Research Article, Arch Transplant Vol: 4 Issue: 5
Does Laterality Influence Outcomes in Paired Expanded Criteria Deceased Donor Kidney Transplantation: It All Depends on which Side You’re on!
Hany El-Hennawy1, Muhammad A Khan1, David Harriman1, Alan C Farney1, Jeffrey Rogers1, Giuseppe Orlando1, Amber Reeves-Daniel2, Michael Gautreaux3, William Doares4, Scott Kaczmorski4 and Robert J Stratta1*
1Department of Surgery, Section of Transplantation; Wake Forest School of Medicine, Winston-Salem, NC, United States
2Department of Internal Medicine, Section of Nephrology, Wake Forest School of Medicine, Winston-Salem, NC, United States
3Department of Pathology, Wake Forest School of Medicine, Winston-Salem, NC, United States
4Department of Pharmacy, Wake Forest School of Medicine, Winston-Salem, NC, United States
*Corresponding Author : Robert J Stratta, MD
Department of Surgery, Section of Transplantation ,Wake Forest School of Medicine, One Medical Center Blvd. Winston-Salem, NC 27157, USA
Tel: 336/716-0548
Fax: 336/713-5055
E-mail: rstratta@wakehealth.edu
Received: March 07, 2018 Accepted: March 21, 2018 Published: March 28, 2018
Citation: El-Hennawy H, Khan MA, Harriman D, Farney AC, Rogers J, et al. (2018) Does Laterality Influence Outcomes in Paired Expanded Criteria Deceased Donor Kidney Transplantation: It All Depends on which Side You’re on!. Arch Transplant 2:1.
Abstract
Introduction: The study purpose was to determine if kidney laterality influences outcomes in expanded criteria donor (ECD) kidney transplants (KTs). Methods: A retrospective, case-control comparison of left versus right mate ECD kidneys transplanted at our center was performed. All patients received similar management protocols. Results: Over a 16-year period, left and right kidney pairs from 118 ECDs were transplanted as single allografts into 236 patients.Mean donor and recipient ages were both 61 years. 209 kidneys (88.6%) were pumped and mean cold ischemia time was 25 hours.Demographic characteristics were similar in both groups. Initial length of stay (mean 6.4 days), readmissions (53%), delayed graft function (24%), primary nonfunction (2.5%), and acute rejection (mean 14%) rates were similar between groups. With a mean follow-up of 80 months, patient (54% vs 59%) and kidney graft survival rates (both 41.5%) were comparable in recipients of left vs right kidneys, respectively. There were no significant differences in mean eGFR (based on MDRD) levels at 24 months (left 41 vs right 43 ml/min/1.73m2) in recipients with functioning kidneys. Conclusion: Based on the above findings, it is apparent that kidney laterality does not have a major impact on medium-term outcomes in recipients of ECD kidneys.
Keywords: Deceased donor; Expanded criteria donors; Import kidneys; Kidney laterality
Abbreviations
BMI: Body Mass Index; CIT: Cold Ischemia Time; CrCl: Creatinine Clearance; DCGS: Death-Censored Graft Survival; DCD: Donation after Cardiocirculatory Death; DD: Deceased Donor; DGF: Delayed Graft Function; DSA: Donor Service Area; DWFG: Death With Functioning Graft; ECD: Expanded Criteria Donor; ESRD: End Stage Renal Disease; GFR: Glomerular Filtration Rate; HLA: Human Leukocyte Antigen; IVC: Inferior Vena Cava; KAS: Kidney Allocation System; KDPI: Kidney Donor Profile Index; KT: Kidney Transplantation; MDRD: Modification of Diet in Renal Disease; PNF: Primary Nonfunction; PRA: Panel Reactive Antibody; SCr: Serum Creatinine; UNOS: United Network for Organ Sharing; US: United States
Introduction
For patients with end stage renal disease (ESRD), kidney transplantation (KT) is considered a superior form of renal replacement therapy [1-4]. One of the major challenges in organ transplantation today is the disparity between kidney supply and demand. To increase the size of the pool of donors, liberalization of deceased donor (DD) criteria have resulted in an upturn in the number of “marginal” DDs such as expanded criteria donors (ECD) [5-7], donation after cardiocirculatory death (DCD) donors [8,9], and donors with prolonged warm or cold ischemia time (CIT) [10-12]. Commensurate with efforts to enlarge the donor pool and with changes in the Kidney Allocation System (KAS) including implementation of the Kidney Donor Profile Index (KDPI), the deceased donor (DD) kidney discard rate in the United States (US) increased from 10% in 1998 to 20% in 2017 [13-20].
The influence of kidney laterality on KT outcomes remains controversial. The left kidney is preferred in living donation KT because of a longer vein, which facilitates implantation [21]. In general, the length of the left renal vein is double that of the right renal vein [22]. Additionally, the right renal vein is about half the length of the right renal artery, which may be prone to kinking because of the disparate length of the two vessels [22]. United Network for Organ Sharing (UNOS) Registry reports of DD kidneys transplanted in the late 1980s and early 1990s suggested that early graft survival was superior with left kidneys [23,24] However, in DDs, the right kidney is usually recovered with a full inferior vena cava (IVC), which can be used as a conduit to lengthen the right renal vein [25-30]. In addition, right kidneys tend to be smaller and more frequently have multiple renal vessels, which may increase the risk of thrombosis [22]. Finally, the right kidney vasculature may be at greater risk for injury during either liver or DCD DD organ recovery [31,32].
Although data are conflicting, a recent large registry analysis of paired (mate) kidneys suggested inferior outcomes in adult recipients of right DD kidneys [33]. To our knowledge, no previous reports have studied the impact of laterality on outcomes in recipients of ECD kidneys. The purpose of this study was to determine if kidney laterality affected outcomes in this setting using a paired-kidney analysis
Materials and Methods
Study design
We conducted a retrospective chart review of all DD KTs performed at Wake Forest Baptist Medical Center from 1/1/02 to 1/1/18. During this 16-year study period, 2080 DD KTs were performed at our center. Specific exclusions included pediatric recipients (younger than age 19 years), dual KT recipients, living donor KT recipients, and recipients of kidneys from standard criteria donors (SCD). A retrospective, case-control cohort study of left versus right mate ECD kidney pairs transplanted at our center was performed. The paired kidney analysis eliminates most donor or preservation factors other than kidney anatomy as potential sources of bias. Standardized donor and recipient selection and management algorithms were followed during the period of study [7,34-37].
