Research Article, J Pharm Drug Deliv Res Vol: 12 Issue: 4
Effect of Penetration Enhancers and Formulation Composition on the Diffusion of Caffeine through Artificial Membranes
Stephen Gichie and Eric Dadey*
Department of Pharmaceutical Sciences, South College, School of Pharmacy, University of Kentucky, Kentucky, United States
*Corresponding Author: Eric Dadey
Department of Pharmaceutical Sciences,
South College School of Pharmacy, University of Kentucky, Kentucky, United
States;
E-mail: edadey@south.edu
Received date: 03 March, 2023, Manuscript No. JPDDR-23-90842;
Editor assigned date: 06 March, 2023, PreQC No. JPDDR-23-90842 (PQ);
Reviewed date: 20 March, 2023, QC No. JPDDR-23-90842;
Revised date: 26 May, 2023, Manuscript No. JPDDR-23-90842 (R);
Published date: 02 June, 2023, DOI: 10.4172/2325-9604.1000235
Citation:Gichie S, Dadey E (2023) Effect of Penetration Enhancers and Formulation Composition on the Diffusion of Caffeine through Artificial Membranes. J Pharm Drug Deliv Res 12:4.
Keywords: Caffeine; Transdermal; Penetration enhancers; Release profiles; In vitro diffusion
Abbreviations:
API: Active Pharmaceutical Ingredient; ECT: Electroconvulsive Therapy; CBD: Cannabidiol; CMC: Carboxymethylcellulose; EMP: Electronic Mortar and Pestle; MCT: Medium Chain Triglycerides; NMSC: Non Melanoma Skin Cancer; PBS: Phosphate Buffered Saline; PEG: Polyethylene Glycol; UV: Ultraviolet; UV-Vis: Ultraviolet-Visible
Introduction
The consumption of caffeine, dates to at least the eighth century CE with the first confirmed report of tea ingestion in 750 CE [1].
Approximately 85% of the U.S. population consumes at least one caffeinated beverage per day, with coffee ranking as the most consumed source of caffeine [2]. Caffeine is found in many natural sources such as coffee beans, cocoa beans, tea leaves, and kola nuts and is well-known for its psychoactive properties. In addition to its mood elevation characteristics, somnolytic properties, and fatigue reduction, consumption of caffeine is reported to produce other pharmacological properties, including improvements in mental alertness and concentration, heightened athletic performance, enhanced weight loss, reduction in the incidence of type II diabetes, and a decrease in the frequency of certain cancers. In an earlier review, Sawynok listed other therapeutic uses of caffeine that include, mitigation of migraine headaches through the constriction of cranial blood vessels, evidence of body weight reduction when used in combination with ephedrine, and the added benefit of seizure prolongation with concomitant improvement of therapeutic outcome when injected intravenously during the course of Electroconvulsive Therapy (ECT), [3].
In addition to enteral delivery, caffeine has been investigated as an intravenous treatment in patients with a history of stimulant drug abuse, and injected intramuscularly (CAFCIT, (Caffeine Citrate; Hikma Pharmaceuticals, PLC) for the treatment of apnea of prematurity [4].
In still other therapeutic applications, caffeine has been applied topically;
• For cosmetic purposes.
• To prevent excessive fat accumulation in skin.
• To promote lymphatic drainage.
• To protect the skin from photo damage [5].
In a recent review, Visconti and colleagues tabulated the results from multiple clinical studies that evaluated caffeine intake as a treatment for Non-Melanoma Skin Cancer (NMSC), rosacea, melanoma facial redness and psoriasis vulgaris [6]. The authors concluded that although caffeine likely offers positive benefits, these results have generated only a few clinical applications and therefore warrant the conduct of additional clinical evaluation of the therapeutic effects of caffeine. Yet other investigators explored the use of topical caffeine for the treatment of atopic dermatitis and psoriasis [7]. Topical application of caffeine at least 30 minutes prior to UV-B exposure was shown to decrease UV-B induced thymine dimer formation and sunburn lesions, creating a sunscreen effect [8].
Other topical formulations of caffeine include cosmetic and over the counter products. In a review of the mechanisms of action for cosmetically formulated products, Herman and Herman indicate that owing to its lipolysis activity, caffeine was incorporated into and used as the active ingredient for the treatment and reduction of cellulite deposits [9].
When taken together, it appears that the topical application of caffeine has the potential to provide therapeutic benefit for a variety of dermatological indications. However, due to the protective properties of the skin, and specifically due to the barrier characteristics of the stratum corneum, the ability of most drugs to penetrate into and through the skin is restricted or limited [10]. Furthermore, there are other physiochemical and physiological factors that may attenuate or exacerbate the rate and extent of transdermal absorption. For example, the weight percent of active ingredient and the amount of the formulation applied to the skin, the physicochemical properties of the API (i.e. hydrophilicity, lipophilicity, and aqueous solubility) are known to influence the transdermal delivery of caffeine. Still yet, the presence or absence of penetration enhancers or other excipients, as well as the presence of open or closed hair follicles affect the rate of transdermal flux [11].
