Research Article, Biomater Med Appl Vol: 7 Issue: 1
Effect of Morphology on the In Vitro Bioactivity and Biocompatibility of Spray Pyrolyzed Bioactive Glass
Tsion Chuni Akililu1, Kena Dachasa1, Tadele Hunde Wondium1,2 and Fetene Fufa Bakare1,2*
1Department of Materials Science and Engineering, Adama Science and Technology University, Adama, Ethiopia
2Department of Advanced Materials Science and Engineering Center of Excellence, Adama Science and Technology University, Adama, Ethiopia
*Corresponding Author: Fetene Fufa Bakare
Department of Materials Science
and Engineering, Adama Science and Technology University, Adama, Ethiopia
Tel: +251-(0)935314103
E-mail: hayu.fufa@gmail.com
Received date: 17 October, 2022, Manuscript No. BMA-22-77427; Editor assigned date: 21 October, 2022, PreQC No. BMA-22-77427 (PQ); Reviewed date: 04 November, 2022, QC No. BMA-22-77427; Revised date: 02 January, 2023, Manuscript No. BMA-22-77427 (R); Published date: 09 January, 2023, DOI: 10.4172/2577-0268.1000511
Citation: Akililu TS, Dachasa K, Wondium TH, Bakare FF (2023) Effect of Morphology on the In Vitro Bioactivity and Biocompatibility of Spray Pyrolyzed Bioactive Glass. Biomater Med Appl 7:1
Abstract
Bioactive Glass (BG) is one of the most remarkable materials in the field of biomedical applications because of its fundamental properties such as bioactivity, biodegradability and biocompatibility, which are mainly used for the applications of bone implants, dermal fillers and drug releasing carriers. Here in this study, simple and continuous methods were employed to produce Hollow Spherical Bioactive Glasses (HSBGs) microspheres. Using a spray pyrolysis method, solid and hollow spherical particles were successfully synthesized and the particle formation mechanism was also discussed in detail. Surface morphologies and inner structures of all Bioactive Glass (BG) powders were examined using scanning electron microscopy and transmission electron microscopy, respectively. In addition, in vitro bioactivity was examined by SEM after soaking in Stimulated Body Fluid (SBF). The cell viability of both BG specimens was evaluated at various extraction concentrations using 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenylte trazolium bromide (MTT assay). The in vitro bioactivity of BG powders was determined by evaluating their apatite forming ability in Simulated Body Fluid (SBF). Based on the Ca/P ratio HSBGs possessed higher hydroxyapatiten forming capacity than SBGs. In addition, the cell viability of both specimens showed that at all extraction concentrations, they pass the standard biocompatibility levels. Therefore, the HSBGs have better biocompatibility and in vitro bioactivity; hence they are promising for future tissue engineering development.
Keywords: Bioactive glasses; Bioactivity; Hollow particles; Spray pyrolysis; Transmission electron microscopy; Cell viability
Introduction
Over the last few decades, Bioactive Glasses (BGs) have received special attention in the applications of bone tissue, drug delivery, tooth implants and dermal fillers [1,2]. Particularly, due to its superior properties such as bioactivity, biodegradability, biocompatibility and osteoconductivity, which stimulate new tissue growth which is similar to compositions within the main inorganic components of human bones [3-6]. Among these properties, bioactivity and biocompatibility have received special attention extensively to enhance the clinical demand for BG's performance [7].
Hence, several factors influence the bioactivity and biocompatibility of bioactive glasses such as compositions morphology and surface areas [8-11]. For example, bioactive glass which contains hollow structures improves several properties such as inject ability, flow ability and drug delivery, which are the most significant in tissue engineering [12-14]. In addition, particles with spherical morphology also enhance the activities of bone tissue regeneration, optimizes drug release and minimizes inflammatory reactions than irregular shape particle [15]. Several research works were reported related to the effect of morphology on bioactivity. For example, Liu et al. reported that the spherical and hollow particles were synthesized using Poly Acrylic Acid (PAA) as a template by sol-gel methods in which the bioactivity of the BGs improvement was reported [16]. In addition, spherical bioactive glasses particles with hollow structures have been attracted, due to owing superior properties such as higher specific surface area, lower density, good flow ability and large porosity [17]. Furthermore, bioactive glass, which contains hollow structures have higher characteristic in vitro bioactivity as compared to a solid structure, due to a higher specific surface area which enhances the contacting area of the fluids and the materials. In addition, the morphologies were directly influencing the biocompatibility of the bioactive glasses. For example, Kuo et al. reported that the spherical and smooth sphere particles synthesized by the spray pyrolysis method result in greater cell viability than irregular and rough surface particles [18].
