Review Article, Biomater Med Appl Vol: 1 Issue: 1
Emulsions Stabilization for Topical Application
Naves LB1-3*, Luis Almeida2, Maria J Marques2, Graça Soares2 and Seeram Ramakrishna3,4
1CAPES Foundation, Ministry of Education of Brazil, Brasília, Brazil
2Centre for Textile Science and Technology, University of Minho, Guimarães, Portugal
3Center for Nanofibers and Nanotechnology, Department of Mechanical Engineering, National University of Singapore, Singapore
4Guangdong-Hongkong-Macau Institute of CNS Regeneration (GHMICR), Jinan University, Guangzhou, China
*Corresponding Author : Seeram Ramakrishna
Center for Nanofibers& Nanotechnology, Singapore
Tel: (65) 6516 2216
E-mail: seeram@nus.edu.sg
Received: August 04, 2017 Accepted: September 19, 2017 Published: September 27, 2017
Citation: Naves LB, Almeida L, Marques MJ, Soares G, Ramakrishna S (2017) Emulsions Stabilization for Topical Application. Biomater Med Appl 1:1.
Abstract
Emulsions developed for topical applications have attracted so much interested over the last years, due to their characteristics, stability and possibility to work as Nano carrier to deliver an active agent to the skin. There are some aspects that need to be taken into considerations when developing an emulsion for topical application, such as: long-term delivery drugs with beneficial properties, have a pleasant feeling to the users, maintaining its properties during the storage, being stable, biocompatible, and not provide skin irritation.In this manuscript, we report the importance of the stabilization emulsions for topical application purposes, which can be achieved by either adding surfactants, solid particles (Pickering emulsions), or bio-mass. Pickering emulsions are stabilized by adding solid particles into the water-in- oil, oil- in water, or both interface. Moreover, this manuscript includes the use surfactant stabilizer, which can be replaced for biomass-based particles, which are biocompatible and eco-friendly agents.
Keywords: Emulsion stabilizations; Solid particles; Micelles; Topical application; Skin
Introduction
Over the last few years, the interest regarding emulsions has increased, once they possess a substantial and vast number of applications [1]. When formulating emulsions, including application for food, paint, agrochemical, pharmaceutical and oil industries [2], some important factors must be taken into consideration, as they must give a pleasant feeling during its application, long-term of the beneficial effect properties, good appearance regarding the original formulation, and above all, maintain its appeal and also the properties during the storage [3]. The original formulation of lotions should focus on the benefits action on the skin concerning the compounds in its formulation that might evaporate the water containing as well as other volatile polymers and chemicals [4]. It was reported by Mezei and Gulasekharam, that they are focusing their attention on the original dispersion structures, as the evaporation behavior is also important. It is known that the stratum corneum (SC) when it is present in its intact form, can be penetrated by liposomes and vesicles action [5]. Some micro emulsion structures are reported to provoke skin irritation, once that they can disorder the SC increasing the penetration. Colloidal micro emulsion, after less than 30 minutes of application, evaporates, not causing any skin irritation. When developing skin care lotions, the emulsifiers used in its formulation play a significant role during the evaporation process either in simple emulsion or colloidal structures [6]. The formulations of emulsions are used in many processes such as pharmaceutical and personal good that beautify and moisturize our skin. The emulsions can be either water drops in oil or oil drops in water, both kinds of emulsions have the need to be stabilized to prevent their formulation from recoalescing.
Emulsions may also be used to deliver multiple active pharmaceutical ingredients, known as (API´s). Therefore, is important to note the importance of solubility, improve the patient compliance, and permeation and/or distribution. Emulsions, in general, are inherently thermodynamically unstable, for this reason, when dealing with emulsions for topical applications it is crucial the theoretical understanding of several factors that may influence emulsion stability and emulsion formulation [7]. The process by which an emulsion completely breaks (coalescence), i.e., the system separates into bulk oil and water phases, is generally considered to be governed by four different droplet mechanisms. These mechanisms are: creaming, sedimentation flocculation, Brownian flocculation, and disproportionation. The emulsions may destabilize by the first three methods, although all four may occur in any order and simultaneously. In this manuscript we aim to explain the methods and test of emulsions stabilization for topical application.