Luo and colleagues tabulated the effect of various types of dosage forms (e.g. emulsions, micro emulsions, nanoparticles, etc.) and the addition of permeation enhancers such as oleic acid, Azone, 1,2- pentanediol, ethanol, and propylene glycol on the absorption of caffeine through human and porcine skin. To this end, the current study evaluates the in vitro absorption of caffeine through an artificial membrane designed to mimic the transdermal delivery of drugs and investigates the effect of various penetration enhancers on the rate and extent of caffeine diffusion. The specific enhancers tested include lauric acid, PEG 3350, tween-80, coconut oil (MCT; Medium Chain Triglycerides) containing cannabidiol, and combinations of these ingredients when incorporated into a carboxymethylcellulose base.
Materials and Methods
Materials
Anhydrous caffeine powder and Polyethylene Glycol (PEG) 3350 were purchased from Letco medical (Decatur, AL) and Carboxymethylcellulose (CMC) from Sigma Aldrich. Lauric acid was acquired from Acros organics (now thermo scientific chemicals; Waltham, MA) and absolute ethanol obtained from fisher bioreagents (Pittsburgh, PA). Cannabidiol tincture was purchased from go green hemp (plantation, FL) and polysorbate 80 (tween 80) from Hawkins, Inc (Roseville, MN).
Procedures
The release and permeation of caffeine from various formulations were assessed using an ILC07 in-line automated diffusion cell system (Permegear, Inc., Hellertown, PA) fitted with 25 mm Strat-M™ Membranes (Merck KGaA, Darmstadt, Germany). Phosphate Buffered Saline (PBS) was pumped at a flow rate of 2.0 mL/hr through each diffusion cell using a calibrated peristaltic pump. All diffusion cells as well as the PBS receiving medium were maintained at 32°C throughout sample collection using a circulating, temperature regulated water bath. Approximately 0.50 g of the selected formulation was weighed into the sample compartment of each of six diffusion cells. Individual 2.0 mL aliquots of the PBS receiving medium were collected into 20 mL scintillation vials during four, one hour intervals (0 hour 1, hour 1 hour 2, hour 2 hour 3, and hour 3 hour 4) using an automatic sampler. Caffeine concentrations of interval were measured using a UV-Vis spectrophotometer with caffeine absorption measured between 274 nm and 306 nm. The cumulative percentage of caffeine diffusing from the formulation through the membrane and into the receiving medium was then plotted as a function of diffusion time.
The diffusion of five caffeine formulations was evaluated in this study. Six replicates of formulation were assessed in parallel with each set of six replicates repeated twice for a total of 12 replicates of each formulation. Briefly, a general preparation of the formulations is outlined below with the full complement formulation used as an example.
Formulations for evaluation were prepared by weighing approximately 3.7 g of aqueous 1.75% Carboxymethylcellulose (CMC) into a 50 mL beaker maintained at 70℃. Anhydrous caffeine powder was weighed and added to the CMC along with an aliquot of water. The resulting mixture was brought to a boil with constant stirring until a homogenous, white to off white suspension was obtained. In a separate beaker, a known amount of lauric acid was dissolved in absolute ethanol and added to the CMC/caffeine solution with constant stirring. Known amounts of CBD oil, PEG 3350, and tween 80 were then added to the caffeine mixture. Approximately 30 g of this mixture were homogenized using an electronic mortar and pestle (EMP; health engineering systems, norman, ok) operating at 5000 rpm for 2 minutes. Finally, the formulation was removed from the EMP, a final aliquot of water added, and the final formulation returned to the EMP and re-homogenized for an additional 2 minutes at 5000 rpm. The cream-like formulations were then stored at room temperature in a 50 g EMP unguator jar. The composition of each formulation is summarized in Table 1. The composition of each formulation was altered sequentially to assess the effect of each ingredient on the release profile of caffeine. For example, formulation 001-79-A-SG assessed the effect of lauric acid on the release of caffeine when compared to the control formulation of caffeine and CMC (i.e., formulation 002-01-B-SG). Conversely, formulation 001-63-A-SG assessed the effect of PEG 3350 on caffeine release when compared to the formulation 002-01-B-SG.
| Formulation ID | Caffeine | CMC | Lauric acid | CBD | PEG 3350 | Tween 80 |
|---|---|---|---|---|---|---|
| Control #1 formulation 002-01-B-SG |
X | |||||
| 001-79-A-SG 001-63-A-SG |
X | X | X | |||
| 001-68-A-SG 001-76-A-SG |
X | X | X | |||
| Control tween 80 002-01-C-SG |
X | X | X | |||
| 001-70-A-SG 001-73-A-SG |
X | X | X | X | X | X |
Table 1: Ingredient composition of each formulation.
