Research Article, Endocrinol Diabetes Res Vol: 8 Issue: 1
Frequency and Associations of Diabetic Peripheral Sensory Neuropathy: an EMR- Based Retrospective analysis
Khushroo Minhas*, Aziz Fatima, Saira Burney, Khadija Irfan Khawaja, Zobia Zafar, Arsalan Nawaz
Department of Endocrinology and Metabolism, Services Hospital, Lahore
*Corresponding Author: Khushroo Minhas
Department of Endocrinology and Metabolism, Services Hospital, Lahore, Pakistan,
E-mail: kminhas1@hotmail.com
Received date: 28 December, 2021, Manuscript No. ECDR-21-29318;
Editor assigned date: 31 December, 2021, PreQC No. ECDR -21-29318 (PQ);
Reviewed date: 11 January, 2022, QC No ECDR -21-29318;
Revised date: 21 January 2022, Manuscript No. ECDR -21-29318 (R);
Published date: 28 January, 2022, DOI: 10.4172/2470-7570.1000169
Abstract
The magnitude of burden of diabetic peripheral neuropathy in south-asian population is undetermined, there being a paucity of large-scale studies. Electronic medical records (EMR) of patients offer a promising avenue by which data analysis can be used for clinical research. Objectives: To determine i) the frequency of peripheral sensory neuropathy and ii) its association with various factors using Electronic Medical Record (EMR) database of diabetic patients. Methods: This was a retrospective cross-sectional study conducted at Diabetes Management Centre (DMC), Services hospital, Lahore which undertook a comprehensive review of electronic medical record (EMR) from first visit of 12,485 diabetic patients, over a three-year period. The frequency of peripheral neuropathy was derived from database analysis of specific signs and symptoms including bilaterally symmetrical symptoms of peripheral numbness or paraesthesia, burning / pins and needles sensation. Positive examination included insensitivity to Semmes-Weinstein monofilament (SWM), pinprick sensation, absence of ankle reflexes and vibration perception threshold using biothesiometer. Data was analyzed in SPSS v.25.Chi square and logistic regression analysis was done for association of DPN with body mass index, waist circumference, blood pressure, type and duration of diabetes, age, gender, HbA1c, LDL levels, Total cholesterol levels and eGFR. Results: The frequency of DPN was 84.6% in a sample size of 12485. More females(61.1%)had neuropathy as compared to males(38.9%), similarly greater number of type2 diabetics(91.6%) were found to be suffering from DPN as compared to type1.The mean±SD age of population was 50.85 ±11.13, duration of diabetes 6.70 years ±6.70, BMI 28.26±4.75, HbA1c 9.25 % ±2.31s . Logistic regression analysis predicted 81% of the model correctly. The factors with a significant association with peripheral sensory neuropathy by applying Chi square test included BMI, waist circumference, duration of diabetes, nephropathy, poor glycemic control and hypertension, while serum LDL did not show a positive relation with neuropathy.
Keywords: Diabetic Peripheral Sensory Neuropathy (DPSN); Electronic Medical Record (EMR); Body Mass Index (BMI); HbA1c, SMBG (self-monitoring blood glucose).
Introduction
Peripheral neuropathy is a microvascular complication of diabetes contributing to significant disability, poor quality of life and substantial increase in health care utilization. The clinical signs and symptoms of DPSN can vary in severity from asymptomatic to mild sensory impairment to intractable pain and frank foot ulcers occasionally necessitating amputation. There are certain clinical predictors of DPSN like poor glycemic control, long duration of diabetes, obesity and hyperlipidemia which can prompt the clinicians to screen for DPSN and undertake timely protective measures with an aim to preventing lifelong disability.
The routine evaluation of DPSN by most clinicians is based on reported patient symptoms like burning, constricting, throbbing, freezing or knife-like pain in feet or negative symptoms including numbness or walking on cotton wool and physical examination findings of absent deep tendon reflexes and / or gross sensory disturbance in feet like failure to elicit touch or pain sensation or proprioception. These clinical findings serve as the basis of therapeutic intervention. In developing countries such as Pakistan, nerve conduction studies for detection and confirmation of DPSN are costly and also not universally available.
World prevalence figures for diabetic peripheral sensory neuropathy (DPSN) reach up to as high as 50 % [1]. Diabetes is one of the major contributors to the national disease burden in Pakistan with 6.9 million cases reported in 2014 anticipated to rise to an alarming 14 million by 2040 [2]. Despite this, data about the frequency of DPSN and its associated risk factors is scanty. Reported frequencies from neighboring countries in the region vary widely from as low as 2.9% to an alarming 77.1% [3,4]. Some international studies vary widely from 7% to 56.1% [4,5]. One of the reasons for this variability could be the small sample size of the studies, which is always a limitation whenever paper-based records and forms are used by researchers. Electronic medical records (EMRs) have the advantage of being more comprehensive and easily searchable for data analysis. They serve as a portal for reliable, efficient and authentic everyday data for addressing research needs and creating intelligent algorithms that facilitate individualized patient care based on real-life health trends derived from ample-sized data [6].
