Research Article, J Chem Appl Chem Eng Vol: 7 Issue: 1
Finite Aluminium Graphite Titanium Triple Layered Composite Sheet for Aeronautic Application
Mahmoud A Rabah*
Department of Chemical and Electrochemical, Central Metallurgical R and D Institute, Cairo, Egypt
- *Corresponding Author:
- Mahmoud A Rabah
Department of Chemical and Electrochemical,
Central Metallurgical R and D Institute,
Cairo,
Egypt;
E-mail: Mrabah010@gmail.com
Received date: 29 August, 2019, Manuscript No. JCACE-19-1842; Editor assigned date: 03 September, 2019, PreQC No. JCACE-19-1842 (PQ); Reviewed date: 17 September, 2019, QC No. JCACE-19-1842; Revised date: 02 February, 2023, Manuscript No. JCACE-19-1842 (R); Published date: 02 March, 2023, DOI: 10.4172/Jcace.1000013.
Citation: Rabah MA (2023) Finite Aluminium Graphite Titanium Triple Layered Composite Sheet for Aeronautic Application. J Chem Appl Chem Eng 7:1.
Abstract
Preparation of translaminar aluminium graphite titanium triple layered composite sheet was studied. A parametric investigation of synthesizing sheet without stresses in a composite delamination modelling has been considered. The triple layered metal sheet was prepared by two methods. By electro less chemical reduction of aluminium and titanium solutions or by hot pressing of aluminium thin plate to a flake graphite polymer composite coated with adhesive. A thin sheet of ≈ 0.5 mm in thickness structured of flake crystalline graphite polymer sheet punched with 1 mm diameter holes. It was inserted in a sac made of a semipermeable membrane. The assembly was placed in an electrolyzing cell filled with an ascorbic acid solution. At the beginning of the experiment, aluminium and titanium ions separately diffuse across the membrane towards the graphite plate surface that is soaking in the ascorbic acid solution. Ascorbic acid reduces the diffusing ions to give free metal nanoparticles that deposit on the graphite surface. After about 6 hours, a sandwich of Al-graphite titanium plate was obtained. The sheet was dried at 80°C for 2 hours and hot pressed under 150 KPa. It was annealed at 550°C for a few minutes. Results revealed that a triple sheet having variant thickness was prepared after ≥ 250 min of diffusion. Temperature help enhancing the rate of diffusion but deteriorates the physicomechanical properties of the prepared sheet.
Keywords: Al-C-Ti, Triple laminar metals, Aerospace metal, Electroless deposition, Nanoparticle deposition
Introduction
Aerospace materials are developed metal alloys for use for aerospace purposes or have come to prominence. Aerospace science often requires very high performance such as mechanical or thermal properties. Other reporters are more suitable for their long term reliability in this safety conscious field, particularly for their resistance to fatigue. Materials engineering is rigid and an important norm within the aerospace application is fully regarded. The international standards bodies maintain standards for the materials and processes involved. Engineers and academicians had performed extensive work for aeronautic materials improvement. The aerospace materials for use may be present in nature and used to the manufacture the early aircraft. Such materials were also utilized after timber in wing structures and fabric and dope to cover them. The material quality was of utmost importance and so the timber would be of carefully selected. Standard specifications were this highly required for the proper selection, manufacture, and use of these materials. Specifications were then developed and updated with the cooperation of manufacturer’s engineers or maybe government technicians and often with the assistance of university engineering departments [1].
Development of materials for use in the aerospace application was to adopt materials such as duralumin which means, hardening of aluminium alloy (s) and scoring of this goal of new and/or unavailable properties. Many of these new materials were studied to report their new properties and how to beneficiate the best use of them. This work was carried out through the financial and technical help and/or supervision of national laboratories and research institutions aluminium alloys for airframes and skin, and composites for structures. Some reporters predicted composite materials and aluminium metal may constitute the roost when it comes to aerospace innovation and similar updated structures. On the other hand, aluminium metal is still a lightweight metal, technically advanced in terms of forming and alloying. It is relatively low cost, especially when compared to aluminium or composites of aluminium alloys [2].