Results
The standard curve produced by plotting UV Absorption Values (AUC) against known caffeine concentrations generated a straight line between 5 μg/mL and 25 μg/mL with a linear regression estimate (r2) of 0.9928.
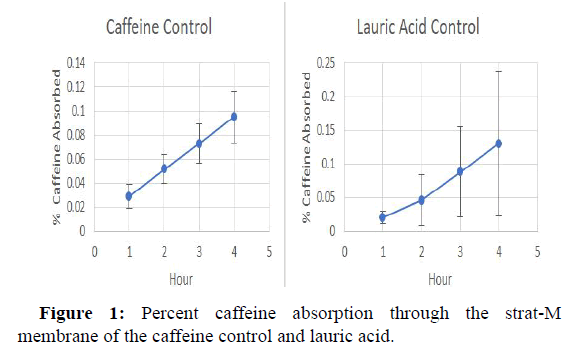
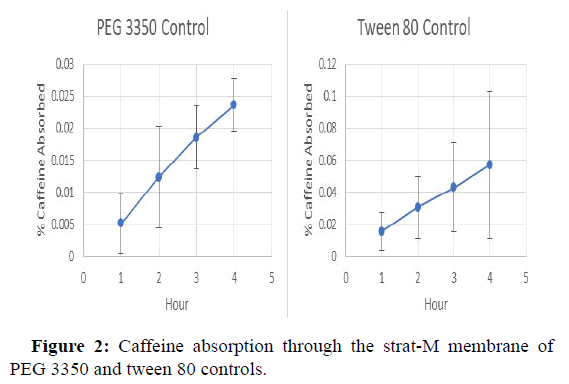
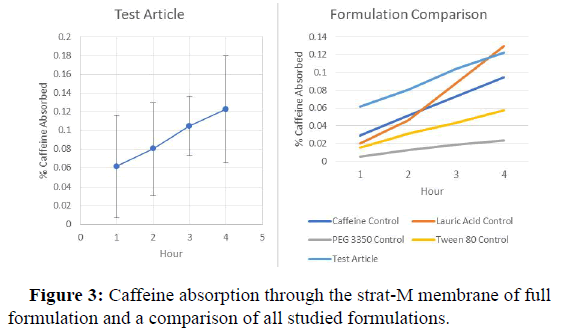
The release profile of caffeine from each formulation containing a single excipient are shown in Figures 1-3, whereas the release profile of the formulation consisting of a mixture of caffeine and all excipients is shown in Figure 3. As a control, the release profile of caffeine alone in aqueous media is shown in Figure 1. The data indicate that caffeine in combination with lauric acid alone demonstrated the highest diffusion with 0.13% of the total caffeine in the formulation passing through the membrane. Whereas caffeine in combination with PEG 3350 alone released the lowest amount, with 0.024% of the caffeine in the formulation ending up in the PBS receiving medium. The rapid absorption of caffeine in Figure 1 is likely due to the absence of carboxymethylcellulose which owning to it known viscosity, may impede caffeine release (Table 2).
| Diffusion time (hours) | Cumulative % caffeine released | ||||
|---|---|---|---|---|---|
| Caffeine control (13.6%)* | Lauric acid (12.3%)* | PEG 3350 (12.5%)* | Tween 80 (12.7%)* | Formulation (8.9%)* | |
| 1 | 0.029 | 0.02 | 0.005 | 0.016 | 0.061 |
| 2 | 0.052 | 0.046 | 0.012 | 0.031 | 0.08 |
| 3 | 0.073 | 0.088 | 0.019 | 0.043 | 0.104 |
| 4 | 0.095 | 0.13 | 0.024 | 0.057 | 0.122 |
*The numbers in parentheses represent the weight % of caffeine in each formulation.
Table 2: Cumulative percent of caffeine released from each formulation during 4 hours of diffusion.
Discussion
According to the diffusion curves illustrated in Figure 3, only lauric acid increased the rate and extent of caffeine diffusion beyond the performance of the caffeine/CMC control formulation while all other individual excipients impeded caffeine release. However, the formulation containing caffeine and all excipients in aqueous CMC exhibited the highest amount of caffeine release during the first three hours and approached the same amount of caffeine release as the lauric acid alone at hour 4. It is hypothesized that this property of the combination formulation is primarily due to the presence of lauric acid. In comparison, the PEG 3350 alone and tween 80 alone formulations in Figures 2 and 3, release lower amounts of the caffeine control. It appears that PEG 3350 and tween 80 inhibit caffeine release. According to Mustapha and colleagues, formulations containing higher concentrations of caffeine lead to a greater in vitro release of caffeine through a poly sulfone membrane, which is of similar composition of the strat-M membranes used in this experiment. Therefore, the lower percentage of caffeine in the full formulation and the corresponding higher caffeine release suggests that unknown secondary release mechanisms play an important role caffeine release.