Definitions
ECDs were defined by UNOS criteria as all DDs aged 60 and older or donors aged 50-59 years with any 2 of the following 3 specific co-morbid conditions: Brain death from cerebrovascular accident, history of hypertension, or a terminal serum creatinine (SCr) level >1.5 mg/dl [5-7]. DCD donor was defined as organ recovery after withdrawal of life support in the absence of brain death [8,9]. Delayed graft function (DGF) was defined as the need for dialysis for any reason in the first week following KT. Primary nonfunction (PNF) was defined as the failure to render the patient dialysis-free following KT or lack of a decline in SCr level in a preemptively transplanted patient. Renal allograft loss was defined as death with a functioning graft (DWFG), allograft nephrectomy, resumption of dialysis, retransplantation, or return to the pretransplant SCr level in patients transplanted preemptively. The KDPI is a numerical scoring system that explicitly compiles 10 donor factors to rank order the quality of kidneys as defined by an aggregate population relative risk (recovered and transplanted deceased donor kidneys from the previous calendar year) to project all-cause allograft survival associated with the use of that particular organ [14,15,18]. The KDPI is based on the Kidney Donor Risk Index (which uses a Cox proportional hazards regression model) and became the basis for the new KAS that was implemented in the US in December 2014 [14,15,38].
Back bench preparation of DD kidneys
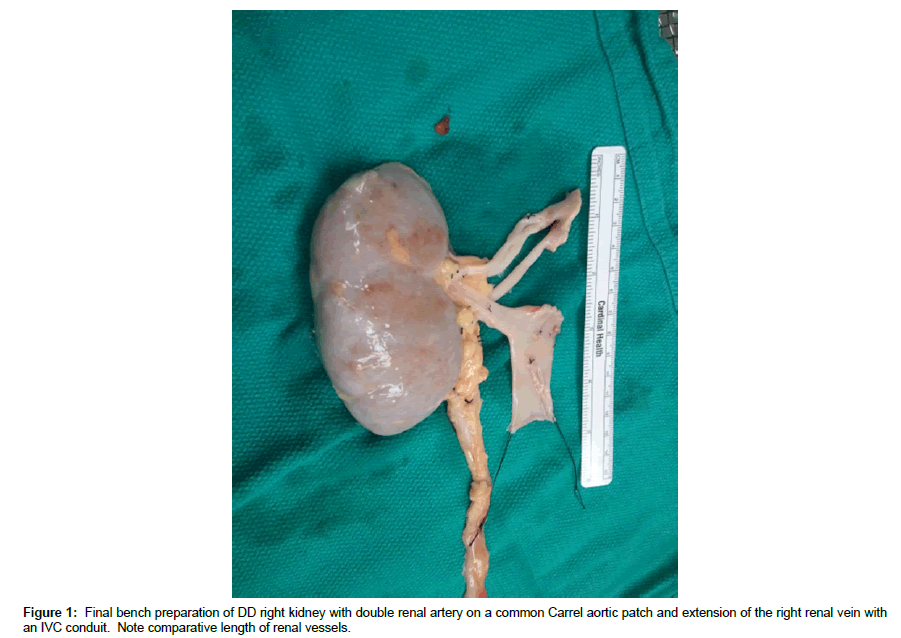
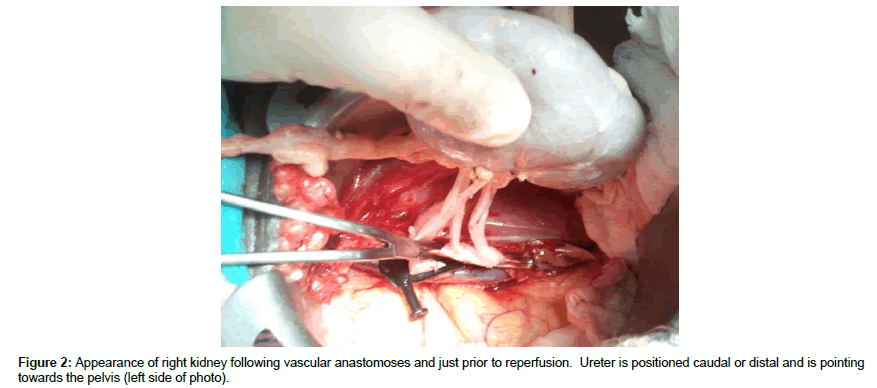
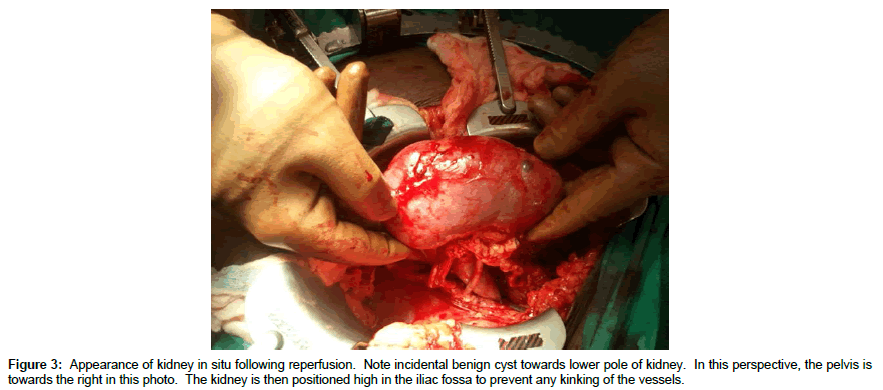
DD right kidneys were reconstructed using the donor IVC as a conduit to lengthen the right renal vein such that it was 1-2 cm longer than the renal artery (Figure 1, note double artery on a Carrel aortic patch) to simplify implantation and avoid kinking of vessels since the external iliac vein is usually deeper and more medial than the iliac artery (Figures 2 and 3) [25-30]. Following dissection and ligation of branches from the IVC and right renal vein, the suprarenal IVC was tailored as needed and then closed with a running 6-0 Prolene overand- over suture. The distal IVC was then cut to length obliquely as described above. Use of the IVC conduit also permitted incorporation of any accessory renal veins as part of the reconstruction. DD left kidneys were similarly prepared on the backbench under cold storage and sterile conditions with the kidney immersed in preservation solution and never in direct contact with iced slush. Conventional hilar and perinephric dissection was performed as the renal vessels were identified, separated and prepared for implantation. Left adrenal, gonadal, and lumbar branches were ligated on the left renal vein in order to maximize its length. Single or multiple renal arteries were prepared by using a cuff of aorta (Carrel aortic patch) to simplify the implantation in the recipient. Vessels were carefully flushed with cold preservation solution to test for leaks and vascular integrity following preparation. Care was taken to preserve peri-ureteral tissue to avoid possible ureteral devascularization. Kidneys were then stored in cold, sterile, preservation solution on the back bench during the vascular dissection in the recipient and prior to implantation.