So far, several methods have been used to synthesize BG nanoparticles, such as sol-gel, spray drying and spray pyrolysis methods. Among those methods, the sol-gel method is the most common method due to its chemical flexibility and low temperature synthesis [19]. However, the whole process takes from 2 days to 3 days and it is batch production. In contrast, the Spray Pyrolysis (SP) process offers the benefits of low cost, short process time and continuous fabrication, which is a suitable form of production [20,21].
In brief, surface morphologies and inner morphologies of the bioactive glasses have been proven capable of influencing the bioactivity and biocompatibility of BGs. In our previous studies, PEG was used as a pore-forming agent who could significantly changes the morphology and the structure of BG partic les properties as well as biodegradation and bioactivity ability. However, little attention has been paid to hollow spherical BG particles and their effect on the bioactivity and biocompatibility of BGs. In this work, we study the effect of morphology on the bioactivity and biocompatibility (cell viability) of BGs using the spray pyrolysis method.
Finally, the resulting powders were characterized by Scanning Electron Microscopy (SEM), Transmission Electron Microscopy (TEM), nitrogen adsorption/desorption method Brunauer-Emmett- Teller (BET) method) and MTT assay for the observation of surface morphology, inner structure, specific surface area and cell viability respectively.
Materials and Methods
Powder preparation
Two BG powders were synthesized by SP. Initially, BG precursors without PEG were prepared using, Tetraethyl Orthosilicate (TEOS, Si(OC2H5)4, 99.9 wt%, Showa, Japan), calcium nitrate tetra hydrate (CN, Ca(NO3)2.4H2O, 98.5 wt%, Showa, Japan) and Triethyl Phosphate (TEP, (C2H5)3PO4, 99.0 wt%, Alfa Aesar, USA) were used as the sources for Si, Ca and P, respectively. The precursor solution was prepared by mixing 37.49 g Tetraethyl Orthosilicate (TEOS), Si(OC2H5)4, 99.9 wt%, Showa, Osaka, Japan), 25.50 g calcium nitrate tetra hydrate (CN, Ca(NO3)2.4H2O, 98.5 wt%, Showa, Osaka, Japan) and 4.37 g Triethyl Phosphate (TEP,(C2H5)3PO4, 99 wt%, Alfa Aesar, Haverhill, MA, USA) into 60.00 g of ethanol with 1.60 g of 0.5 M HCl. Secondly, for PEG derived BG particles, the precursor solutions were prepared by adding additional polyethylene glycol (PEG, 95.0 wt %, molecular weight of 600 g/mol, Showa, Tokyo, Japan) Si, Ca and P precursor mixture was dissolved in 60.00 g ethanol combined with 1.00 g 0.5 M HCl and stirred at room temperature for 24 h to form the final precursor solution. For the SP process, the precursor solution was dispersed into fine droplets using an ultrasonic nebulizer (King Ultrasonic Co., Taiwan) at a frequency of 1.65 MHz. The droplets went through the thermal treatments of preheating, calcining and cooling in a tube furnace (D110, Dengyng, Taiwan) at 250°C, 550°C and 350°C, respectively. The surfaces of the particles were charged by electrons released from tungsten corona wire at high voltage (16 kV). After that, the negative charged powders were neutralized and condensed in an earthed stainless steel collector [22].
Characterization
For TGA analysis (TGA, perkin-elmer model TGA-7) the percussor were placed under nitrogen flow to examine the decomposition temperature, in which the heating rate was 20°C/min. In addition, surface morphology and inner structure were examined by Scanning Electron Microscopy (SEM) and Transmission Electron Microscopy (TEM), respectively. For TEM characterization powders were prepared by dispersing the particles in acetone using an ultrasonic bath for around 5 min and then depositing a drop of suspension onto holey carbon film grids. The solvent on the carbon grids was evaporated at room temperature. Furthermore, for the evaluation of bioactivity, Simulated Body Fluid (SBF), which has a similar ionic concentration as human plasma, was used according to Kokubo’s protocol. The resulting powders were washed with deionized water and acetone three times and then dried in an oven at 70°C. Then, to analyze the bioactivity of each specimen SEM was employed. Finally, the assessment of cell viability was carried out using the experimental procedure reported.