Emulsions Stabilization
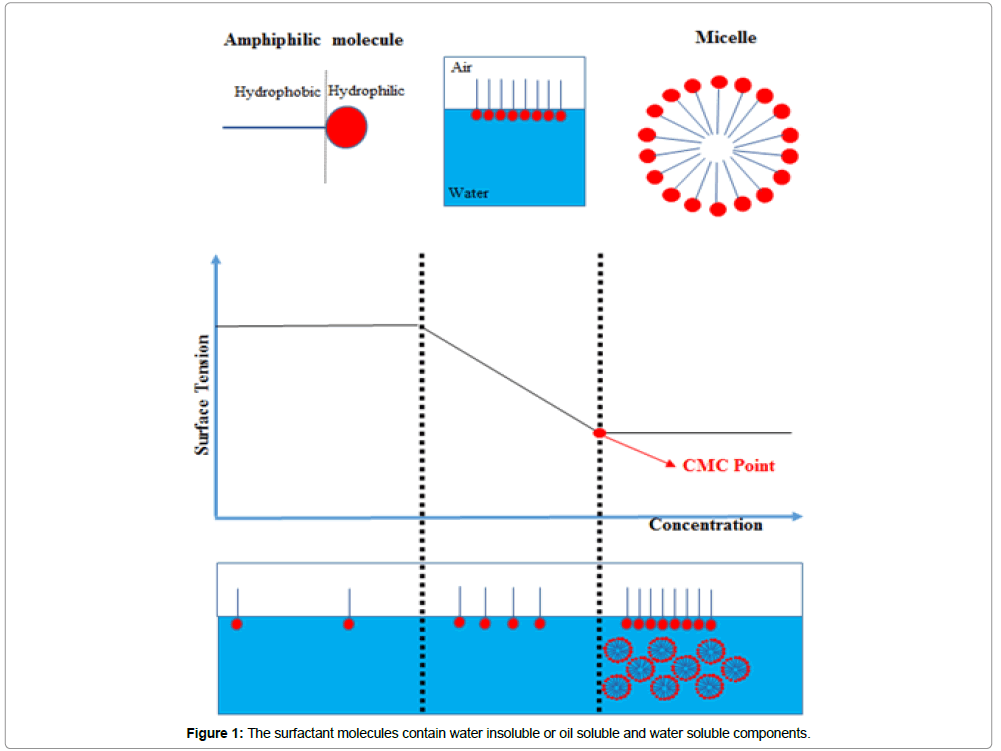
The stabilization of droplet found in the emulsion interface is achieved through the addition of emulsifier which acts increasing the electrostatic repulsion between droplets, steric hindrance and by decreasing the phase interfacial tension. Another way to stabilize the emulsions is by adding amphiphilic molecules such as surfactant (Figure 1). The properties of the emulsion are directed related to the molecular surfactant structures. The molecular structure is decisive for the emulsion phase condition. Surfactants are classified into two groups: non-soluble in water (forming a cloudy dispersion even at a concentration as low as 1%) and water soluble (as the result is obtained a transparent emulsion, when the surfactant concentration varies up to 25%); their designation comes from the appearance in aqueous dispersion. An important factor on the emulsion stabilization influenced by micellization or also described as critical micelle concentration (CMC), the CMC depends on electrolytes, temperature, pressure and the concentration and presence of other active surface substances. The surface of the element changes strongly upon varying the surfactant concentration [8]. However, when the CMC is reached, the tension at the surface remains relatively stable or might change in a lower slope [9], this can be observed in Figure 1. Emulsifiers also play an important long-term effect even in the simple colloidal structures solutions formulations [6]. In addition to small surfactant molecular weight, solid colloidal particles, polymeric emulsifiers and soluble proteins, can be used as emulsion stabilizer by intermediating the wetting properties [10].
As shown in Figure 1, the surfactant molecules contain water insoluble or oil soluble and water soluble components. When these molecules are on oil-water or air- water interface, the hydrophobic molecule part is in air or oil phase and the hydrophilic portion is located in the water phase., When the micelles are in water solution, the hydrophilic head group protects the hydrophobic tails, which is a surfactant energical favorable form. The graphic surface tension versus concentration shows the CMC point, found in logarithmic scale [11].
The stability testing is an integral part of the development of emulsions. The emulsion formulator is generally concerned with understanding the effects that include storage and shipping condition on shelf-life which may include exposure to sunlight, extreme temperatures, humidity and vibration. In Table 1 we list important properties to fully understand important stability and characteristics of an emulsion formulation Table 1.
| Property | Test method |
|---|---|
| Active ingredient(s) | Chemical or bio-assay |
| Color | Visual or colorimeter |
| Conductivity | Conductivity meter |
| Droplet size distribution | Microscopic examination (image analysis) and instrumental |
| Flow behavior | Oscillatory shear viscosity with a cone/plate Rheometer |
| Odor | Organoleptic |
| pH | pH meter |
| Preservation | Microbial challenge and/or assay |
| Separation | Creaming value - visual or instrumental |
| Specific gravity | Pycnometer |
| Tack/Texture | Extensional and compressional deformation |
| Viscosity | Rotational viscometer |
Table 1: Emulsion properties to be monitored during stability test.