Donor selection
No specific DD age limits were excluded from consideration; the oldest DD in this study was 77 years and the highest KDPI was 97%. In general, ECDs with other risk factors (positive hepatitis B or C serology, high-risk social/sexual behavior, central nervous system malignancy) were excluded from consideration. A history of diabetes or longstanding hypertension was not a contraindication to using an ECD kidney, unless the donor had documented proteinuria or a decline in renal function. The Cockcroft-Gault formula was employed to estimate adult DD creatinine clearance (CrCl), using both the admission and terminal donor SCr level and adjusted body weight to calculate a range of DD kidney function in order to determine optimal kidney utilization [7,9,34-37]. If the terminal SCr was >2.0 mg/dl, then the kidney(s) from an ECD were not used routinely. If the estimated DD CrCl was >65 ml/min, then a single KT was usually performed. If the estimated DD CrCl was 40-65 ml/min, then an adult dual KT was performed, preferably into an older recipient with a body mass index (BMI) <30 kg/m2 [7,34-37]. If the estimated DD CrCl was <40 ml/min, then the kidney(s) were not transplanted at our center.
Kidney assessment
Donor kidney biopsy was used to assist in the evaluation of preexisting and terminal renal parenchymal injury. Renal cortical wedge biopsies for frozen section were performed and evaluated for the presence and degree of glomerulosclerosis, interstitial fibrosis, chronic interstitial inflammation, tubular atrophy, and vascular hyalinosis or sclerosis [7,9,34-37]. In general, <20% glomerulosclerosis and absence of moderate to severe tubular, vascular and parenchymal changes were considered acceptable for kidney utilization. Whenever possible, ECD kidneys were placed on hypothermic machine perfusion preservation to minimize preservation injury, maintain functional reserve and endothelial integrity, and provide another means of assessment [7,9,34-37]. Although pump parameters were not exclusively used to discard kidneys, a flow rate >80 ml/min and a resistance < 0.40 mm Hg/ml/min after a minimum of 6 hours on the machine perfusion apparatus were considered thresholds for single kidney utilization [35]. If the kidney(s) were pumping well, CITs up to 40 hours and beyond were considered acceptable (the longest CIT in this study was 47 hours). In addition, the logistics of transplanting the kidney with an acceptable (<36 hours) CIT was a consideration, particularly if the kidney was not placed on machine preservation locally and was being imported from another Donor Service Area (DSA) [37]. Whenever possible, DD kidneys were accepted by our center with a minimum of “pump and anatomic waivers" and usually full waivers. A “waiver” implies that a kidney acquisition charge does not have to be paid by the accepting center if the kidney is not transplanted on condition that documentation is provided as to why the kidney was considered unusable (for example, poor pump parameters or unexpected anatomic findings). However, with application of the above donor selection, biopsy, and pump criteria, it was unusual (probably <5%) that accepted kidneys were ultimately discarded although we do not specifically track this data.
Recipient evaluation and selection
At our center, no specific upper age limit was an absolute contraindication to DKT; the oldest recipient in this series was 79 years. All patients underwent a comprehensive pre-transplant medical, psychosocial, and financial evaluation, with emphasis placed on the cardiovascular system to determine operative risks and physiologic age [7,37,39-41]. Non-contrast abdominal/pelvic computerized tomographic imaging (to assess iliac artery calcifications) and cardiac stress testing were performed in all patients. In general, elderly patients needed to be reasonably well compensated, active and functional, not have multiple comorbidities, and have a solid social support system [7,37,39-41]. All patients age 70 years and older also underwent carotid and iliac artery duplex ultrasonographic imaging, cardiology consultation, and heart catheterization. Specific exclusion criteria in the elderly included the presence of dementia, nursing home residence, poor overall functional status or frailty, lack of social support, advanced disease or organ failure in an extra-renal organ system, recent malignancy, or severe cardiac or vascular disease [39-41].
With ECD kidneys, recipient selection was usually not by standard kidney allocation but based on older age (>40 years) and smaller size (BMI <30 kg/m2) matching and identifying low immunological risk patients such as primary KT, human leukocyte antigen (HLA) matching, low panel reactive antibody level (PRA, usually 0%), and informed consent [7,37,39-41] In addition, age matching between donor and recipient was a consideration as we tried to avoid age mismatches >15 years.
Immunosuppression
KT recipients received depleting antibody induction with either multi-dose rabbit antithymocyte globulin or a single dose of alemtuzumab 30 mg intravenous [36]. From 2002-2005, patients received multiple (3-5) doses of rabbit antithymocyte globulin 1.5 mg/kg administered as a 4-6 hour infusion on alternate days (1st dose administered intra-operatively) for induction therapy. From February 2005 to October 2008, patients received either multi-dose rabbit antithymocyte globulin or single dose alemtuzumab as part of a randomized trial [36]. Subsequent to this trial, all patients received alemtuzumab 30 mg intravenous as a single intraoperative dose administered as a 2-3 hour infusion. Maintenance immunosuppression consisted of tacrolimus, mycophenolate mofetil (2 gm/day), and either rapid tapering doses of steroids or early steroid withdrawal based on immunological risk stratification. Target 12 hour tacrolimus trough levels were 6-10 ng/ml; recipients aged 60 years and over received half dose mycophenolate mofetil (1 gm/day) in 2 divided doses [36,39-41]. Early steroid withdrawal was performed in low-risk patients whereas steroids were continued in high immunologic risk patients such as patients receiving retransplants, patients with a current PRA level >20%, and patients experiencing prolonged DGF [36].