The aluminium producer Co, Alcoa, in 2013, for example, predicted around 6% more aluminium will be used in planes compared to 2011. The company, also claimed that the current fleet of airliners and military jets are the main users of aluminium, and newer designs continue to specify lots of this metal. It was reported that one of the largest passenger airliners in the world, contains 10 times the amount of aluminium used as compared to the less updated model of the airliner. Also, Boeing’s 787 dreamliner, are structured containing 20% aluminium alloy 7085 (by weight), which was a relatively new aluminium alloy. In Japan, Mitsubishi, for example, go equipped with aluminium wings and they would be a better overall solution. Other companies supplied aluminium composites to commercial aircraft manufacturers. Aluminium was used in the manufacture of J-35 joint strike fighter. It makes up six forged bulkheads [3].
Pure aluminium has a tensile strength property of about 1000 kg/cm square. Aluminium is alloyed with some nonferrous elements such as manganese, silicon, aluminium, magnesium, or zinc and antimony to further increase its strength. Cold working is a technique used to increase the strength of these alloys. However, some alloys are further strengthened and hardened by heat treatments. Aluminium matrix composites (MMCs). These consist of metal alloys reinforced with fibres, whiskers, particulates, or wires. Alloys used as matrices to date are of aluminium, aluminium, magnesium and aluminium. For example, according to NASA space shuttle, 240 struts were made of aluminium reinforced with boron fibers. The following table shows the alloying elements with aluminium.
Series primary alloying element
• Aluminium 99.00% or greater
• Aluminium
• Manganese
• Silicon
• Magnesium
• Magnesium and silicon
• Zn
Alloying, cold working and heat treatment improve the microstructure and modified the mechanical properties. In this regard, researcher reported the influence of some alloying elements on the microstructures and mechanical properties of aluminum alloys and aluminum alloy composites. Aluminum also has its own anticorrosion mechanism. When oxidized, aluminum forms a hard, microscopic oxide protective coating from the environment. The tight chemical oxide bond is the reason aluminum is not found free in nature; it exists only as compound. The most common aluminum zinc alloy 7075, is mostly used in aerospace. Al-Zn alloy 7075 is strong as compared to steel. The alloy includes 5.6%-6.1% zinc, 2.1%-2.5% magnesium, 1.2%-1.6% aluminium. It is commonly produced in several heat temper grade. But aluminum alloy is superplastic formable, high strength, make, it available for structural applications, Initial cost of 7475 is high. On the other hand, the cost of finished parts is usually lower than that of 7075 alloy because of the savings involved ++-toweight and stiffness to weight ratios. With proper selection and placement of carbon fibers, the composites of aluminium can be stronger and stiffer than steel parts with similar thicknesses of 40% to 70% light weight. Fatigue resistance of continuous carbon fiber composites is preferable. The chemical resistance is much better than that of glass reinforced composites. Particularly in alkaline medium however, carbon composites are relatively brittle. They have no yield behavior and their resistance to impact is low [4].
Thermal characteristics of carbon fibers differ as compared to other materials. This property helps making the design of structures with zero or very low linear and planar thermal expansion. The following Table 1 gives the comparing aerospace composites followed by precipitation as hydroxide using ammonium hydroxide solution. It was filtered and the hydroxide gel was reduced with ascorbic acid or hydrazine hydrate. The aim of this paper is to prepare a triple layered aluminium carbon aluminium sheet and examine its properties for possible application in aerospace. The influence of some parameters on the end products such as temperature, concentration of soluble reactants, time and pH value were studied. Table 1 shows the characteristics of different composites used [5].