The development of lotions for treatments of any disorder includes a two phase formulation; this formulation is not often used on the skin care development, here we describe the stabilization of two and three-phase emulsions
Two-phase emulsion stabilization
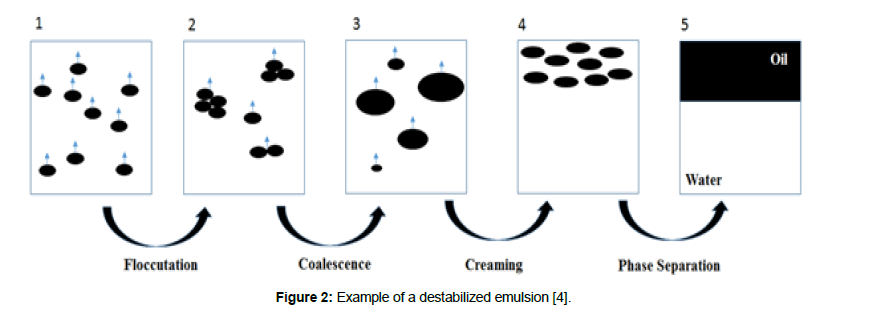
Sometimes referred as the stability of emulsions or stabilization of emulsions, expressed by the monomer, the stability degree of emulsions is in fact measured through their instability; while the destabilization process is undergoing, the rate is given in a quantitative number, called as colloidal stability. The primordial factor that influences the colloidal stability is the dispersion of non-stable particles, because as these particles do not possess inter-forces, they become attached permanently at the touching moment. By adding electrolytes, the stabilization is lost, as electrolyte compresses the electric double layer of different charges, this loss is directly related to the concentration of the electrolytes, also referenced as the rule of Schulze- Hardy [4]. The destabilization is achieved when the compression of electric double layer potential leads to total repulsive potential cancellation. As an alternative for the particle, stabilization is dealing with polymers chain distended from the particle surface into the surrounded liquid [12]. The DLVO theory states that the excess addition of electrolytes concentration may lead to the cancellation of a stable double layer. Although the addition of electrolytes may cancel the stabilization forces, the incensement of salts into the emulsion may interfere to Schulze- Hardy limit, leading to enhancement of stabilization. The stabilization mechanism is the inverse of the destabilization one. The destabilization process is described in four phases (Figure 2). The first step is shown in Figure 2, where there is an aggregation of the droplets, in this period the degree of destabilization can increase addition of salt in excess accordingly. The second phase is named coalescence (Figure 2), the flocculated drops burst, followed by the formation of larger droplets, in this phase if the electrolyte is added, is possible to reduce the surfactant repulsion in the interfacial layer. Therefore, the solution becomes stable and denser. The third phenomena is the gravitational droplet transportation, known as creaming or sedimentation depending upon the density of the continuous and dispersed phase (Figure 2) this step only occurs for macro emulsion droplets, once that in the micro emulsion the Brownian motion is the determinant phenomenon instead of gravitational transportation. The last phase happens when the sedimentation or creaming droplets lead to the formation of a concentrated layer of the emulsion, the coalescent is enhanced. As a result, the coalescence is completed, followed by phase separation (Figure 2) [4].
Figure 2: Example of a destabilized emulsion [4].
Three-phase emulsion stabilization
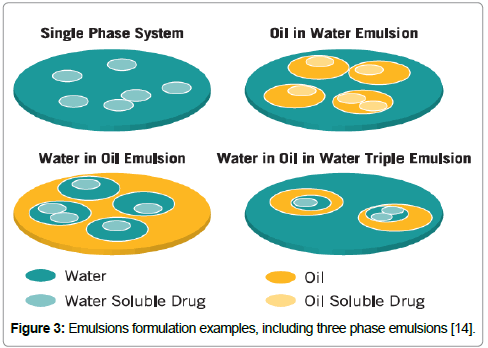
In the three-phase stabilization, the stability is played not only for the surfactant molecules, as well as to liquid crystalline phase [13]. In this process, the limitation of CMC does not exist. The vesicles are found in this stage, which is characterized by the thickness of the shell and the multilayers surrounding it and the radius of the water core. The stability of three-phase system consists in water-particleoil, basically the system comprehends oil and a solid phase dispersed in aqueous solution. It is more complex, as “triple emulsions” have dispersed droplet of continuous phase materials. The three primary interests are the amount of water needed to have the vesicle formation, the fraction of the surfactant concentration versus the total vesicle fraction, and last but not least the ratio between the amount of liquid crystals and the surfactant concentration in the emulsion. Two main destabilization phenomena have an impact on the homogeneity of dispersions: liquid particles and migration of solid, creaming and sedimentation, and aggregation in combination with corresponding/ subsequent increase of particle size, i.e., coalescence and flocculation. Creaming is a common mechanism of instability of emulsions or suspensions; it is observed when the density of the particles is lower than that of the liquid. Sedimentation can occur if the density of the dispersed phase is greater than that of the continuous phase. From the physicochemical point of view, coalescence and flocculation/ coagulation phenomena may occur; they both lead to a shift of size distribution of the dispersed phase towards coarser values. Frequently it is noted not one destabilization mechanism, but a combination of and even interrelation among different ones. Therefore, the presence of more than two phases additionally multiplies and complicates the identification of destabilization mechanisms in a dispersed system. In this case there are three possibilities
Solid particles are well dispersed in (oil) droplet without any attraction to the interface (immersion mechanism);
The solid phase is dispersed in the continuous (aqueous) phase and has no attraction to (oil) droplets (hydrophilic particles)
Solid particles gather at the interface (distribution mechanism).