Post-transplant management
All patients received surgical site prophylaxis with a firstgeneration cephalosporin for 24 hours, anti-fungal prophylaxis with nystatin or fluconazole for 1-2 months, and anti-Pneumocystis prophylaxis with sulfamethoxazole-trimethoprim (Dapsone® if allergic to sulfa) for at least 12 months. Antiviral prophylaxis consisted of oral valganciclovir for 3-6 months, depending on donor and recipient cytomegalovirus serologic status. Specifics regarding drug dosing and duration have been published previously [7,9,34-37,39-41]. Most patients received aspirin prophylaxis. Treatment of hypertension, hyperlipidemia, anemia, diabetes, and other medical conditions was initiated as indicated, aiming to maintain the blood pressure <140/90 mm Hg, fasting serum cholesterol <200 mg/ dl, hematocrit >27%, and fasting blood sugar <126 mg/dl. Posttransplant renal allograft function was evaluated by measuring SCr levels as well as calculating glomerular filtration rate (GFR) using the abbreviated Modification of Diet in Renal Disease (MDRD) formula.
Statistical Analysis
Endpoints included patient survival as well as uncensored and death-censored graft survival (DCGS). Other study endpoints included early graft loss, PNF, DGF and renal allograft function (based on SCr level and estimated GFR. Data were compiled from both prospective and retrospective databases, with confirmation by medical record review in accordance with local Institutional Review Board guidelines and approval.
Results
From 1/1/02 to 1/1/18, 118 left and right kidney pairs from ECDs were transplanted as single allografts into 236 patients at our center. In looking at consecutive 4-year periods, 27 mate ECD kidney transplants were performed from 2002-2006, 43 from 2006-2010, 34 from 2010-2014, and 14 from 2014-2018 (mean 8.7 ECD pairs transplanted per year during the first 3 periods versus 3.5 ECD pairs per year in the final 4-year period). Mean donor age was 61.4 years, mean KDPI was 84%, and mean estimated DD CrCl was 84 ml/min (Table 1). There were 10 (8.5%) DCD donors (DCD/ECD) and 45 kidney pairs (38.1%) were imported from outside our DSA. A total of 209 kidneys (88.6%) were placed on machine preservation and mean cold ischemia time was 25.2 hours (Table 1) and was similar according to kidney laterality. There was a near equal distribution in terms of whether the left or right kidney was transplanted “first” in mate recipients. Other donor and preservation characteristics are shown in Table 1.
| Mean ± SD or n (%) P=NS | N=118 Donors | Left Kidney N=118 | Right Kidney N=118 |
|---|---|---|---|
| Donor age (years) | 61.4 ± 6.4 | ||
| Donor weight (kg) | 87.7 ± 19.2 | ||
| Donor BMI (kg/m2) | 28.2 ± 6.0 | ||
| Donor gender: Male | 69 (58.5%) | ||
| Donor: African American | 8 (6.8%) | ||
| Donor: Hypertension | 91 (77.1%) | ||
| Donor: Diabetes | 38 (32.2%) | ||
| Donor category: DCD/ECD | 10 (8.5%) | ||
| Cause of death: Trauma Cerebrovascular Anoxia |
14 (11.9%) 92 (78.0%) 12 (10.1%) |
||
| Imported kidneys | 45 (38.1%) | ||
| Kidney Donor Profile Index (%) | 84 ± 10 | ||
| Terminal CrCl (ml/min) | 84 ± 29 | ||
| Terminal SCr (mg/dl) | 1.1 ± 0.5 | ||
| Machine preservation | 209/236 (88.6%) | 105 (89.0%) | 104 (88.1%) |
| Pump time (hours) | 14.8 ± 6.8 | 14.7 ± 7.0 | 14.9 ± 7.0 |
| Pump flow (ml/min) | 116 ± 29 | 113 ± 27 | 119 ± 31 |
| Pump resistance (mm Hg/ml/min) | 0.25 ± 0.08 | 0.27 ± 0.07 | 0.24 ± 0.09 |
| Cold ischemia time (hours) | 25.2 ± 7.6 | 25.4 ± 7.5 | 25.0 ± 7.7 |
Table 1: Donor and Preservation Characteristics.
Demographic and transplant characteristics were similar in recipients of left versus right kidneys (mean age 61.5 years, 55.5% male, 39% African American, 41% diabetic, 6.8% retransplants) (Table 2). There were numerically more retransplants in recipients of left kidneys (12 left vs 4 right, p=0.067) and more African-American recipients of right kidneys (40 left vs 52 right, p=0.14). There was one simultaneous kidney-pancreas transplant recipient and one 3rd kidney transplant recipient in each group. The mean HLA-mismatch was 4.1 and the majority of recipients (84%) had a PRA level of 0%. Mean dialysis vintage was 3 years and 22 patients (9.3%) were transplanted preemptively (Table 2).
| Mean ± SD or n (%) P=NS | Total Group N=236 | Left Kidneys N=118 | Right Kidneys N=118 |
|---|---|---|---|
| HLA-mismatch | 4.1 ± 1.4 | 4.2 ± 1.4 | 4.1 ± 1.3 |
| Zero HLA-mismatch | 5 (2.1%) | 3 (2.5%) | 2 (1.7%) |
| 0% PRA | 199 (84.3%) | 98 (83.1%) | 101 (85.6%) |
| PRA ≥ 20% | 22 (9.3%) | 12 (10.2%) | 10 (8.5%) |
| Retransplant | 16 (6.8%) | 12 (10.2%) | 4 (3.4%) |
| Preemptive transplant | 22 (9.3%) | 13 (11.0%) | 9 (7.6%) |
| Months on dialysis pretransplant | 36.5 ± 32 | 35.7 ± 25 | 37.9 ± 31 |
| Waiting time (months) | 17.4 ± 16 | 17.1 ± 13.5 | 17.7 ± 18 |
| Recipient age (years) | 61.5± 10 | 61.0 ± 10.0 | 62 ± 9.5 |
| Recipient age ≥ 70 years | 51 (21.6%) | 27 (22.9%) | 24 (20.3%) |
| Recipient weight (kg) | 74.0 ± 14.7 | 73.3 ± 14.4 | 74.7 ± 15 |
| Recipient BMI (kg/m2) | 25.9 ± 4.6 | 25.5 ± 4.3 | 26.3 ± 4.9 |
| Recipient gender: Male | 131 (55.5%) | 67 (56.8%) | 64 (54.2%) |
| Recipient: African American | 92 (39.0%) | 40 (33.9%) | 52 (44.1%) |
| Recipient: Diabetes | 97 (41.1%) | 47 (39.8%) | 50 (42.4%) |
| Primary cytomegalovirus Exposure (Donor +/recipient -) | 31 (13.1%) | 14 (11.9%) | 17 (14.4%) |
Table 2: Recipient and Transplant Characteristics.
