to active mammalian muscles [20,21]. According to current and previous research in blood vessels, K+ coupling in skeletal muscles is likewise mediated by activating vascular KIR channels and Na+/K+ ATPases.
For the first time, the study's use of K+ sensitive electrodes has demonstrated the validity of the fictitious "K+ cloud effect". As a result, the (K+) at the myocyte layer's surface rose by around 10 mM when U46619 was present. According to previous research, this rise in (K+) that was seen in the presence of U46619 when using K+ selective electrodes was iberiotoxin-sensitive. This suggests that the cloud's K+ ions came from myocyte BKCa channels, which were opened by the rise in intracellular (Ca2+) in myocytes caused by U46619. Additionally, after the cloud was abolished by the inclusion of iberiotoxin in the Krebs solution, calindol-induced relaxations of precontracted vessels were obtained on a routine basis.
Under the organ bath conditions in which the effects of vasorelaxants on markedly pre-constricted vessels are typically investigated, the presence of a K+ cloud almost certainly favours endothelial–myocyte communication via gap junctions rather than through the involvement of ATPases and inward rectifiers, which are effectively saturated by the high K+ concentrations present in the spasmo-generated K+ cloud. Whether marked pre-constriction should be regarded as ‘non-physiological’ is a matter for debate, but, in vivo, blood vessels are only substantially constricted in pathological or diseased circumstances, such as vasospastic angina or Raynaud’s disease. Electrophysiological findings in ZDF containers. The effects of calindol in ZDF arteries were compared with those in matched ZL controls to see if the reduced relaxant effects in diabetic arteries had an electrical foundation. The myocyte hyperpolarizations caused by calindol in the ZDF and ZL rats were prevented by Calhex 231 and eliminated by TRAM-34 or by first removing the endothelium. These results demonstrate that endothelium IKCa channels are activated by calindol to produce its hyperpolarizing effects (as in non-diabetic rat and pig arteries).
Moreover, mesenteric arteries from ZDF rats had considerably less endothelium-dependent myocyte hyper-polarizations triggered by calindol than those from control lean (ZL) animals. We concluded that this might point to a decrease in the expression of the IKCa or CaR proteins, or both, in the diabetic arteries. We looked at the amount of CaR and IKCa proteins in the ZDF and ZL arteries to evaluate this.
CaR and IK1 protein expression altered in ZDF vessels comparing the arteries of ZDF rats to ZL controls, Western blot analysis revealed a significant decrease in the expression of CaR protein. This discovery implies that the decreased electrical responses to calindol in the ZDF arteries are caused, at least in part, by this reduced expression of CaR protein and is therefore consistent with the myograph and electrophysiological results. It is noteworthy that an animal model of low-insulin (type I) diabetes likewise has down regulated renal CaR expression. This could suggest that the body's overall CaR downregulation is triggered by hyperglycemia, which would have broad ramifications for the numerous metabolic disorders that accompany both types of I and II.
A noteworthy observation from the western blot examination of the ZDF and ZL vessels was the existence of an extra band exhibiting immunoreactivity to the IK1 antibody, in addition to a decrease in the quantity of IK1 (IKCa channel a-subunit) protein in ZDF samples as compared to ZL controls. In contrast to the other band, which matched the expected size of the IK1 monomer (48 kDa), this one represented a protein of 40 kDa. The lighter protein may be a misfolded protein, a breakdown product of IK1, or a shortened form resembling the SK3 channel a-subunit that previously reported.
It is obvious that the decreased quantity of "authentic" IK1 protein could be accountable for the decreased hyperpolarizations brought on by calindol that were seen in the ZDF vessels. Nevertheless, in the diabetic arteries, responses to three doses of the IKCa activator 1- EBIO were not diminished. Based on this, it is possible that the 40 kDa species is a modified form of IK1 protein that can be put together with "normal" IK1 subunits to form functional tetrameric IKCa-like channels. Whatever the real reason, taken as a whole, these data suggest that the most plausible reason for the observed decrease in calindol's hyperpolarizing and dilator activities in the type II diabetes model is the decreased expression of the CaR protein.