| Material type | Nomenclature | Tensile strength\(Psi) | Modulus (Psi) | Strain (%) |
|---|---|---|---|---|
| Carbon/Epoxy | T300/934 | 245 | 20 | 1.0-1.2 |
| IM7/85517 | 400 | 24 | 1.62 | |
| P75/934 | 135 | 44 | 0.2-0.5 | |
| AS4/35016 | 100 | 10 | 1 | |
| IM6/35016 | 330 | 23 | 1.5 | |
| Glass/Epoxy | Eggless/934 | 150-170 | 68 | 2.75 |
| Kevlar/Epoxy | K49/7934I | 30-85 | 4 | 1.85 |
| Carbon/PEE | M7/APC2 | 410 | 24 | 1.6 |
| Carbon/Phenolic | FM5055 | 15-20 | 2.6-2.8 | 1.0-1.2 |
Table 1: The characteristics of different composites.
Materials and Methods
Experimental
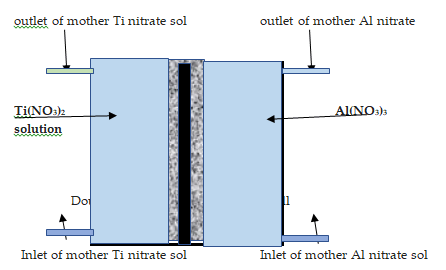
Soft polymer sheet doped with flake graphite powder (S1) measuring 80 × 200 mm and 0.2 mm) in thickness was cut from a mother roll made by ringsdorf, Germany. Alternatively, a thin plate of electro graphite 80 mm × 120 mm long (S2) was used for deposition of aluminium and aluminium metals. The plate sample was heated at 250°C to get rid of oil impregnant. The sample plate was punched with 2 mm diameter hard chrome die with a spacing of 10 mm between the center of two adjacent pores. The graphite plate was washed and dried at 110°C for 4 h before storing in a horizontally mounted perspex cell, 6 mm in thickness fitted with a lid. Figure 1 shows a schematic diagram of the graphite sheet [6].
Description of titanium
Preparation of titanium metal was applied according to the method given by Rabah. Graphite sheet was prepared according to the method [7].
Preparation of the triple layer metal composite sheet
Samples were prepared by two alternative ways; the first was chemical reduction of the metal ions and the second by hot pressing in steel die of aluminium, graphite composite and aluminium sheets followed by heating at 500°C for 5 minutes.
Figure 2 shows a schematic diagram of the test rig used in the first method. Table 2 shows the proper ties of the chemicals used in this work.
| Chemical | Source | Purpose | Property |
|---|---|---|---|
| Nitric acid | SP.GR.1.18 (AR) | - | ADWIC Riedel- de Hein |
| Min. assay 36% | |||
| Fuming 69% | |||
| H2SO4 | H2SO4 95%-97% | Leaching process | ADWIC |
| Extra pure | |||
| SP.GR.1.18 (AR) | |||
| HCl Ca carbonate, EJSF2 Ca oxide | Green Egypt | Synthesis process | 99.3, 1.6 um |
| Sigma aldrich | 3.34 g/cm3, 1.57 um | ||
| NaOH (Sodium hydroxide) Ammonium hydroxide | United Co. for chemicals and med. preparations | - | Pure reagent for analysis |
| AgNO3 (Sliver nitrate) | - | Chloride ion determination | 25% Pure reagent for analysis |
| Mono-distilled water | - | Chemical reactions | Pure reagent for analysis |
| Tap water | - | Other purposes | - |
Table 2: Properties of the chemicals.
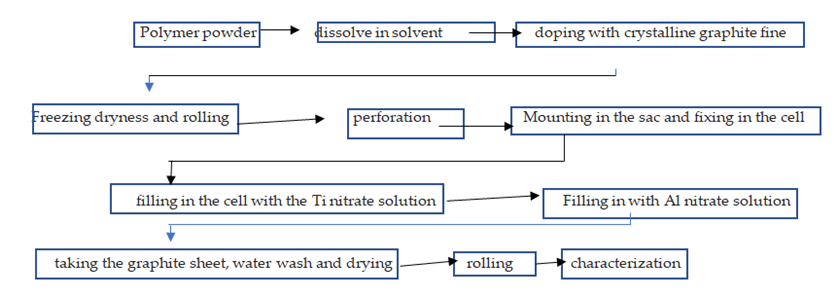
Figure 3 shows the sequential process flow sheet for the preparation of the triple aluminium-C-titanium sheet samples.