With a mean follow-up of 80 ± 48 months (minimum one-year follow-up), actual patient (54.2% left vs 59.3% right) and kidney graft survival rates (both 41.5%) were comparable in recipients of left vs right kidneys (Table 3). Among the 118 pairs, during the period of follow-up, in 44 instances both kidneys failed, in 49 one kidney failed, and in the remaining 25 both kidneys continue to function. In the first year post-KT, there were numerically more deaths (4 left vs 10 right, p=0.166) and graft losses (9 left vs 20 right, p=0.046) in recipients of right kidneys (Table 3). In addition, death-censored early (within 12 months) graft losses were more prevalent (6 left vs 14 right, p=0.063) in recipients of right kidneys. One-year patient (96.9% left vs 91.5% right), kidney graft (94.8% left vs 83.0% right), and death-censored graft survival rates (94.8% left vs 87.5% right) were numerically higher in recipients of left kidneys. However, there were 3 cases of primary nonfunction in each group and the incidence of DGF (24%) was comparable (Table 3). In recipients of left kidneys, the 4 early (within one year of KT) deaths included 2 deaths secondary to sepsis and 2 related to cardiac events. In recipients of right kidneys, the 10 early deaths included 5 secondary to sepsis, one related to CMV disease, one cardiac event, one pulmonary embolism (in a previous liver transplant recipient), one stroke, and one liver failure in a patient with Hepatitis C. Of the 6 patients with PNF in both groups, 4 died within one year, including all three who received right kidneys. Of the 9 graft losses that occurred in the first year in recipients of left kidneys, there were 3 DWFG, 3 PNF, 2 acute rejection, and one polyomavirus nephropathy. Of the 20 early graft losses in recipients of right kidneys, there were 6 DWFG, 3 PNF, 6 acute rejection (all in African American recipients, 3 of which were related to overt noncompliance), 4 AKI/acute tubular necrosis, and one pseudoaneurysm that resulted in allograft nephrectomy. There were no differences in 1- and 3-month patient survival, graft survival, death-censored graft loss, or renal function between the two groups.
| Mean (range) or n (%) | Total Group N=236 | Left Kidney N=118 | Right Kidney N=118 | P-Value |
|---|---|---|---|---|
| Actual Patient Survival | 134 (56.8%) | 64 (54.2%) | 70 (59.3%) | 0.51 |
| One Year Patient Survival | 222 (94.1%) | 114 (96.9%) | 108 (91.5%) | 0.166 |
| Five Year Patient Survival | 185 (78.4%) | 91 (77.1%) | 94 (79.7%) | NS |
| Actual Graft Survival | 98 (41.5%) | 49 (41.5%) | 49 (41.5%) | NS |
| One Year Graft Survival | 207 (87.7%) | 109 (92.4%) | 98 (83.0%) | 0.046 |
| Five Year Graft Survival | 153 (64.8%) | 76 (64.4%) | 77 (65.3%) | NS |
| Follow-up (months) | 80 ± 48 | 78 ± 45 | 83 ± 51 | NS |
| Death with functioning graft | 67 (28.4%) | 39 (33.1%) | 28 (23.7%) | 0.15 |
| DCGS | 98/169 (58.0%) | 49/79 (62.0%) | 49/90 (54.4%) | 0.35 |
| Death-Censored Early Graft Loss (in 1st 12 months) | 20/227 (8.8%) | 6/115 (5.2%) | 14/112 (12.5%) | 0.063 |
| Initial graft function: Immediate Slow Delayed |
112 (47.5%) 68 (28.8%) 56 (23.7%) |
55 (46.6%) 34 (28.8%) 29 (24.6%) |
57 (48.3%) 34 (28.8%) 27 (22.9%) |
NS |
| Primary nonfunction/Poor initial graft function | 6 (2.5%) | 3 (2.5%) | 3 (2.5%) | NS |
| # Days to SCr < 3.0 mg/dl | 6.6 ± 5.1 | 6.3 ± 4.9 | 6.8 ± 5.3 | NS |
| Initial length of stay (days) | 6.4 ± 2.8 | 6.5 ± 2.8 | 6.3 ± 2.7 | NS |
| Readmissions in 1st year | 126 (53.4%) | 62 (52.5%) | 64 54.2%) | NS |
| Acute Rejection in 1st year | 33 (14.0%) | 16 (13.6%) | 17 (14.4%) | NS |
| 24 month SCr (mg/dl) | 1.9 ± 0.7 | 1.9 ± 0.75 | 1.8 ± 0.7 | NS |
| 24 month GFR (ml/min/1.73m2) | 42 ± 17 | 41 ± 15 | 43 ± 19 | NS |
Table 3: Results.
The overall DCGS rate (62.0% left vs 54.4% right, p=0.35) was slightly higher in recipients of left kidneys. However, 5-year patient (77.1% left vs 79.7% right) and kidney graft survival rates (64.4% left vs 65.3% right) were similar because of fewer late deaths (50 left vs 38 right, p=0.22) and fewer DWFGs (39 left vs 28 right, p=0.15) in recipients of right kidneys. In recipients of left kidneys, DWFG accounted for 72.2% of deaths and 56.5% of graft losses. In comparison, in recipients of right kidneys, DWFG accounted for 58.3% of deaths and 40.6% of graft losses. In patients (both groups) who experienced graft loss independent of DWFG, their subsequent mortality was 49.3% and death occurred at a mean of 29 months following graft loss (40% of these deaths occurred with one year of graft loss). Causes of death in both groups were equally distributed and included cardiac events, infection/sepsis, respiratory failure, malignancy, and stroke in descending order of frequency.