The volume and structure considerations in emulsions containing liquid crystals are interesting in two situations, the emulsion behavior during the evaporation process followed by stable surfactant emulsions with an excess of Krafft point at room temperature. It is important to mention that emulsions when stabilized by any surfactant with an excess of Krafft point at room temperature, they do not have liquid crystals at this temperature. The surfactant is found in hydrated crystalline form, monomolecular layers only, poor stabilization and very low concentration excess covering the interface [14]. The greater stability can only be achieved at high temperature, in which the surfactants form liquid crystals with water [15]. The emulsions are cooled to room temperature, the lamellar liquid crystals structures at the interface change to gel layered phase, which provides an excellent stabilization. At this point the layered structure with water is destroyed. Therefore, the needle-formed crystals are found instead of water layers, once that this last layer has been squeezed from the structure at the interface. Now the presence of surfactant contributes to the emulsion stabilization, instead of the reverse.
Evaporation Process and Structural Changes
This process is paramount when dealing with emulsion; it has been widely investigated by many researchers depending on their applications. It is possible to find some studies reporting that the solid particle size into the emulsion may delay the evaporation process. Skin care emulsion does not have the significant concern regarding the particles shape and size and the evaporation rate associated with surfactant structures and volatile compounds. The primary factor of W/O emulsion is that after topical application, 90% of the containing water will evaporate, leading to the structural changes [6]. Hence, a liquid crystal with high viscosity is formed, as the water drops in few minutes a fluid crystalline particle is formed. On the other hand, the liquid crystalline material when replaced can result in an emulsion with oil in oil of liquid surfactant, both dissolved. Surprisingly, these changes affect the original emulsion composition. For skin lotions, as they do not contain a significant amount of volatile oils, the evaporation path is useful. We can point that the essential aspect of emulsions for topical application regarding the distillation process effect and behave is that the skin care formulation most of the time undergoes significant structural changes in evaporation process [16], even with the evaporation there is no potential chemical change, so, there is no tendency to cause skin irritations if the initial formulation of the emulsion is non-toxic for dermal human fibroblasts (DHF).
Pickering Emulsions
This kind of emulsions are stabilized by solid particles, as nanoparticles, instead of surfactants, they can be found in any type, water-in-water (W/O), oil-in-water (O/W) or in multiple phases (Figure 4) [17]. These emulsions have been reported for the first time in 1907 by Pickering [18], who published that O/W emulsion may be stable by adding solid particles, which are later absorbed in the surface of oil droplets; in this study he reported one of the major features of O/W emulsions stabilized by solid particles. Particles for emulsions stabilization are possible due to the properties of the particles, which is a combination of partial dual wettability of water and oil phase, size, and shape. The most commonly used particles for Pickering emulsion stabilization are hitherto included synthetic/inorganic particles such as alumina, latex, titanium oxides, clays and silica [2]. Among all benefits of emulsion stabilization by solid particles, the most important characteristics are that these emulsions have a high resistance to coalescence and Ostwald ripening [10]. By eliminating the emulsifier (surfactant), these emulsions become much more attractive for different kinds of applications, especially in pharmaceutics and cosmetic industries where surfactant based emulsions might possess some adverse effects such as hemolytic behavior and cytotoxic. The solid particle size can stabilize the emulsion, when the particles are at the Nano scale, size range from 1-100 nm, or even when these particles are sized at sub-micro-size ~ 100 nm. The smaller the particle size, the smaller is the droplet that can be stabilized, for example, micronsized particles can stabilize larger droplets up to few millimeters in diameter [19].
Figure 3: Emulsions formulation examples, including three phase emulsions [14].
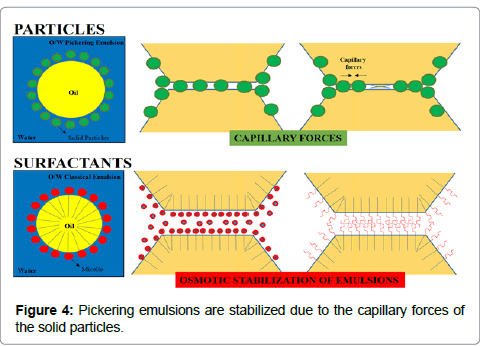
Pickering emulsions, are stabilized due to the capillary forces of the solid particles, as we can observe at the images above. In the surfactantbased emulsions the micelles are absorbed at O/W interface, stabilizing the emulsion through osmotic process (Figure 4) [19,20].