This study was designed with a two-fold objective: to use a large electronic database of diabetic subjects attending diabetes management centre, Services hospital, Lahore, Pakistan, firstly, to determine the frequency of peripheral sensory neuropathy and secondly, to study the possible associations of DPSN among these subjects.
Materials/Methods
Prior to commencement of research, ethical approval was taken from the Ethical Review Committee of Services Institute of Medical Sciences, Lahore. This was an EMR-based, retrospective, case control study conducted at Diabetes Management Centre, Services hospital, Lahore. A network-based expert system software developed through Microsoft ASP.NET using MVC architecture based user-specific interface was deployed for documentation of anthropometric and biochemical parameters in electronic medical record; each record being saved in back-end database. On each visit, multiple information were secured including personal bio data, SMBG results brought by the patient, specific laboratory reports, vital signs and anthropometric measurements, subjective and objective clinical data to define the metabolic control and any complications and co- morbidities to help in decision-making regarding treatment.
Total 12484 patients were selected to include in study. To obtain the final data for this study, records of diabetic patients over a three-year period were tapped from Microsoft SQL server (2014) based database. A query was generated to select the required data. Of those records retrieved, duplicate visits of the same patient were filtered out to obtain the final dataset of 12484 patients, later used for analysis. The patients enrolled in the study were each assigned a PIN number for identification purposes.
The primary variable of interest of this study was diabetic peripheral sensory neuropathy (DPSN). We reviewed the database for individual patient records having clinical features of diabetic peripheral sensory neuropathy. A diagnosis of DPSN was made by the evaluating physician through simple yes or no answers from patients about symptoms of bilateral symmetrical peripheral numbness or paraesthesia including burning and / or pins and needles sensation and positive clinical examination for one or more of insensitivity to Semmes-Weinstein monofilament (SWM), pinprick sensation, absence of ankle reflexes and vibration perception threshold using biothesiometer; all findings were then entered in EMR.
The variables selected for association analysis of DPSN were: age, gender, duration and type of diabetes, anthropometric measures of body mass index (kg/m2), waist circumference (cm), blood pressure (mm Hg), HbA1c %, fasting lipid levels and eGFR as derived from Cockcroft Gault formula:( 140-age) x (Weight in Kg) x 0.85 (If female)/72 x s. creatinine (mg/dl).
Variable for association were further stratified and grouped. Duration of diabetes was grouped as less than 5 years, 5-10 years and more than 10 years. Metabolic control was defined as controlled and uncontrolled diabetes on the basis of the cut off value of Hba1c at 7.5%.BMI was defined as normal, overweight and obese by applying cut off values of less than 24, 24-30 and >30 respectively.
The resultant dataset was exported to SPSS version 25 for statistical analysis. The results were summarized as percentages and proportions. Chi square test was used to analyses the association of age, BMI, gender, age, duration and type of diabetes, waist circumference (cm), blood pressure (mm Hg), HbA1c %, fasting lipid levels and eGFR with the presence of neuropathy. P <0.05 was considered statistically significant. Logistic regression analysis was applied to these variables with peripheral neuropathy (yes/no) as a dependent variable, to predict the accuracy of the model.
Results and Disscussions
The current study used EMR repository containing data from visits of scores of type 2 diabetic patients to amass a large pool of data. An exhaustive analysis of this EMR database showed a significant burden of peripheral neuropathy based on patients’ clinical responses along with a positive association with longer duration and poor control of diabetes, high BMI and waist circumference as well as with nephropathy. Although prevalence of diabetes in Asian subcontinent parallels, rather exceeds that of some of the highest ranking countries in the world, yet figures for DPSN frequency from this part of the world are scant. Even where these are present, official figures tend to underestimate the true frequency and therefore may lead to inappropriately low allocation of resources for countering the phenomenal costs this ubiquitous complication poses both to the individual and the society at large. The alarmingly high frequency of diabetic peripheral sensory neuropathy of 84.6% in our study might reflect that DPSN is ubiquitous among the diabetic population presenting in public tertiary care setting. This may indicate a referral bias as government facilities such as ours receive a lot of referred patients with complicated diabetes accounting for a falsely high frequency of neuropathy. In support of this view, a recent review cites parallel frequencies of 77.1% and 74% from tertiary care settings in neighboring countries of India and Sri Lanka [4]. On the other hand, this might represent a true trend which is unique to the regional population as tertiary centers are almost always located in major cities with poor environmental and lifestyle factors conducive to inadequate metabolic control and therefore a higher risk of related complications. Similar studies from Lahore report matching prevalence statistics of 68.5% and 74.8% [7,8].