The percent of caffeine diffused through the membrane for each formulation over 4 hours are presented in Table 2. In comparison, most over the counter topical containing caffeine are formulated with 3% caffeine. Therefore, increasing the weight % caffeine to 12%-14% and the addition of excipients will likely increase the ability of the formulation to protect the skin against the exposure to UV-B radiation.
Conclusion
Due to its low water solubility and its physiochemical properties, caffeine is not ideally suited for transdermal delivery. Therefore, formulation excipients such as surfactants, polymers and oils are needed to assist the delivery of caffeine through skin. Furthermore, other factors such as the site of application, the age of the patient, and presence and density of hair follicles all play a role in the rate and extent of drug absorption the through skin.
Studies in mice demonstrated that topical application of caffeine 30 minutes prior to high UVB irradiation decreases UVB induced thymine dimer formation and sunburn lesions, thereby creating a sunscreen effect. Furthermore, models using UV-B radiated mice have shown that carcinogenesis can be reduced by approximately 70%. Therefore, increasing the rate and extent of transdermal absorption of caffeine will likely increase the protection against UVB induced skin cancers.
The current study evaluated the effect of lauric acid, PEG 3350, tween 80 and a natural product consisting of cannabidiol in MCT oil on the in vitro diffusion of caffeine from a carboxymethylcellulose base. These results were then compared to the diffusion of caffeine from a full formulation containing all components. A comparison among the release profiles showed that a formulation consisting of the caffeine dispersed in 1.75% aqueous carboxymethylcellulose and lauric acid resulted that the largest amount of caffeine released over 4 hours. However, each of the remaining excipients reduced the rate of caffeine diffusion, resulting in lower amounts of caffeine released over 4 hours.
References
- Mitchell DC, Knight CA, Hockenberry J, Teplansky R, Hartman TJ (2013) Beverage caffeine intakes in the U.S. Food Chem Toxicol 63:136-142.
[Crossref] [Google Scholar] [PubMed]
- Sawynok J (1995) Pharmacological rationale for the clinical use of caffeine. Drugs 49:37-50.
[Crossref] [Google Scholar] [PubMed]
- Rush CR, Sullivan JT, Griffiths RR (1995) Intravenous caffeine in stimulant drug abusers: Subjective reports and physiological effects. J Pharmacol Exp Ther 273:351-358.
[Google Scholar] [PubMed]
- Luo L, Lane ME (2015) Topical and transdermal delivery of caffeine. Int J Pharm 490:155-164.
[Crossref] [Google Scholar] [PubMed]
- Visconti MJ, Haidari W, Feldman SR (2020) Therapeutic use of caffeine in dermatology: A literature review. J Dermatol Dermatol Surg 24:18-24.
- Alashqar MB (2019) Caffeine in the treatment of atopic dermatitis and psoriasis: A review. Skin 3:59-71.
- Lu YP, Lou YR, Xie JG, Peng QY, Zhou S (2006) Caffeine and caffeine sodium benzoate have a sunscreen effect, enhance UVB induced apoptosis, and inhibit UVB induced skin carcinogenesis in SKH-1 mice. Carcinogenesis 28:199-206.
[Crossref] [Google Scholar] [PubMed]
- Herman A, Herman AP (2013) Caffeine’s mechanisms of action and its cosmetic use. Skin Pharmacol Physiol 26:8-14.
[Crossref] [Google Scholar] [PubMed]
- Abd E, Roberts MS, Grice JE (2016) A comparison of the penetration and permeation of caffeine into and through human epidermis after application in various vesicle formulations. Skin Pharmacol Physiol 29:24-30.
[Crossref] [Google Scholar] [PubMed]
- Mustapha RB, Lafforgue C, Fenina N, Marty JP (2011) Influence of drug concentration on the diffusion parameters of caffeine. Indian J Pharmacol 43:157-162.
[Crossref] [Google Scholar] [PubMed]
- Otberg N, Patzelt A, Rasulev U, Hagemeister T, Linscheid M, et al. (2007) The role of hair follicles in the percutaneous absorption of caffeine. Br J Clin Pharmacol 654:488-492.
[Crossref] [Google Scholar] [PubMed]
 Spanish
Spanish  Chinese
Chinese  Russian
Russian  German
German  French
French  Japanese
Japanese  Portuguese
Portuguese  Hindi
Hindi