Designation of the prepared samples
The following Table 3 shows the composition of the prepared samples. Hot pressing was carried out using chromium die steel at pressures up to 105 KPa. Pressing was performed for 5 minutes.
| Samples | Chemical deposition, thickness, mm | Hot pressing, up to 105 KPa thickness, mm | ||||
|---|---|---|---|---|---|---|
| Al | C | Ti | Al | C | Ti | |
| 1 | 0.23 | 0.1 | 0.2 | - | - | - |
| 2 | 0.34 | 0.2 | 0.36 | - | - | - |
| 3 | 0.8 | 0.2 | 0.55 | - | - | - |
| 4 | 1.01 | 0.2 | 0.55 | - | - | - |
| 5 | - | - | 0.18 | 0.1 | 0.2 | |
| 6 | - | - | 0.52 | 0.2 | 0.4 | |
| 7 | - | - | 0.85 | 0.2 | 0.55 | |
| 8 | - | - | 1.1 | 0.2 | 0.55 | |
Table 3: Summary of the composition of the prepared samples.
Results
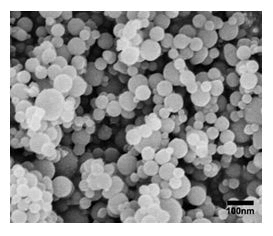
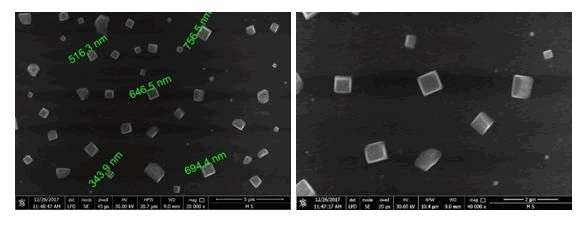
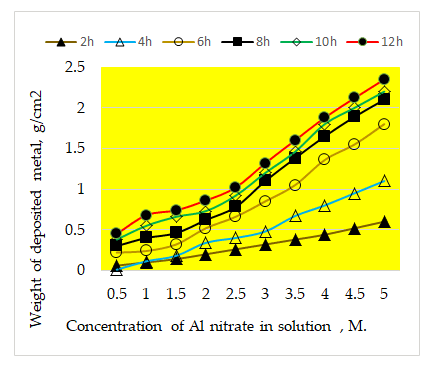
Figure 4 through 6 show SEM images of the graphite-doped polymer sheet SEM image of Al nanoparticles (Figure 5) and SEM image of the deposited Ti after 10 minutes of deposition (Figure 6).
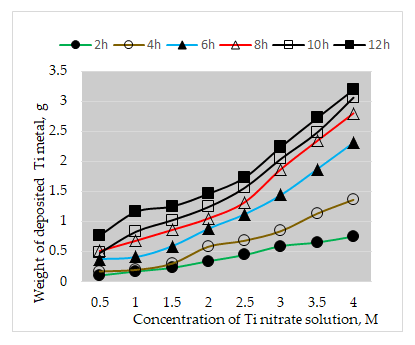
Figure 7 shows the effect of concentration of aluminium nitrate solution in the electroless cell on the weight of aluminium nanoparticles deposited on the graphite plate for different times up to 12 hours [8]. It can be seen that the weight of the deposited aluminium particles increases with increase in concentration of the aluminium nitrate up to 5 M and time of the electro less reduction process up to 12 h. The weight of the deposited aluminium particles per 1 square centimeter of the graphite plate surface amounts to 0.32 g, 0.85 g and 1.32 g with 3 M solution after 2 h, 6 h and 12 h respectively. The corresponding values with 5 M solution amount to 0.6 g, 1.8 g and 2.35 g after 2 h, 6 h and 12 h respectively [9].