Polymerization- pickering emulsions
Different types of materials either hybrid, organic or even inorganic can be coated with solid particles which make them tightly attached at the oil interface [21]. Polymerization of the conventional emulsion can be done using as stabilizer, solid particles, instead of usual methods as copolymerization of a hydrophilic comonomer, emulsifier absorption or ionic initiator resulting in electrostatic repulsion. The utilization of solid particles instead of conventional stabilization does not change the polymerization [22]. By grafting into the surface of the solid particles an organic monomer, the solid particles surface presents a more hydrophobic behavior, which contributes to the solid particles absorption to the monomer droplets [23]. Miniemulsion polymerization stabilization can be achieved by solid nanoparticles [24]. In this process the solid particles aim the stabilization of the monomer as well the polymer droplets. The stabilization of the monomer droplets with a submicron-size is difficult, once that most monomers are very soluble in water, causing de- stabilization. Porous materials can be done by using as template the Pickering emulsions droplet. Polymerization in Pickering emulsion followed by a drying process can develop porous inorganicorganic hybrid materials. In concentrated emulsions, the porous materials wall is formed by the polymerization in a continuous phase, where are immersed the solid particles. These materials are known as Polymerized High Internal Phase ratio Emulsion, Poly- HIPE [25]. In the emulsion continuous phase, the reinforcement of the wall by the monomer polymerization leads to an improvement in the mechanical strength of porous materials; similar characteristic can be achieved by mixing solid particles (with opposite electrical charge) with surfactant [26].
Nanoparticles Interfaces and their Absorption
When solid particles are in O/W interface, it is necessary a partial wetting of these particles, which can be done by water and oil. This phenomenon is an interfacial energy of three different interfaces, namely, oil-water, solid -oil and solid-water, respectively ᵧo-w, ᵧs-o and ᵧs-w as described by Chevalier & Bolzigner, already mentioned in this manuscript. They reported that the partial wetting of solid particles by water when in an oil medium is needed the spreading coefficient of water, S (W/O), which is negative and the energy-water adhesion, EAdh (W/O) which is positive:
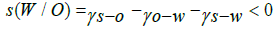 (1)
(1)
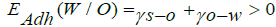 (2)
(2)
By one side, if the solid particles have a very hydrophilic surface, as a consequence of the hydrophilic behavior they will be thoroughly wetted by water interface, afterwards solid particles will no longer be absorbed once that they remain dispersed in the aqueous emulsion phase. By the other side particles with hydrophobic behavior are wetted by the oil. The solid particle absorption under wetting conditions is increased. Free energy adsorption is related to interfacial size and tension of the particles, where the free energy absorption is characterized by the free energy input necessary to the solid particles be released through the surface, either in oil or aqueous phase [27]. The higher absorption rate is found at the 90º angle of contact, corresponding to a maximum emulsion stabilization [28]. The absorption free energy rate is higher in those particles which possesses a larger area (larger particle size) in contact with water and oil.
Particles Stabilization
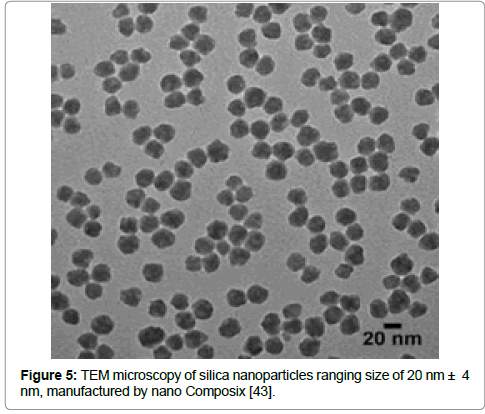
In 1923, Finkle and coworkers were the first researchers to report the correlation between the particles ability to stabilize the emulsion and their wettability; this is called Finkle´s rule. The Finkle´s rule says that the droplet system will be favored either water-in-oil or oil-in-water, which depends on the angle of contact at the interface of oil-particle-water [29]. The stabilization through particles use is not theoretically different from macro-molecular emulsifiers such as hydrocolloids or proteins, by absorbing the oil-water interface, the particles became trapped, as a result of the free energy detachment [30]. Either organic or inorganic, many particles can achieve a greater wetting condition in oil interface. As an example of these particles we can mention: laponite [31], micelles copolymers blockers [32], carbon nanotubes [33], carbon black, barium sulfate and calcium carbonate [34]. However, for Pickering emulsions, the best particle stabilizers are spores [35], hydrophobic bacteria [36] and cationic Nano crystals [37]. Hydrophobic particles meet the perfect conditions for partial wetting by oil and water, to enhance the hydrophobicity behavior surface modification of solid particles. Previous studies reported that oil content changes in the emulsion, for example, may lead to an inversion of the phase interface of Pickering emulsions [38]. The dispersed phase fraction level was described by Binks & Lumsdon, who reported that the inversion occurs depending on the affinity of the particles for the initial continuous phase in Pickering emulsion [39]. Some inorganic particles might have hydrophilic surface characteristics, in this case, to ensure a better wetting by oil and water, is required a hydrophobic coating (silica is one of the most used as particle stabilizer). Instead of adsorption, chemical grafting is preferred, even though it can present some limitations, once that this method involves reactions with the groups at solid surface; for chemical grafting the chemicals should be stable against hydrolysis and such reaction is preferably done by dry organic solvent, in a suspension, or on the dry powder of the solid materials, in an aqueous medium these chemicals reactions are not compatible. Absorption of organic polymers can make the surface of the particles more hydrophobic [40]. Pickering emulsion stabilization can be achieved due to the solid particles flocculation, thus neutralization of different charge, as a result of multivalent ions absorption of different charge at the solid surface takes place, as an example the lanthanum cations onto silica [41]. Silica particles have been done from some manufacturers as Cabot, Wacker, Evonik and nano Composix [42] (Figure 5), this is the most used solid material due to its surface that can quickly become hydrophobic by organosilanes grafts; the surface coverage, known as grafting degree plays a role in the hydrophobic characteristics of silica, as well the wetting capacity in oils and water. In Pickering emulsions, the droplets size is related either to the emulsification energy intensity in a high concentration of solid particles or to the amount of particles available, dispersed in the phase to stabilize the emulsion interface at lower concentration of solid particles (Tables 1 and 2) Figure 5 [19,20,43].