Results and Discussion
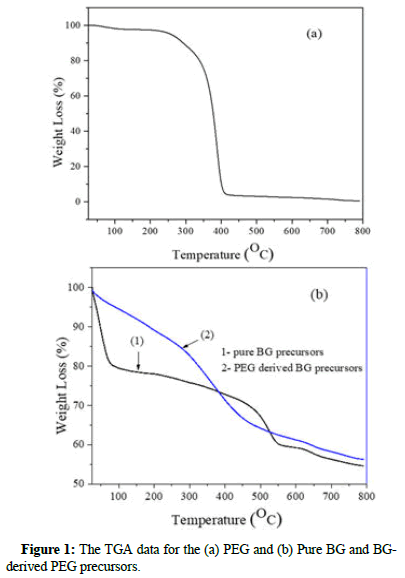
The TGA result of PEG, pure BG and BG-derived PEG precursor was shown in Figure 1. As shown, at ~400°C the sharp peak is associated with the mass loss for PEG in the particles. For pure BG precursors, as shown in Figure 1. With the temperature ranges of ~50°C to ~200°C and 550°C to 800°C can be attributed to dehydration and the formation of calcium oxide, respectively. In addition, BGderived PEG precursors showed that some PEG residues might be within particles, even at 400°C temperature. The second mass loss stage at ~550°C is associated with the decomposition of Ca(NO3)2 and no PEG is remaining in the particles at this temperature.
The specific surface area and pore volume for BG derived-PEG powders are 92.5 ± 0.3 and 0.27 ± 0.01 respectively, which are higher than those of pure BG (41.2 ± 0.1 for specific surface area and 0.22 ± 0.01 for pore volume) (Table 1).
| Materials | Specific surface area (m2g-1) | Pore volume (cm3g-1) |
|---|---|---|
| Pure BG | 41.2 ± 0.1 | 0.22 ± 0.01 |
| BG derived-PEG | 92.5 ± 0.3 | 0.27 ± 0.01 |
Table 1: The specific surface area and pore volume comparison of pure BG and BG derived-PEG powders.
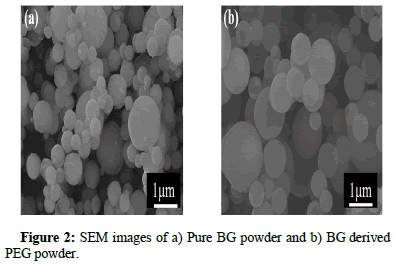
The surface morphology of both BG powders was observed using SEM images, as shown in Figure 2. This shows that solid spherical BG powder was spherical and smooth surface morphology was obtained. Similarly, the SEM images of the BG derived PEG powder also exhibited spherical and smooth surface morphology.
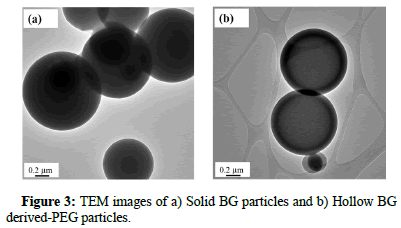
For the inner morphology, Figure 3 shows the TEM images of both BG particles. For the pure BG powder, only the particles with continuous contrast were found, which indicates that there is no thickness variation within the particles. Thus, it can be recognized as a solid particle while Figure 3b, within a hollow sphere that contains a single pore. In summary, by combining both SEM and TEM images, pure BG particles exhibit only one morphology of a solid sphere, whereas the BG derived PEG particles exhibit hollow structures.
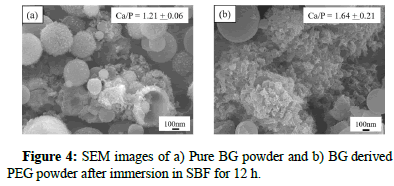
Furthermore, for the evaluations of in vitro bioactivity, both BGs powders were carried out after immersing in SBF for 12 hr. Figure 4, shows the SEM images of both BG powders after immersing in SBF for 24 h. It can be seen from the images that the formation of a needle shape was observed on the surface of each BGs powder.
As shown, (inset) the Ca/P ratio was calculated from SEM-EDS spectra and the higher Ca/P ratio was obtained for BG derived-PEG powders compared to pure BG powders which induce better in vitro bioactivity formation.