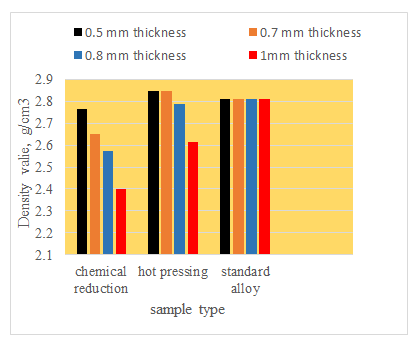
Figure 8 shows the effect of concentration of titanium nitrate solution in the electro less cell on the weight of the deposited titanium nanoparticles deposited on the graphite plate for different times up to 12 hours. It can be seen that the weight of the deposited Ti nanoparticles.
It can be seen that the density value of the samples prepared by chemical method are lighter as compared to the samples prepared by hot pressing method. Both samples are slightly denser than the standard Al alloy 7075.
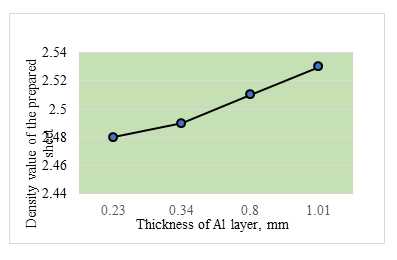
Figure 9 shows the density of the triple sheet prepared with different thickness of aluminium and constant thickness of titanium at 0.2 mm. It can be seen that the density value increases very slightly with increase in thickness of Al layer approaching the value of the standard Al7075 alloy of 2.96 g/cm3 increases with the increase in the concentration of the aluminium ions up to 3 M [10].
Figure 10 the density of the triple sheet prepared with different thickness of aluminium and constant thickness of titanium at 0.2 mm. It is seen that the density value increases slightly with the increase in the thickness of aluminium layer. Density attains a value of 2.55 g/cm3 with samples having about 1 mm aluminium.
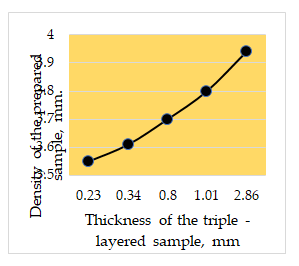
Figure 11 shows the effect of thickness of the prepared triplelayered Al-C-Ti sample on its apparent density value. It can be seen that the density value displays the same trend as shown in Figure 10 provided that the value of the density is comparatively higher [11].
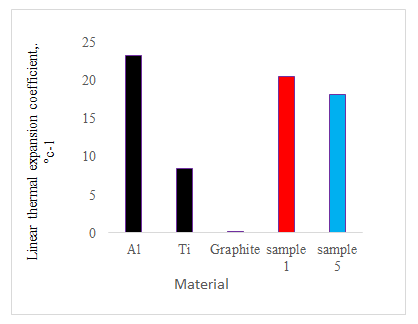
Figure 12 shows the Linear Thermal Expansion Coefficient (LTEC) of the prepared samples by the chemical reduction deposition. It can be seen that the LTEC value increases in the order Al, Ti sample 1, sample 5 and graphite. Table 4 shows character of the finished samples of triple-layered Al-C-Ti sheets [12].
