Case Report, Arch Transplant Vol: 1 Issue: 1
De Novo Eosinophilic Myocarditis after Heart Transplantation
Mahmoud H Abdou*, M Azam Hadi, Angela Brittsan and Marco A Caccamo
Division of Cardiology, Indiana University School of Medicine, Indianapolis, IN, USA
*Corresponding Author : Mahmoud H Abdou
Division of Cardiology, Indiana University School of Medicine, 401 N. Senate Ave #363. Indianapolis, IN 46204, USA
Tel: (503)737-8367
Fax: (317)962-9704
E-mail: mhra78@gmail.com
Received: May 11, 2017 Accepted: May 31, 2017 Published: June 06, 2017
Citation: Abdou MH, Hadi MA, Brittsan A, Caccamo MA (2017) De Novo Eosinophilic Myocarditis after Heart Transplantation. Arch Transplant 1:1.
Abstract
Eosinophilic myocarditis (EM) is a rare cause of cardiac arrest and cardiogenic shock. However, in the cardiac transplant population on immunosuppressive therapy this phenomenon has never been described. We report a case of a patient 3 years after heart transplantation who suffered a cardiac arrest and had allograft biventricular failure with histological evidence suggestive of EM.
Keywords: Eosinophilic, Myocarditis
Introduction
Eosinophilic myocarditis (EM) is a rare cause of cardiac arrest and cardiogenic shock. However, in the cardiac transplant population on immunosuppressive therapy this phenomenon has never been described. We report a case of a patient 3 years after heart transplantation who suffered a cardiac arrest and had allograft biventricular failure with histological evidence suggestive of EM.
Case Report
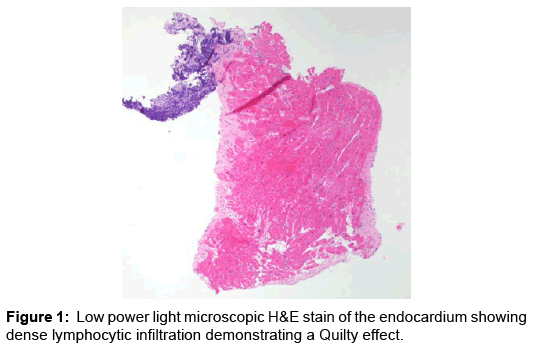
62-year-old African American male post orthotopic heart transplantation performed 3 years prior to presentation. He had a longstanding history of chronic systolic heart failure due to non-ischemic cardiomyopathy thought to be secondary to hypertensive heart disease for at least 10 years prior to cardiac transplantation. He was maintained on appropriately dosed and surveyed immune suppressive therapy. The patient presents after a witnessed ventricular fibrillation (VF) arrest, for which bystander cardiopulmonary resuscitation (CPR) was performed with appropriate electrical defibrillation upon arrival of Emergency Medical System (EMS) personnel. During transfer, he had recurrent VF requiring CPR and eventual return of spontaneous circulation (ROSC). Upon arrival, he underwent emergent left and right heart catheterization and endomyocardial biopsy, which revealed angiographically normal coronary arteries and severely reduced cardiac output with elevated filling pressures consistent with cardiogenic shock. Emergent echocardiogram demonstrated newly reduced biventricular failure (Figure 1).
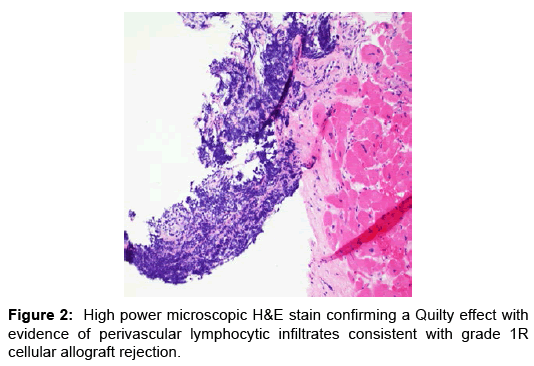
He was subsequently placed on hypothermia protocol and intra-aortic balloon pump counter pulsation (IABP) for mechanical circulatory support and inotropic therapy. He was empirically treated for acute cellular rejection with high dose parenteral steroids and intravenous anti-thymocyte polyclonal antibodies. The results of endomyocardial biopsy subsequently showed evidence of mild (1R) rejection with abundance of eosinophils with negative immunofluorescence (Figures 2 and 3). Hemodynamically he significantly improved and was weaned off IABP, inotropic agents and normalization of blood pressure with evidence of good peripheral perfusion.