Conclusion
Diabetes type II and the CaR: Whether the vascular CaR–IKCa pathway contributes to the contractile state of is one of the study's main questions. Blood vessels in vivo, and if so, may the type II diabetes patients' elevated diastolic blood pressure have something to do with the disruption of this interrelationship shown in ZDF vessels? According to our research, the CaR–IKCa pathway's contribution to vascular tone in vivo will be inversely correlated with the extent of prevalent vascular tone and the ensuing size of the K+ cloud that is related. Nonetheless, the existence of this inhibitory route tends to contribute to a "background vasodilator effect" by holding myocyte membrane potential at a more negative level. The IKCa knockout mouse, whose basal blood pressure is higher than that of matched controls, may provide evidence for this. If the CaR–IKCa pathway is disrupted, as this study's type II diabetes model suggests, this will probably favor the long-term development of a higher basal tone, and as a result might be involved in the vascular issues linked to type II diabetes. The vascular CaR's physiological role. The physiological function of the vascular CaR and the endogenous ligand(s) that may activate it are fundamental questions. The CaR's ability to function as a plasma Ca2+ sensor seems improbable because the plasma (Ca2+) is often under good control. Nonetheless, we propose that there may be notable increases in (Ca2+) in the blood's myoendothelial gaps based on an analogy with the K+ clouds found in this investigation.
In these conditions, the CaR–IKCa pathway may provide a potential negative feedback mechanism on the myocytes, amplifying the impact of other 'switch-off' processes inside these cells. A variety of other substances, such as amino acids, can also activate the CaR in addition to Ca2+. The phenomena of post-prandial hyperaemia is widely acknowledged, and there is ongoing research exploring the possibility of amino acid activation of the vascular CaR (or the nearly similar receptor, designated GPRC6A).
References
- Hofer AM, Brown EM (2003) Extracellular calcium sensing and signalling. Nat Rev Mol Cell Biol 4: 530–538.
[Crossref] [Google Scholar] [PubMed]
- Breitwieser GE (2006) Calcium sensing receptors and calcium oscillations: Calcium as a first messenger. Curr Top Dev Biol 73: 85–114.
[Crossref] [Google Scholar] [PubMed]
- Kessler A, Faure H, Petrel C, Ruat M, Dauban P, et al. (2004) N2-benzyl-N1-(1-(1-naphthyl)ethyl)-3-phenylpropane-1,2-diamines and conformationally restrained indole analogues: Development of calindol as a new calcimimetic acting at the calcium sensing receptor. Bioorg Med Chem Lett 14: 3345–3349.
[Crossref] [Google Scholar] [PubMed]
- Weston AH, Richards GR, Burnham MP, Vanhoutte PM, Edwards G, et al. (2002) K+ induced hyperpolarization in rat mesenteric artery: Identification, localization and role of Na+/K+ ATPases. Br J Pharmacol 136: 918–926.
[Crossref] [Google Scholar] [PubMed]
- Wulff H, Miller MJ, Hansel W, Grissmer S, Cahalan MD, et al. (2000) Design of a potent and selective inhibitor of the intermediate-conductance Ca2+ activated K+ channel, IKCa1: A potential immunosuppressant. Proc Natl Acad Sci USA 97: 8151–8156.
[Crossref] [Google Scholar] [PubMed]
- Weston AH, Absi M, Ward DT, Ohanian J, Dodd RH, et al. (2005) Evidence in favor of a calcium-sensing receptor in arterial endothelial cells: Studies with calindol and Calhex 231. Circ Res 97: 391–398.
[Crossref] [Google Scholar] [PubMed]
- Petrel C, Kessler A, Maslah F, Dauban P, Dodd RH, et al. (2003) Modeling and mutagenesis of the binding site of Calhex 231, a novel negative allosteric modulator of the extracellular Ca2+ sensing receptor. J Biol Chem 278: 49487–49494.