| Storage conditions | Storage Period |
|---|---|
| Ambient temperature | 25ºC for 3 years (or projected shelf-life of the product) |
| Cycling chamber | 4ºC to 45ºC in 48 hours for 1 month |
| Elevated temperature | 37ºC for 6 months and 45ºC for 6 months |
| Freeze/thaw cycles (5) | Approximately -10oC to ambient |
| Light exposure | 1 month exposure to north-facing daylight or light cabinet |
| Refrigerator | Approximately 4oC for 3 months |
Table 2: Most used test conditions for emulsion stability testing.
Figure 5: TEM microscopy of silica nanoparticles ranging size of 20 nm ± 4 nm, manufactured by nano Composix [43].
Biomass Stabilization
In the oil-water interface, the size and the angle of contact, including other particles properties will directly influence the wettability and the eventual emulsion stability. The surface roughness and morphology may change the emulsifying behavior of the solid particles. It was reported that by increasing the roughness surface of these particles, consequently, increase the particles emulsification capacity. This behavior of the solid particles can reach a so high level, where the roughness surface degree of the particles can no longer be wetted homogeneously by the liquids interface. Hence, the resulting emulsions stability is drastically decreased [44]. An eco-friendly alternative option to this issue might be the bio-mass based particles, which mainly consists of particles that are often made from bio-degradable and renewable resources, presenting nonspherical morphology, however many literature [45-47] reported that according to capillary interaction of anisotropic particles curved fluid interfaces may have different shapes, as pentagons, ellipses, stars, and dumbbells. These particles can be synthesized from molecules extracted from biomass (e.g. crystallization, coacervation, aggregation, precipitation or crosslink), obtained by isolating their intrinsic biological structures or decreasing the size of the existing structures. As an example of biomass-based solid particles we can mention the Nan rods, which are made from cellulose (cotton, bacteria and algae), they are found in various ratios [48]. When approaching biomass particles synthetically, we can exemplify chitin nan crystals [49], flavonoid particles [50], chemically modified Nano spheres [51],fat crystals and solid lipid particles [52]. On the other hand, when particles are obtained by the breakdown of larger structures (milling and hydrolysis), these include: ethyl cellulose particles [53], cellulose Nan crystals [54] and keratin wool powder [55].
Conclusion
The emulsions stability is related to the surfactant behavior in diluted aqueous solution. The micelles, formed by the surfactants are absorbed to the W/O interface in monolayers form. In addition to that, the surfactant concentration at the interface is limited to the amount at its CMC. Vesicles form is caused by the high energy of emulsification-dispersed to lamellar liquid crystals. During the evaporation process the emulsions undergo crucial changes in their formulations; these changes must be taken into consideration, when developing emulsion for topical applications. We also point out that not only surfactant but also nanoparticles can be used as a stabilizer. Pickering emulsions have been for a long period left without any scientific interested, however now this situation has changed. The scientific community worldwide has increased the interest in Pickering emulsions, once they possess an excellent stability against the coalescence, making possible the development of stable double emulsions. They can be used in many different fields, porous materials and drug delivery systems are examples of it. Because of solid nanoparticles coating the surface of the emulsion droplets a rigid shell is formed, acting as a barrier against materials transferring through the surface and against deformation. By using Pickering emulsion, it is possible to decrease or eliminate the usage of active agent at the surface. Instead of using surfactant, it can be replaced by eco-friendly agents and more biocompatible as biomass- based particles, which are defined as materials made from renewable resources. The stable emulsions can be used as Nano carriers for topical application; a promising strategy is the design of advanced target Nano carriers. A rational design of Nano carriers should ensure a skin permeability, penetration and biocompatibility producing a local pharmacological action decreasing the side effect. It has been shown in this manuscript that polymer characteristics, as well particle size, surface modification among other factors may affect directly the efficiency, bio distribution and safety, therefore, the availability of the Nano carrier. In this context, stands out the design of polymeric nanoparticles by nan-emulsion as a versatile and safe technique for the Nano carriers preparation.
Acknowledgments
Authors want to acknowledge financial support from CAPES foundation for the PhD grant with process number 13543/13-0, Brazilian Ministry of Education- Brazil. Authors also like to thank FEDER funds through the Competitivity Factors Operational Programme - COMPETE and national funds through FCT – Foundation for Science and Technology (POCI-01-0145- FEDER-007136).