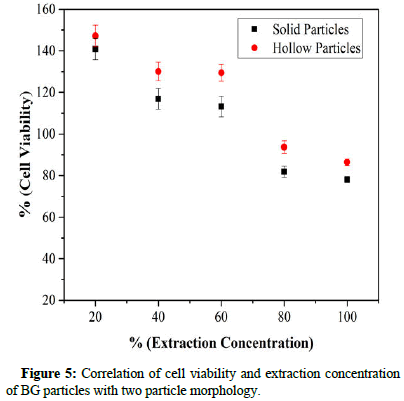
Evaluation of cell viability of the solid and hollow containing a particle of BGs was carried out by MTT assay and the results are seen in Figure 5. Cell viability was measured as the percentage of living cells for each of the different extract concentrations. As seen, both BGs specimens (solid and hollow particles) passed the standard cell viability level of 70% at extraction concentrations of 20%, 40%, 60%, 80% and 100% indicating that all specimens were nontoxic. BG specimens with hollow particles were better in biocompatibility compared to solid particles in all extraction concentrations.
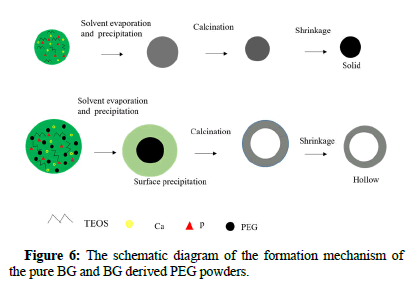
Based on the SEM images of as prepared BG powders, it has been demonstrated that the particle morphology is spherical for both BG powders. This is due to the typical particle formation mechanism of the spray pyrolysis method, similar to the “one-particle per-drop”. Moreover, it can be seen from the TEM prepared BG powders, that pure BG powders exhibit solid whereas BG derived PEG powders exhibit hollow structures. The mechanism of hollow particle formation is expected to be sequential PEG decomposition and BG particle precipitation. Furthermore, temperature increases can lead to pyrolysis of the PEG core, leaving only the BGs shell, as shown in Figure 6.
Conclusion
In this work, solid and hollow spherical BG particle structures were successfully formed within spray pyrolyzed BGs. BG derived PEG powders have a larger specific surface area and performed better in bioactivity as compared to pure BG powders. Both the prepared powders were passing the minimum standard values of cell viability. From this study, the morphology directly influenced the bioactivity and biocompatibility of BG powders. Thus, it can be concluded that BGs with hollow spherical particles are a promising material for future tissue engineering development.
Acknowledgment
We acknowledge Adama science and technology university for funding. The authors also acknowledge the national Taiwan university of science and technology for materials support and characterization of the samples and cytotoxicity test.
Author Contributions
The data collection was performed by Fetene Fufa Bakare. The data analysis was performed by Fetene Fufa Bakare, Tsion Chuni Akililu, Kena Dachasa and Tadele Hunde Wondium. The first draft of the manuscript was written by Fetene Fufa Bakare and all authors read and approved the final manuscript. Writing original draft, Fetene Fufa Bakare and Fekadu Gochole Aga; writing review and editing, Fetene Fufa Bakare.
Data Availability
The data used to support the findings of this study are included in the article.
Conflicts of Interest
The authors declare that they have no conflicts of interest.
References
- Lei XB, Chen X, Koh YH (2011) Effects of acidic catalysts on the microstructure and biological property of sol-gel bioactive glass microspheres. J Sol-Gel Sci Technol 58:656-663.
- Lei B, Chen X, Wang Y, Zhao N, Du C, (2010) Surface nanoscale patterning of bioactive glass to support cellular growth and differentiation. J Biomed Mater Res A 94:1091-1099.
[Crossref] [Google Scholar] [PubMed]
- Hadush Tesfay A, Chou YJ, Tan CY, Fufa Bakare F, Tsou NT, et al. (2019) Control of dopant distribution in yttrium-doped bioactive glass for selective internal radiotherapy applications using spray pyrolysis. Materials (Basel) 12:986.
[Crossref] [Google Scholar] [PubMed]
- Bakare FF, Chou YJ, Huang YH, Tesfay AH, Moriga T, et al. (2019) Correlation of morphology and in-vitro degradation behavior of spray pyrolyzed bioactive glasses. Materials (Basel) 12:3703.
[Crossref] [Google Scholar] [PubMed]
- Bellucci D, Salvatori R, Giannatiempo J, Anesi A, Bortolini S, et al. (2019) A new bioactive glass/collagen hybrid composite for applications in dentistry. Materials (Basel) 12:2079.