Comprehensive infectious workups including viral and parasitic etiologies, in addition to vasculitis workup were unremarkable. Notably, the absolute peripheral eosinophilic count on presentation was significantly elevated to approximately 2700/mm3 that was further elevated from 1000/mm3 three months prior in a routine office visit. Absolute peripheral eosinophilic count was back to zero upon initiation of steroids. A diagnosis of eosinophilic myocarditis was given, in absence of any other obvious or more common etiologies that could explain presence of this significant elevated level of peripheral eosinophilia, and treatment with high dose prednisone was continued.
Interestingly, the hospital course was complicated by development of difficult to treat encephalopathy; MRI brain and brain enolase were not suggestive of significant anoxic brain injury that could be related to downtime during cardiac arrest. Eventually, the patient’s neurologic status recovered, with minimal left side muscle weakness, he was discharged from the hospital to an acute rehabilitation facility for physical reconditioning with a prolonged oral steroid taper regimen. The usual immune suppression regimen with tacrolimus and mycophenolate mofetil were also continued.
Discussion
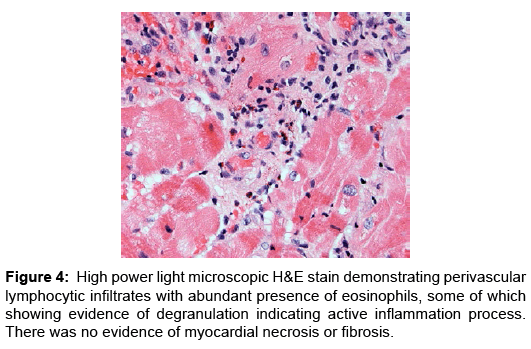
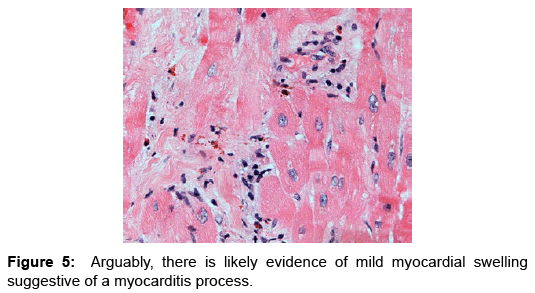
Eosinophilic myocarditis (EM) or hypersensitivity myocarditis affects the heart as a target organ and is considered an allergic reaction to a drug or a substance. Usually it manifests in association with classic allergic symptoms, symptoms of serum sickness, or signs of infectious disease process; more commonly being viral or parasitic in etiology [1,2]. Commonly patients present with signs and symptoms of heart failure that evolves over weeks to months, arrhythmias as a result of fibrosis of the conduction system (Figures 4 and 5), or myocardial infarction as a result of embolism from the left ventricular cavity [3]. It has been reported in heart transplant patients as frequent as 2-7% in few studies and case series. These patients had evidence of EM prior to heart transplantation, majority of which were detected by histological examination of explanted hearts. Interestingly despite the frequency of this finding, none of the patients described in these case series and studies had clinically detectable disease nor was the condition suspected clinically [2,4-6]. Nonetheless, the vast majority of cases manifested peripheral blood eosinophilia. A number of these patients reported to receive drugs known to be associated with EM; loop and thiazide diuretics, angiotensin converting enzyme inhibitors, digoxin and/or dobutamine, drugs commonly used standardly in treatment of advanced stage heart failure [7-9].
However, in these case series, there was no report of development of post-transplant EM and the survival of these patients was similar up to 8 years after heart transplant, entailing that preoperative EM does not confer a poor prognosis nor appears to affect survival after heart transplantation [2].
In our case, the patient did have mild peripheral eosinophilia prior to heart transplantation, but absolute eosinophilic count did not exceed 500/mm3. Furthermore, histological examination of the explanted heart did not reveal evidence of eosinophilic infiltration. This makes the likelihood of the findings in our patient mostly a de novo disease process of development of EM post heart transplantation, as the histological appearance of the endomyocardial biopsy of extensive eosinophilic infiltrate within the myocardium has been described in prior reports as the typical appearance of EM.
However, we did find that the absolute eosinophilic count started to rise prior to his presentation on a routine office visit, and since he was asymptomatic and was doing well clinically, no action was taken at that time. In EM, the eosinophils may be associated with myocardial inflammation in three distinct forms. The first is a hypersensitivity reaction to a foreign antigen known as allergic esosinophilic myocarditis and is often drug induced. Myocardial inflammation may also be triggered in association with systemic eosinophilc disorders such as Churg-Strauss. Lastly, EM may present in the form of fulminant necrotic myocarditis of unclear etiology [10]. As mentioned in the case presentation, a comprehensive work up for common causes of eosinophilia and EM was performed with unremarkable results including viral infections, fungal infections, and lack of use of drugs commonly known to be associated with EM post transplantation. Moreover, the immune suppression regimen post transplantation was also reviewed, and the patient was on appropriate doses of standard immune suppression agents including calcineurin inhibitor (tacrolimus) and cell cycle inhibitor (mycophenolate mofetil), making the etiology in this case unlikely to be related to a manifestation of any form of allograft rejection. The etiology of development of EM in this patient remains unclear especially with absence of any reported cases similar to this one, which makes this case unique. Interestingly in this case, the peripheral eosinophilia improved with a steroid pulse, making this a steroid responsive immunologic process. Eosinophilic myocarditis can present as an acute condition, or chronic, as in connective-tissue diseases, hypereosinophilic syndrome, eosinophilic leukemia, and druginduced or infection-related settings. This patient presented with an acute case manifested by cardiac arrest. Interestingly, despite being on standard post-transplant immunosuppressive therapy.