Conflict of Interests
Authors declare that there is no competing interest associated with this manuscript.
References
- Sjöblom JH ( 2001) Encyclopedic handbook of emulsion technology. New York.
- Aveyard R, Binks BP, Clint JH (2003) Emulsions stabilised solely by colloidal particles. Adv Colloid Interface Sci 102: 503-546.
- Roguet R (2002) The use of standardized human skin models for cutaneous pharmacotoxicology studies. Skin Pharmacol Appl Skin Physiol 15: 1-3.
- Al-Bawab A, Friberg SE (2006) Some pertinent factors in skin care emulsion. Adv ColloidInterface Sci 123: 313-322.
- Mezei M ,Gulasekharam V (1980) Liposomes - a selective drug delivery system for the topical route of administration I Lotion dosage form. Life Sci 26: 1473-1477.
- Friberg SE, Al-Bawab A,Odeh F, Bozeya A, Aikens PA, et al. (2009) Emulsion evaporation path. A first comparison of experimental and calculated values Colloids Surfaces. A PhysicochemEng Asp.338: 102-106.
- Brief T (2011) Emulsion stability and testing.Sci Drug Dev Serv2: 1-2.
- Aranberri I,Binks B, Clint J,Fletcher P (2006) Elaboración y caracterización de emulsiones estabilizadas por polimeros y agentes tensioactivos. Rev Iberoam Polímeros 7: 21.
- Mc Naught AD,Wilkinson A (2012) Compendium of Chemical Terminology-Gold Book Iupac 1670.
- Rayner M, Marku D, Eriksson M, Sj M, Dejmek P, et al. (2014) Biomass-based particles for the formulation of Pickering type emulsions in food and topical applications.Colloids Surfaces A Physicochem Eng Asp458: 48-62.
- Sigma A, Concept of Critical micelle. Attension TN. 2-3.
- Buscall R, Corner T (1982) The Phase-separation behaviour of aqueous solutions of polyacrylic acid and its partial sodium salts in the presence of sodium chloride. Eur Polym J 18: 967-974.
- Friberg S, Mandell L, Larsson M (1969) Mesomorphous phases a factor of importance for the properties of emulsions. J Colloid Interface Sci 29: 155-156.
- Drug development services (2009) Emulsions and Emulsification. Part Sci -Tech 9.
- Larsson K (1994) Lipids - Molecular organization physical functions and technical applications.The Oily Press Ltd : 5.
- Al-Bawab A, Barber JL, Friberg SE, Aikens PA (1998) Phase Equilibria in the Systems of Acetic and Glycolic Acid With Water and Laureth 4.J Dispers Sci.Technol19: 399-420.
- Binks BP (2007) Colloidal particles at liquid interfaces.Phys Chem Chem Phy 96: 6298-6299
- Pickering SU (1907) CXCVI-Emulsions. J Chem Soc Trans 91: 2001-2021.
- Chevalier Y, Bolzinger MA (2013) Emulsions stabilized with solid nanoparticles Pickering emulsions.Colloids Surfaces A Physicochem Eng Asp 439: 23-34.
- Tcholakova S, Denkov ND, Lips A (2008) Comparison of solid particles globular proteins and surfactants as emulsifiers. Phys Chem Chem Phys 10: 1608-1627.
- Arditty S, Schmitt V, Giermanska-Kahn J, Leal-Calderon F (2004 ) Materials based on solid-stabilized emulsions. J Colloid Interface Sci 275: 659-664.
- Herrera NN, Letoffe JM, Putaux JL, David L, Bourgeat-Lami , et al. (2004) Aqueous dispersions of silane-functionalized laponite clay platelets.A first step toward the elaboration of water-based polymer/clay nanocomposites. Langmuir 20: 1564-1571.
- Teixeira RFA, McKenzie HS, Boyd AA, Bon SAF (2011) Pickering emulsion polymerization using Laponite clay as stabilizer to prepare armored soft polymer latexesMacromolecules.44: 7415-7422.
- Tiarks F, Landfester K, Antonietti Silica M (2001) Nanoparticles as Surfactants and Fillers for Latexes Made by Miniemulsion Polymerization Silica Nanoparticles as Surfactants and Fillers for Latexes Made by Miniemulsion Polymerization.Langmuir 17: 5775-5780.
- Destribats M, Faure B, Birot M, Babot O, Schmitt V, et al. (2012) Tailored silica macrocellular foams Combining limited coalescence-based pickering emulsion and Sol-Gel process. Adv Funct Mater 22 :2642-2654
- Simovic S, Heard P, Prestidge CA (2010) Hybrid lipid-silica microcapsules engineered by phase coacervation of Pickering emulsions to enhance lipid hydrolysis. Phys Chem Chem Phys 12: 7162-7170.
- Tadros TF, Vincent B (1983) Emulsion stability in Encyclopedia of Emulsion Technology. 1:129-285.
- Binks BP, Lumsdon SO (2000) Influence of particle wettability on the type and stability of surfactant-free emulsions.Langmuir 16: 8622-8631.