| Samples | Metal thickness, mm | Porosity, % | Weight, g/m2 | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Al | C | Ti | Finished sample | Al | C | Ti | sample | ||
| Density of free solid metal, g/cm3 | 2.65 | 2.25 | 4.5 | ||||||
| 1 | 0.1 | 0.5 | 0.1 | Not pressed | 46 | 4 | 52 | 49.2 | 5.25 |
| 2 | 0.2 | 0.5 | 0.1 | Not pressed | 46 | 4 | 52 | 49.2 | 7.75 |
| 3 | 0.1 | 0.5 | 0.1 | 50 KPa | 21 | 4 | 23.5 | 24 | 7.25 |
| 4 | 0.2 | 0.5 | 0.2 | 50 KPa | 21 | 4 | 23.5 | 24 | 22.15 |
| 5 | 0.3 | 0.5 | 0.2 | 50 KPa | 21 | 4 | 23.58 | 22 | 26.35 |
| 6 | 0.5 | 0.5 | 0.5 | 50 KPa | 20 | 4 | 21 | 18.5 | 39.5 |
| 7 | 1 | 0.5 | 0.5 | 100 KPa | 12 | 4 | 13 | 12.5 | 57.2 |
| 8/ | 2 | 1 | 1 | 100 KPa | 9 | 4 | 11 | 10 | 114 |
| 9 | 2 | 1 | 2 | 150 kPa | 4 | 4 | 5 | 5 | 182 |
| 10 | 2 | 2 | 2 | 150 kPa | 4 | 3 | 4 | 4 | 208 |
Table 4: Characters of the finished triple layered Al-C-Ti sheets.
Discussion
This study deals with a composite aluminium-graphite-titanium triple-layered metal sheet that would be regarded as a composite anisotropic and inhomogeneous material. The metals of aluminium and titanium were deposited in nanoparticle size on the surface of graphite-polymer sheet using the cell. The nanoparticles of aluminium are nearly spherical whereas titanium particles are larger (≈ 500 nm) and decahedral in shape. Spherical particles have less contact surface area as compared to decahedral ones. Under the experimental technique used in this work, shape deformation of aluminium particle would take place by the action of hot pressing so that solid metal layer generates. According to Richard Collins and others, materials for making aircraft are highly recommended to be lighter. In this context, this work is a step forward to develop metals composite for possible use in an aerospace application. The designed end products of A-C-Ti triple sheet include aluminium that is the classic metal in this industry and titanium which has a good thermal resistant and is stiff material together with graphite composite that is a distinguished material improving resistant to corrosion and high thermal chock [13].
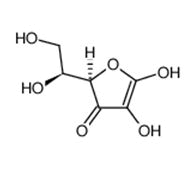
The method of preparation of the triple-layered metal sheet involved two alternative ways. The first method involves the chemical reduction of the metal ions in a solution using ascorbic acid. Ascorbic acid has the following structure (Figure 13).
The reaction with aluminium or titanium nitrate or hydroxide Ti(OH)2 to give nanoparticles of free metal, would take place according to.
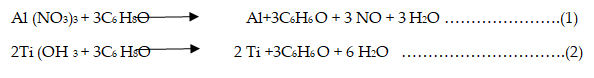
Similarly, the reaction of reducing Al(OH)3 would proceed in a similar way. It follows that regimenting metal particles would be pressed for cementing. The rate of particle formation and its flow ability to the graphite sheet would help to improve the mechanical strength of the prepared triple-layered metals sheet after pressing and sintering in particular. The mechanism of shaping the triple-layered sheet would take place similar to the technology of shaping metal powders. The force of pressing would be uniformly distributed to the particles. A major part of the pressing forces is retained at the intraparticle surface at certain active sites. Also, hot pressing of the metal particles with the graphite composite helps good binding the two metals; aluminium and titanium to the graphite composite plate. Carbon-reinforced fiber plastic and glass-fiber-reinforced aluminium composites with carbon nanotubes are types of composite materials typical to metal-matrix and ceramic-matrix composites that have a broad range in engineering applications. The effect of pressing force on the apparent density value of the triple-layered Al-C-Titanium can be understood on the dislocation phenomenon of the metal powders with subsequent alignment to form metal with loss porosity. Rate of die filling the die is based largely on the flowability of the powder. Pressing aluminium powder generally requires lower force while pressing titanium powder requires relatively higher force. Powder characteristics determine the extent of pressing force. Additives also have a role in determining the desired density. The friction force between metal particles resists movement of particles during pressing, therefore lubrication can reduce the required pressing force and also cause a more uniform distribution of particles during pressing. Hot pressing decreases spaces, bridges and gaps in the solid metal and density increases due to a more efficient packing of the particles. Density measurement given in Figure 9 shows that density of the prepared triple-layered Al-C-Ti sheet decreases in the order standard, hot pressed (and annealed) and the sample prepared by chemical reduction without pressing. The findings are in agreement with the model given above. Table 4 shows the characters of the finished triplelayered Al-C-Ti sheets. It is seen that not pressed sample has a very high porosity extent on basis of it is loose particles. The density decreases from ≈ 50% with increasing the pressing force to 4% porosity with 150 KPa.