[Crossref] [Google Scholar] [PubMed]
- Kaya S, Cresswell M, Boccaccini AR (2018) Mesoporous silica-based bioactive glasses for antibiotic-free antibacterial applications. Mater Sci Eng C Mater Biol Appl 83:99-107.
[Crossref] [Google Scholar] [PubMed]
- Wu C, Chang J (2012) Mesoporous bioactive glasses: Structure characteristics, drug/growth factor delivery and bone regeneration application. Interface Focus 2:292-306.
[Crossref] [Google Scholar] [PubMed]
- Xia W, Chang J (2008) Preparation, in vitro bioactivity and drug release property of well-ordered mesoporous 58S bioactive glass. J Non-Cryst Solids 354:1338-1341.
- Hench LL (2006) The story of bioglass. J Mater Sci Mater Med 17:967-978.
[Crossref] [Google Scholar] [PubMed]
- Mackovic M, Hoppe A, Detsch R, Mohn D, Stark WJ, et al. (2012) Bioactive glass (type 45s5) nanoparticles: In vitro reactivity on nanoscale and biocompatibility. J Nanopart Res 14.
- Rottensteiner U, Sarker B, Heusinger D, Dafinova D, Rath SN, et al. (2014) In vitro and In vivo biocompatibility of alginate dialdehyde/gelatin hydrogels with and without nanoscaled bioactive glass for bone tissue engineering applications. Materials (Basel) 7:1957-1974.
[Crossref] [Google Scholar] [PubMed]
- Kretlow JD, Klouda L, Mikos AG (2007) Injectable matrices and scaffolds for drug delivery in tissue engineering. Adv Drug Deliv Rev 59:263-273.
[Crossref] [Google Scholar] [PubMed]
- Paul W, Sharma CP (1999) Development of porous spherical hydroxyapatite granules: Application towards protein delivery. J Mater Sci Mater Med 10:383-388.
[Crossref] [Google Scholar] [PubMed]
- Xu C, Lei C, Yu C (2019) Mesoporous silica nanoparticles for protein protection and delivery. Front Chem 7:290.
[Crossref] [Google Scholar] [PubMed]
- Liu T, Li Z, Ding X, Zhang L, Zi Y (2017) Facile synthesis of hollow bioactive glass nanospheres with tunable size. Mater Lett 190:99-102.
- Sharma MK, Rohani P, Liu S, Kaus M, Swihart MT (2015) Polymer and surfactant-templated synthesis of hollow and porous zns nano and microspheres in a spray pyrolysis reactor. Langmuir 31:413-423.
[Crossref] [Google Scholar] [PubMed]
- Kuo CK, Chen LG, Tseng CF, Chou YJ (2021) Influences of acid catalysts on the microstructure, bioactivity and cytotoxicity of bioactive glass nanoparticles prepared by spray pyrolysis. J Non-Cryst Solids 560:120710.
- Yan X, Yu C, Zhou X, Tang J, Zhao D (2004) Highly ordered mesoporous bioactive glasses with superior in vitro bone-forming bioactivities. Angew Chem Int Ed Engl 43:5980-5984.
[Crossref] [Google Scholar] [PubMed]
- Ningsih HS, Bakare FF, Liu YC, Chou YJ (2021) Preparation and characterization of spray-pyrolyzed porous SiO2 nanoparticles for low dielectric constant applications. J Aust Ceram Soc 57:1301-1307.
- Chou YJ, Hong BJ, Lin YC, Wang CY, Shih SJ (2017) The correlation of pore size and bioactivity of spray-pyrolyzed mesoporous bioactive glasses. Materials (Basel) 10:488.
[Crossref] [Google Scholar] [PubMed]
- Hong BJ, Hsiao CW, Bakare FF, Sun JT, Shih SJ (2018) Effect of acetic acid concentration on pore structure for mesoporous bioactive glass during spray pyrolysis. Materials (Basel) 11:963.
[Crossref] [Google Scholar] [PubMed]
- Kokubo T, Shigematsu M, Nagashima Y, Tashiro M, Nakamura T, et al. (1982) Apatite and wollastonite-containg glass-ceramics for prosthetic application. Bull Inst Chem Res 60:260-268.
 Spanish
Spanish  Chinese
Chinese  Russian
Russian  German
German  French
French  Japanese
Japanese  Portuguese
Portuguese  Hindi
Hindi