[Crossref] [Google Scholar] [PubMed]
- Edwards G, Dora KA, Gardener MJ, Garland CJ, Weston AH (1998) K+ is an endothelium-derived hyperpolarizing factor in rat arteries. Nature 396: 269–272.
[Crossref] [Google Scholar] [PubMed]
- Fukao M, Hattori Y, Kanno M, Sakuma I, Kitabatake A (1997) Alterations in endothelium-dependent hyperpolarization and relaxation in mesenteric arteries from streptozotocin-induced diabetic rats. Br J Pharmacol 121: 1383–1391.
[Crossref] [Google Scholar] [PubMed]
- Vijan S, Hayward RA (2003) Treatment of hypertension in type 2 diabetes mellitus: blood pressure goals, choice of agents, and setting priorities in diabetes care. Ann Intern Med 138: 593–602.
[Crossref] [Google Scholar] [PubMed]
- Ward DT, Yau SK, Mee AP, Mawer EB, Miller CA, et al. (2001) Functional, molecular, and biochemical characterization of streptozotocin-induced diabetes. J Am Soc Nephrol 12: 779–790.
[Crossref] [Google Scholar] [PubMed]
- Edwards G, Gardener MJ, Thollon C, Vanhoutte PM, Weston AH, et al. (1999) Role of gap junctions in the responses to EDHF in rat and guinea-pig small arteries. Br J Pharmacol 128: 1788–1794.
[Crossref] [Google Scholar] [PubMed]
- Gardener MJ, Johnson IT, Burnham MP, Edwards G, Heagerty AM, et al. (2004) Functional evidence of a role for two-pore domain potassium channels in rat mesenteric and pulmonary arteries. Br J Pharmacol 142: 192–202.
[Crossref] [Google Scholar] [PubMed]
- Laemmli UK (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227: 680–685.
[Crossref] [Google Scholar] [PubMed]
- Boettger MK, Till S, Chen MX, Anand U, Otto WR, et al. (2002) Calcium-activated potassium channel SK1 and IK1 like immunoreactivity in injured human sensory neurones and its regulation by neurotrophic factors. Brain 125: 252–263.
[Crossref] [Google Scholar] [PubMed]
- Strobaek D, Teuber L, Jorgensen TD, Ahring PK, Kjaer K, et al. (2004) Activation of human IK and SK Ca2+ activated K+ channels by NS309 (6,7-dichloro-1H-indole-2,3-dione 3-oxime). Biochim Biophys Acta 1665: 1–5.
[Crossref] [Google Scholar] [PubMed]
- Richards GR, Weston AH, Burnham MP, Feletou M, Vanhoutte PM, et al. (2001) Suppression of K+ induced hyperpolarization by phenylephrine in rat mesenteric artery: Relevance to studies of endothelium-derived hyperpolarizing factor. Br J Pharmacol 134: 1–5.
[Crossref] [Google Scholar] [PubMed]
- Bolton TB, Clapp LH (1984) The diverse effects of noradrenaline and other stimulants on 86Rb and 42K efflux in rabbit and guinea-pig arterial muscle. J Physiol 355: 43–63.
[Crossref] [Google Scholar] [PubMed]
- Edwards G, Weston AH (2004) Potassium and potassium clouds in endothelium-dependent hyperpolarizations. Pharmacol Res 49: 535–541.
[Crossref] [Google Scholar] [PubMed]
- Armstrong ML, Dua AK, Murrant CL (2007) Potassium initiates vasodilatation induced by a single muscle contraction in hamster cremaster muscle. J Physiol 581: 841–852.
[Crossref] [Google Scholar] [PubMed]
- Kirby BS, Carlson RE (2008) Potassium, contracting myocytes and rapid vasodilatation: peaking more than just our interest? J Physiol 586: 315–317.
[Crossref] [Google Scholar] [PubMed]
 Spanish
Spanish  Chinese
Chinese  Russian
Russian  German
German  French
French  Japanese
Japanese  Portuguese
Portuguese  Hindi
Hindi