Initial length of stay (mean 6.4 days), readmissions (53%), delayed graft function (mean 24%), days to reach a serum creatinine level <3.0 mg/dl (mean 6.6 days), and acute rejection (mean 14%) rates were similar between groups (Table 3). There were no significant differences in either mean SCr (left 1.9 vs right 1.8 mg/dl) or eGFR (based on MDRD) levels at 24 months (left 41 left vs right 43 ml/min/1.73m2) in recipients with functioning kidneys.
Discussion
In our own experience, it is apparent that the number of paired ECD kidneys either available or acceptable for mate KT at our center has decreased over time. Prior to the implementation of the new KAS in the US in December 2014, our center on average performed 8-9 paired ECD KTs annually for more than a decade. In the past 4 years, we have performed on average only 3-4 paired ECD KTs annually. This marked reduction in mate ECD KT activity at our center may be related to a number of factors including implementation (and the perceived negative labelling effect) of the KDPI scoring system, aging of the donor population, regional sharing of ECD kidneys (as mandated by the new KAS), and increased risk aversion given the current regulatory environment [13-20]. Centers (and patients) are less willing to consider a kidney from a high KDPI donor compared to an ECD. In addition, with regionalization of ECD sharing, the goal is timely placement of the kidney, which is a positive outcome irrespective of which center receives the kidney. However, in the absence of this occurrence, an unintended consequence is a delay in kidney placement to aggressive centers, extended cold ischemia, and an increase in kidney discards [13-20]. Moreover, in response to increasing regulatory scrutiny and requirements, it has become increasingly difficult for centers (including our own) to “take a chance” on both kidneys from an ECD, particularly if placement is delayed and the kidneys are not managed with machine preservation initially. During this same period, we have likewise seen a marked reduction in our adult dual KT activity, again for the same reasons [34].
Data on the influence of laterality on KT outcomes are conflicting. If there exists an anatomic difference between transplanting left and right kidneys, one would expect the influence on outcome to be primarily a technical issue, which therefore would manifest in the early post-transplant period. In 1992, Gjertson analyzed UNOS Registry data of 35,625 DD KTs performed in the US between 1988 and 1991 and reported that 3-month graft survival rates were superior in recipients of left kidneys compared with right kidneys (90.4% left vs 85% right, p=0.0005) [23] However, 1- and 2-year graft survival rates were noted to be comparable. In 1994, Feduska and Cecka performed another UNOS Registry analysis of 48,541 DD KTs performed in the US between October 1987 - November 1994 and again demonstrated a small benefit of left vs right KT on 1-year graft survival rates (83% vs 81%, respectively, p=0.028), but this apparent improvement was not seen at 2, 3, or even 4 years after KT [24,42]. In 1998, Lechevallier and colleagues performed a retrospective single center study of 257 recipients and reported that right kidney recipients were up to 3 times more susceptible to DGF [43] However, differences in DD characteristics may have confounded their findings because left and right kidneys were not paired in this study. Consequently, it is uncertain whether left KT continues to be a contemporary, independent predictor of early post-transplant graft survival in the new millennium in the setting of substantial improvements in donor and recipient selection and management, organ preservation, surgical techniques, and advances in immunosuppression.
In 2006 using a study design similar to our report, Johnson and colleagues retrospectively analyzed 201 kidney pairs transplanted at their center [44]. They reported outstanding results including rates of DGF of 4% for left vs 6% for right kidneys and DCGS rates of 100% at 1 year and 97.9% at 3 years. Also in 2008, in a similar albeit smaller study, Salehipour and colleagues analyzed 60 kidney pairs and again found no difference in outcomes although the rates of DGF were low and mean CITs were only 3 hours [45]. In a paired kidney analysis using the UNOS database [46], Kayler et al. reported that the longer cold ischemia requisite with the “second” kidney transplanted from an ECD was associated with a higher rate of DGF but this did not appear to have an effect on graft survival [46]. Unfortunately, kidney laterality was not addressed in this study. In 2009, Phelan and colleagues performed a retrospective single center analysis of 323 transplanted left–right DD kidney pairs in Ireland between January 1, 1998 and December 31, 2008 [47]. The incidence of DGF was 16% in both groups and there were no significant differences in acute rejection episodes or serum creatinine levels from 1 month to 8 years post-KT. There were 47 death-censored allograft failures in the leftsided group compared to 57 in the right-sided group (P=0.24). The authors concluded that kidney laterality does not appear to influence outcomes in DD KT. With the findings of the above single center studies, the issue of kidney laterality appeared to be “left behind”.
However, in 2013, Vacher-Coponat and colleagues performed a retrospective paired kidney analysis of 4900 single KTs in adult recipients from 2450 heart-beating DDs in Australia and New Zealand spanning the period from 1995 to 2009 [33]. In this registry study, right kidneys were associated with a higher rate of DGF (21% left vs 25% right) and a lower one-year kidney graft survival rate (91% left vs 89% right, both p<0.01). The authors attributed these inferior early outcomes to a higher rate of surgical complications with right kidneys that resulted in graft loss (n=35 [1.4%] for left vs 66 [2.7%] for right kidneys). Beyond the first year, however, kidney laterality was not associated with disparate graft function or survival outcomes.
A paired DD kidney analysis eliminates donor (and to some extent preservation) factors other than kidney anatomy as potential sources of bias. Kidney laterality is usually not an exclusive consideration in the allocation decision although kidney size, anatomy, biopsy findings (if known), and pump parameters (if available) may certainly influence the choice of which kidney to accept. If the above findings are largely equal, most surgeons prefer the left kidney because it usually requires less “work” or reconstruction on the back bench, particularly if one is creating an inferior vena cava conduit to lengthen the right renal vein [25-30]. Alternatively, in obese recipients or in patients with a deep pelvis, the right kidney may on occasion be preferred because the longer renal vessels may simplify the implantation. However, in the absence of extending the right renal vein, the longer right renal artery may be prone to kinking because a short right renal vein is being anastomosed to an iliac vein that is usually deeper than the iliac artery. Another consideration is the greater susceptibility of the right kidney to injury during either liver or DCD DD organ recovery because of the proximity of the right renal artery to the superior mesenteric artery and the potential for traction injury to the renal vasculature during rapid mobilization of the liver and kidneys [31,32,48]. In addition, right kidneys tend to be smaller and more frequently have multiple renal vessels, which may increase the complexity of the back bench preparation, the recipient implantation, and possibly increase both anastomosis and warm ischemia times in the process [22].