Despite having a prolonged hospital course complicated by neurological manifestation post cardiac arrest, our patient had an early remarkable recovery of cardiac function and hemodynamic profile after initiation of high dose steroid and aggressive immune suppression. Notably, genetic testing for common genetic mutations of myeloproliferative neoplasms which has been associated with EM [11,12] such as JAK2, MPL, CALR and PDGFRA were negative. In addition, a muscle biopsy was also negative for eosinophilic infiltration; this was tested given the profound muscle weakness developing during the patient’s hospital course.
Conclusion
EM is a rare inflammatory condition that can result in mortality and significant morbidity. It has been reported in pre heart transplant patients as frequent as 2-7% in few studies and case series. However, in these case series, there was no report of development of posttransplant EM. However, as illustrated in the case of our report de novo EM can develop in patient post transplantation while on immune suppression therapy. Endomyocardial biopsy remains the definitive gold standard for diagnosis of noninfectious eosinophilic myocarditis. The absence of steroids at the start of the eosinophilia with prompt response of his clinical course and laboratory studies after initiation of glucocorticoids suggest this response was purely a steroid response. As it is the case with pre-transplant patients, EM is usually associated with or preceded by peripheral eosinophilia, a finding that should prompt a more thorough investigative approach, to lead to more aggressive treatment to prevent progression into a morbid presentation of this condition.
References
- Burke AP, Saenger J, Mullick F VR (1991) Hypersensitivity myocarditis. Arch Pathol Lab Med115: 764-769
- Luthringer DJ, Quartel AW, Mirocha J, Queral CA, Blanche C, Trento A (2004) Eosinophilic myocarditis in patients awaiting heart transplantation 32: 714-721
- Mankad R, Bonnichsen C, Mankad S (2016) Hypereosinophilic syndrome : cardiac diagnosis and management 102: 100-106
- Gravanis MB, Hertzler GL, Franch RH, Stacy LD, Ansari AA, et al. (1991) Hypersensitivity myocarditis in heart transplant candidates. J Hear Lung Transplant 10:688-97
- Lewin D, d’Amati G LW (1992) Hypersensitivity Myocarditis: Findings in Native and Transplanted Hearts. Cardiovasc Pathol 1: 225-229
- Yoshizawa S, Kato S, Mancini D, Marboe CC (2003) Hypersensitivity myocarditis and outcome after heart transplantation. J Hear Lung Transplant 32: 553-559
- Ali AM Al, Frcpc LPS, Allard MF, Frcpc API, Ali AM Al, et al. (2006) Eosinophilic myocarditis: Case series and review of literature. Can J Cardiol 22: 1233-1237
- Fenton M, Burch M, Sebire N (2005) A Dobutamine paradox : eosinophilic myocarditis in the explanted heart of a 9-year-old girl undergoing cardiac transplantation 15: 520-522
- Aranda JM, Woo G, Pauly DF, Rodriguez E, Hill JA (2004) Schofield RS. Patient Awaiting Cardiac Transplantation : the Eosinophilic Dilemma 260-261
- Liu P, Baughman K (2012) Myocarditis L: Braunwald’s Heart Disease: A Textbook of Cardiovascular Medicine. Philadelphia: Elsevier Saunders 1595-1610
- Legrand F, Renneville A, Macintyre E, Mastrilli S, Ackermann F, et al. (2013) The Spectrum of FIP1L1-PDGFRA-Associated Chronic Eosinophilic Leukemia New Insights Based on a Survey of 44 Cases 92: 1-9
- Klion AD, Noel P, Akin C, Law MA, Gilliland DG, et al. (2003) Plenary paper Elevated serum tryptase levels identify a subset of patients with a myeloproliferative variant of idiopathic hypereosinophilic syndrome associated with tissue fibrosis , poor prognosis , and imatinib responsiveness 101: 4660-4666
 Spanish
Spanish  Chinese
Chinese  Russian
Russian  German
German  French
French  Japanese
Japanese  Portuguese
Portuguese  Hindi
Hindi