- Finkle P, Draper HD, Hildebrand JH (1923) The Theory of Elmusification. J Am Chem Soc 45: 2780-2788.
- Binks BP (2002) Particles as surfactants-similarities and differences.Curr Opin Colloid Interface Sci 7: 21-41.
- Ashby NP, Binks BP (2000) Pickering emulsions stabilised by Laponite clay particles. Phys Chem Chem Phys 2: 5640-5646.
- Laredj-Bourezg F, Chevalier Y, Boyron O, Bolzinger MA (2012) Emulsions stabilized with organic solid particles. Colloids Surfaces A Physicochem Eng Asp 413: 252-259.
- Wang H, Hobbie EK (2003) Amphiphobic carbon nanotubes as macroemulsion surfactants. Langmuir 19:3091-3093.
- Levine S, Sanford E (1985) Stabilisation of emulsion droplets by fine powders. Can J Chem Eng 63: 258-268.
- Binks BP, Clint JH, Mackenzie G, Simcock C, Whitby CP, et al. (2005) Naturally occurring spore particles at planar fluid interfaces and in emulsions. Langmuir 21-8161-8167.
- Dorobantu LS,Yeung AKC, Foght JM, Gray MR (2004) Stabilization of oil-water emulsions by hydrophobic bacteria. Appl Environ Microbiol 70: 6333-6336.
- Schelero N, Lichtenfeld H, Zastrow H, Möhwald H, Dubois M, et al. (2009) Single particle light scattering method for studying aging properties of Pickering emulsions stabilized by catanionic crystals.Colloids Surfaces A Physicochem Eng Asp 337: 146-153.
- Tzoumaki MV, Moschakis T, Kiosseoglou V, Biliaderis CG (2011) Oil-in-water emulsions stabilized by chitin nanocrystal particles. Food Hydrocoll 25: 1521-1529.
- Binks BP, Lumsdon SO (2000) Catastrophic Phase Inversion of Water-in-Oil Emulsions Stabilized by Hydrophobic Silica. Langmuir 16: 2539-2547.
- Midmore BR(1999) Effect of Aqueous Phase Composition on the Properties of a Silica-Stabilized W/O Emulsion. J Col Interf Sci 213: 352-359.
- Binks BP, Lumsdon SO (1999) Stability of oil-in-water emulsions stabilised by silica particles. Phys Chem Chem Phys 1: 3007-3016.
- Barthel H (1995) Surface interactions of dimethylsiloxy group-modified fumed silica Colloids Surfaces. A Physicochem Eng Asp 101: 217-226.
- Nano Composix (2016) NanoXact.
- San-Miguel A, Behrens SH (2012) Influence of nanoscale particle roughness on the stability of pickering emulsions. Langmuir 28: 12038-12043.
- Le Cheng T, Wang YU Shape-anisotropic particles at curved fluid interfaces and role of Laplace pressure A computational study. J Colloid Interface Sci 402: 267-278.
- Loudet JC, Alsayed AM, Zhang J, Yodh AG (2005) Capillary interactions between anisotropic colloidal particles. Phys Rev Lett 94: 9957-9971.
- Günther F, Janoschek F, Frijters S, Harting J (2013) Lattice Boltzmann simulations of anisotropic particles at liquid interfaces. Comput Fluids 80: 184-189.
- Kalashnikova I, Bizot H, Bertoncini P, Cathala B, Capron I, et al. (2013) Cellulosic nanorods of various aspect ratios for oil in water Pickering emulsions. Soft Matter 9: 952-959
- Tzoumaki MV, Moschakis T, Scholten E, Biliaderis CG (2013) In vitro lipid digestion of chitin nanocrystal stabilized o/w emulsions. Food Funct 4: 121-129.
- Luo Z, Murray BS, Ross AL, Povey MJW, Morgan MRA, et al. (2012) Effects of pH on the ability of flavonoids to act as Pickering emulsion stabilizers. Colloids Surfaces B Biointerfaces 92: 84-90.
- Tan Y, Xu K, Liu C, Li Y, Lu C, et al. (2012) Fabrication of starch-based nanospheres to stabilize pickering emulsion. Carbohydr Polym 88: 1358-1363.
- Gupta R, Rousseau D (2012) Surface-active solid lipid nanoparticles as Pickering stabilizers for oil-in-water emulsions. Food Funct 3: 302-311.
- Jin H, Zhou W, Cao J, Stoyanov SD, Blijdenstein TBJ, et al. (2012) Super stable foams stabilized by colloidal ethyl cellulose particles.Soft Matter 8: 2194-2205.
- Kalashnikova I, Bizot H, Cathala B, Capron I (2011) New Pickering emulsions stabilized by bacterial cellulose nanocrystals. Langmuir 27: 7471-7479.
- Hikima T, Nonomura Y (2011) Pickering emulsions and capsules stabilized by wool powder particles. J Oleo Sci 60: 351-354.
 Spanish
Spanish  Chinese
Chinese  Russian
Russian  German
German  French
French  Japanese
Japanese  Portuguese
Portuguese  Hindi
Hindi