In our study, using a paired kidney analysis exclusive to adult recipients of ECD kidneys, we were not able to demonstrate any sustainable impact of kidney laterality on medium-term outcomes. We elected to focus exclusively on ECD kidneys because of our conjecture that minor differences in outcomes might be amplified in the setting of marginal donor organs. It is important to emphasize that many of these ECD pairs were imported from other DSAs and that CITs were prolonged [37]. However, the majority of these kidneys were placed on machine preservation prior to KT, which might both reduce and mitigate any potential differences in the incidences of DGF according to kidney laterality. In addition, we routinely employed the use of an IVC extension graft with the right renal vein, which also might diminish any variances in early surgical complications and technical graft losses that might be attributed either to disparate vessel length or complexity of transplanting a short right renal vein. We concede that a selection bias in this experience may exist, as kidneys with complex anatomy, complicated injuries, or those unable to be placed on the machine preservation pump were largely excluded [35]. An apparent trend towards increased rates of early (<1 year) death and graft losses in recipients of right kidneys can be largely explained by recipient factors including pre-existing liver disease (in 2 cases) and immunologic graft losses secondary to noncompliance (in at least 3 cases). Moreover, the similar rates of PNF and DGF and similar renal function at 1- and 3-month follow-up suggest that ECD laterality did not have an effect on initial graft outcomes.
In summary, the critical shortage of donor kidneys compounded by the increasing rate of kidney discards defines two of the major challenges in organ transplantation today. It is interesting to note that our study findings appeared to corroborate previous literature in terms of both similarities and differences in outcomes according to kidney laterality “depending on which side you’re on”. With current preservation and surgical techniques, it is reassuring to know that the right kidney from ECDs should not be “left behind” because of concerns that early technical and functional considerations may have an impact on longer-term outcomes.
References
- Ojo AO, Hanson JA, Meier-Kriesche H, Okechukwu CN, Wolfe RA, et al. ( 2001) Survival in recipients of marginal cadaveric donor kidneys compared with other recipients and wait-listed transplant candidates. J Am Soc Nephrol 12: 589-597.
- Merion RM, Ashby VB, Wolfe RA, Distant DA, Hulbert-Shearon TE , et al. (2005) Deceased-donor characteristics and the survival benefit of kidney transplantation. JAMA 294: 2726-2733.
- Tonelli M, Wiebe N, Knoll G, Bello A, Browne S et al. (2011) Systematic review: Kidney transplantation compared with dialysis in clinically relevant outcomes. Am J Transplant 1: 2093-2109.
- Rana A, Gruessner A, Agopian VG, Khalpey Z, Riaz IB, Kaplan B, et al. (2015) Survival benefit of solid-organ transplant in the United States. JAMA Surgery 150: 252-259.
- Metzger Ra, Delmonico FL, Feng S, Port FK, Wynn JJ, et al. (2003) Expanded criteria donors for kidney transplantation. Am J Transplant 3: 114-125.
- Port FK, Bragg JL, Metzger RA, Dykstra DM, Gillespie BW, et al. (2002) Donor characteristics associated with reduced graft survival: An approach to expanding the pool of kidney donors. Transplantation 74: 1281-1286.
- Stratta RJ, Rohr MS, Sundberg AK, Farney AC, Hartmann EL, et al. (2006) Intermediate-term outcomes with expanded criteria deceased donors in kidney transplantation. Ann Surg 243: 594-601.
- Abt PL, Fisher CA, Singhal AK (2006) Donation after cardiac death in the US: History and use. J Am Coll Surg 203: 208-225.
- Farney AC, Hines MH, Al-Geizawi S, Rogers J, Stratta R (2011) Lessons learned from a single center's experience with 134 donation after cardiac death donor kidney transplants. J Am Coll Surg 2012: 440-453.
- Roodnat JI, Mulder PGH, Riemsdijk IC, IJzermans JNM, Gelder T, et al. (2003) Ischemia times and donor serum creatinine in relation to renal graft failure. Transplantation 75: 799-804.
- Kayler LK, Srinivas TR, Schold JD (2011) Influence of CIT-induced DGF on kidney transplant outcomes. Am J Transplant 11: 2657-2664.
- van der Vliet JA, Warle MC, Cheung CLS, Teerenstra S, Hoitsma AJ (2011) Influence of prolonged cold ischemia in renal transplantation. Clin Transplant 25: E612-E616.
- Organ Procurement and Transplantation Network (OPTN) Data 2018 -data-reports
- Formica Jr RN, Friedewald JJ, Aeder M (2016) Changing the kidney allocation system: A 20-year history. Curr Transpl Rep 3: 39-44.
- Stewart DE, Kucheryavaya AY, Klassen DK, Turgeon NA, Formica RN, et al. (2016). Changes in deceased donor kidney transplantation one year after KAS implementation. Am J Transplant 16: 1834-1847.
- Sung RS, Christensen LL, Leichtman AB, Greenstein SM , Distant DA, et al. (2008) Determinants of discard of expanded criteria donor kidneys: Impact of biopsy and machine perfusion. Am J Transplant 8: 783-792.
- Hall IE, Schroppel B, Doshi MD, Ficek J, Weng FL, Hasz RD, et al. (2015) Associations of deceased donor kidney injury with kidney discard and function after transplantation. Am J Transplant 15: 1623-1631.
- Bae S, Massie AB, Luo X, Anjum S, Desai NM, et al. (2016) Changes in discard rate after the introduction of the Kidney Donor Profile Index (KDPI). Am J Transplant 16: 2202-2207.
- Reese PP, Harhay MN, Abt PL, Levine MH, Halpern SD (2016) New solutions to reduce discard of kidneys donated for transplantation. J Am Soc Nephrol 11: 317-323.