Conclusion
Results show that triple-layered Al-C-Ti sheet synthesized from aluminium, flake natural graphite polymer and titanium has been prepared applying two alternative methods. The effect of the experimental parameters that determine the characteristics of the end products was studied. The sample prepared by chemical reduction of the nitrate ions using ascorbic acid were little weak as compared to the cemented metals sheets with graphite plate. Hot pressing and annealing of the green samples were found highly recommended to yield a triple-layered A;-C-Ti sheet with outstanding physical and thermal properties. The ions of aluminium and titanium were reduced and deposited in nanoparticle size on the surface of graphite-polymer sheet. The weight of the deposit is a time dependent process, the rate determining step is the diffusion rate of the ions towards the graphite surface. The characters of the finished triple-layered Al-C-Ti sheets are shown in a table. A triple sheet having variant thickness was prepared after ≥ 250 min of diffusion. Temperature help enhancing the rate of diffusion but deteriorates the physicomechanical properties of the prepared sheet.
References
- Rana RS, Purohit R, Das S (2012) Reviews on the influences of alloying elements on the microstructure and mechanical properties of aluminum alloys and aluminum alloy composites. Int J Sci Res Publ 2: 1-7.
- Wang Q, Gao J, Wang R, Hua Z (2001) Mechanical and rheological properties of HDPE/graphite composite with enhanced thermal conductivity. Poly Comp 22: 97-103.
- Huang R, Riddle M, Graziano D, Warren J (2016) Energy and emissions saving potential of additive manufacturing: The case of lightweight aircraft components. J Clean Prod 135: 1559-1570.
- Zhang X, Chen Y, Hu J (2018) Recent advances in the development of aerospace materials. Prog Aerosp Sci 97: 22-34.
- Amir SMM, Sultan MTH, Jawaid M, Ariffin AH (2019) Nondestructive testing method for Kevlar and natural fiber and their hybrid composites. Wood Publ. 367-388.
- Prasad NE, Wanhill RJ (2017) Aerospace materials and material technologies. Spr Sci Rev 1: 29-52.
- Zhang X, Chen Y, Hu J (2018) Recent advances in the development of aerospace materials. Progr Aeros Sci 97: 22-34.
- Kotadia HR, Gibbons G, Das A, Howes PD (2021) A review of laser powder bed fusion additive manufacturing of aluminium alloys: Microstructure and properties. Addit Manuf 46: 102155.
- Samal P, Vundavilli PR, Meher A, Mahapatra MM (2020) Recent progress in aluminum metal matrix composites: A review on processing, mechanical and wear properties. J Manufac Proc 59:131-52.
- Shim DJ, Alderliesten RC, Spearing SM, Burianek DA (2003) Fatigue crack growth prediction in glare hybrid laminates. Comp Sci Technol 63:1759-1767.
- TrzepieciÅ?ski T, Najm SM, Sbayti M, Belhadjsalah H, Szpunar M, et al. (2021) New advances and future possibilities in forming technology of hybrid metal-polymer composites used in aerospace applications. J Comsp Sci 5:217.
- Seo H, Hundley J, Hahn HT, Yang JM (2010) Numerical simulation of glass-fiber-reinforced aluminum laminates with diverse impact damage. AIAA J 48:676-687.
- Alderliesten R, Rans C, Benedictus R (2008) The applicability of magnesium based fibre metal laminates in aerospace structures. Comp Sci Technol 68:2983-2893.
 Spanish
Spanish  Chinese
Chinese  Russian
Russian  German
German  French
French  Japanese
Japanese  Portuguese
Portuguese  Hindi
Hindi