- Stewart DE, Garcia VC, Rosendale JD, Klassen DK, Carrico BJ (2017) Diagnosing the decades-long rise in the deceased donor kidney discard rate in the US. Transplantation 101: 575-587.
- Satyapal KS, Kalideen JM, Singh B, Haffejee AA, Robbs JV (2003) Why we use the donor left kidney in live related transplantation. S Afr J Surg 41: 24-26.
- Janschek EC, Rothe AU, Holzenbein TJ, Langer F, Brugger PC, et al. (2004) Anatomic basis of right renal vein extension for cadaveric kidney transplantation. Urology 63: 660-664.
- Gjertson DW (1992) Multifactorial analysis of renal transplants reported to the United Network for Organ Sharing Registry. Clin Transpl 299-317.
- Feduska NJ Jr, Cecka JM (1994) Donor factors. Clin Transpl 381-394.
- Barry JM, Fuchs EF (1978) Right renal vein extension in cadaver kidney transplantation. Arch Surg 113: 300.
- Corry RJ, Kelley SE (1978) Technic for lengthening the right renal vein of cadaver donor kidneys. Am J Surg 135: 867.
- Taylor RJ, Hakala TR, Rosenthal JT (1985) Use of vena cava to extend the right renal vein in cadaveric transplants. Surg Gynecol Obstet 160: 279-280.
- Chopin DK, Popov Z, Abbou CC, Auvert JM (1989) Use of vena cava to obtain additional length for the right renal vein during transplantation of cadaveric kidneys. J Urol 141: 1143-1144.
- Tan LC, Rahman AU, Walters AM, Sadek SA (2000) The inferior vena caval conduit-a neglected technique in transplantation of the right cadaveric kidney? Transpl Int 13: S60-S63.
- Santangelo M, Spinosa G, Grassia S, Clemente M, Caggiano M, et al. (2008) In situ elongation patch in right kidney transplantation. Transplant Proc 40: 1871-1872.
- Barry JM, Lemmers MJ (1995) Patch and flap techniques to repair the right renal vein defects caused by cadaver liver retrieval for transplantation. J Urol 153: 1803-1804.
- Watson CJE, Harper SJF (2015) Anatomical variation and its management in transplantation. Am J Transplant 15: 1459-1471.
- Vacher-Coponat HV, McDonald S, Clayton P, Loundou A, Allen RDM (2013) Inferior early posttransplant outcomes for recipients of right versus left deceased donor kidneys: An ANZDATA registry analysis. Am J Transplant 13: 399-405.
- Stratta RJ, Farney AC, Orlando G, Farooq U, Al-Shraideh Y, et al. (2016) Dual kidney transplants from adult marginal donors successfully expand the limited deceased donor organ pool. Clin Transplant 30: 380-392.
- Stratta RJ, Moore PS, Farney AC, Rogers J, Hartmann EL, et al. (2007) Influence of pulsatile perfusion preservation on outcomes in kidney transplantation from expanded criteria donors. J Am Coll Surg 204: 873-884.
- Farney AC, Doares W, Rogers J, Hartmann EL, Sundberg A, et al. (2009) A randomized trial of alemtuzumab versus antithymocyte globulin induction in renal and pancreas transplantation. Transplantation 88: 810-819.
- Khan MA, El-Hennawy H, Farney AC, Rogers J, Orlando G, et al. (2017) Analysis of Local versus Imported Expanded Criteria Donor (ECD) Kidneys: A Single Center Experience with 497 ECD Kidney Transplants. Clin Transplant 31: e13029
- Rao PS, Schaubel DE, Guidinger MK, Andreoni KA, Wolfe RA, et al. (2009) A comprehensive risk quantification score for deceased donor kidneys: The Kidney Donor Risk Index. Transplantation 88: 231-236.
- Moore PS, Farney AC, Hartmann EL, Rogers J, Doares W, et al. (2007) Experience with deceased donor kidney transplantation in 114 patients over age 60. Surgery 142: 514-523.
- Al-Shraideh YA, Farooq U, Farney AC, Palanisamy A, Rogers J, et al. (2014) Influence of recipient age on deceased donor kidney transplant outcomes in the expanded criteria donor era. Clin Transplant 28: 1372-1382.
- Farooq U, Al-Shraideh Y, Farney AC, Palanisamy A, Rogers J, et al. (2016) Deceased donor kidney transplantation in patients aged 70 and older: Is 70 the new 50? J Gerontol Geriatr Res 5: 1-8.
- Hariharan S, Johnson CP, Bresnahan BA, Taranto SE, McIntosh MJ, et al. (2000) Improved graft survival after renal transplantation in the United States, 1988 to 1996. N Engl J Med 342: 605-612.
- Lechevallier E, Dussol B, Luccioni A, Thirion X, Vacher-Copomat H, et al. (1998) Posttransplantation acute tubular necrosis: Risk factors and implications for graft survival. Am J Kidney Dis 32: 984-991.
- Johnson DW, Mudge DW, Kaisar MO, Campbell SB, Hawley CM, et al. (2006) Deceased donor renal transplantation: Does side matter? Nephrol Dial Transplant 21: 2583-2588.
- Salehipour M, Bahador A, Jalaeian H, Salahi H, Nikeghbalian S, et al. (2008) Comparison of right and left grafts in renal transplantation. Saudi J Kidney Dis Transpl 19: 222-226.
- Kayler LK, Magliocca J, Zendejas I, Srinivas TR, Schold JD (2011) Impact of cold ischemia on graft survival among ECD transplant recipients: A paired kidney analysis. Am J Transplant 11: 2647-2656.
- Phelan PJ, Shields W, O’Kelly P, Pendergrass M, Holian J, et al. (2009) Left versus right deceased donor renal allograft outcome. Transplant Int 22: 1159-1163.
- Sagban TA, Baur B, Schelzig H, Grabitz K, Duran M (2014) Vascular challenges in renal transplantation. Ann Transplant 19: 464-471.
 Spanish
Spanish  Chinese
Chinese  Russian
Russian  German
German  French
French  Japanese
Japanese  Portuguese
Portuguese  Hindi
Hindi