A female preponderance (61.1 %.) was noted in this study which was similar to a recent study showing higher frequency of neuropathic symptoms in females (68% in females vs. 53% in males) [9]. Mean age of patients was 50.85+/- 11.1, while a sub-group analysis showed higher frequency of peripheral neuropathy in older age group subjects. 49.1% in in age group of > 50 years as compared to the younger age groups. Advancing age was strongly associated with neuropathy.
Data analysis of our study participants showed a strong correlation between duration of diabetes and development of DPSN. Subgroup with longest duration (more than 10years) of diabetes, more patients i.e. 23.7% had neuropathy compared to 18% with no neuropathy. Group with lesser duration (<5 years) showed more patients with no neuropathy (60.7%) compared to those who had neuropathy (52.0%) This is in accordance with extensive literature from across the world supporting a positive relationship between DPSN and duration of diabetes [10,11].
Obese type 2 patients formed the bulk of our study population (with mean BMI of 28.26 +- 4.75). Multiple studies have shown metabolic syndrome and its various components as being associated with peripheral neuropathy including obesity and prediabetes [12]. Obesity thus may account for the frequency of DPSN in our dataset which showed that increased body adiposity as measured by BMI and waist circumference had a stronger association with DPSN. Increased waist circumference was found in 90.13% of patients with DPSN while 58.6% of patients had BMI category of obese .Similarly another explanation might involve metformin and insulin, which were frequent among our retrieved record prescriptions. Use of both drugs has been linked to worsening of diabetic peripheral neuropathy [13,14].
A strong correlation between peripheral neuropathy and degree of glycemic control is well established [15]. Our EMR analysis also elicited a strongly significant association between DPSN and HbA1c in confirmation of this relationship with frequency of neuropathy being 24.8% in controlled versus 75.1% in those with uncontrolled metabolic profiles. Diabetic nephropathy as documented by eGFR showed positive association with diabetic peripheral sensory neuropathy. Hypertension showed statistic association with the presence of DPSN in our dataset matches with the positive association elsewhere in the region [1]. LDL levels were not found to be significantly associated with a diagnosis of diabetic neuropathy (p=0.208). (Results in table 1)
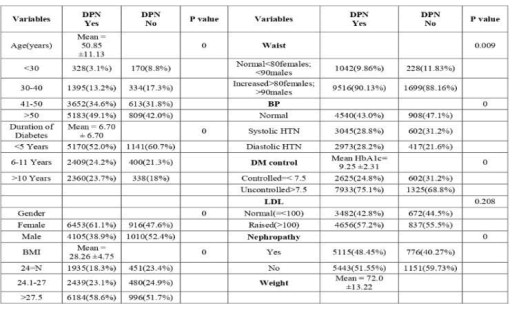
Table 1: diagnosis of diabetic neuropathy
The major strength of this study is the large scale normal data from a public hospital setting in the context of diabetic neuropathy statistics thereby being a more reliable representation of the local population. Limitations include the cross sectional study design as well as the generalizability of these results to other populations being unclear. Furthermore, the diagnosis of DPSN was clinical and not confirmed by nerve conduction studies.
Conclusion
This study highlights the use of specific EMR database to allow improved surveillance of diabetic peripheral sensory neuropathy and also suggests that the duration of diabetes, age, HbA1c, and high BMI are associated with significantly increased risks of DPSN among diabetic patients. A follow up study of these patients after glycemic control and appropriate treatment is warranted to better understand the dynamics of DPSN in order to formulate efficient control strategies. High-quality prospective cohort studies in the region to ascertain if this frequency translates into equally large prevalence figures are warranted for devising preventive and therapeutic strategies for this disabling complication of diabetes.
References
- Gogia S, Rao CR (2017) Prevalence and risk factors for peripheral neuropathy among type 2 diabetes mellitus patients at a tertiary care hospital in coastal Karnataka. Indian J Endocrinol Metab 21:665-669. [Crossref] [Google Scholar]
- International Diabetes Federation (2015). IDF Diabetes Atlas Seventh Edition.
- Simoneau A, Monlun M, Poupon P, Baillet-Blanco L, Alexandre L, et al. (2017) Prevalence of and Risk Factors for Diabetic Peripheral Neuropathy in Youth With Type 1 and Type 2 Diabetes: SEARCH for Diabetes in Youth Study. Diabetes Care 40: 1226-1232. [Crossref] [Google Scholar]
- Almuhannadi H, Ponirakis G, Khan A, Malik RA (2018), Diabetic Neuropathy and painful diabetic neuropathy: Cindrella complications in South East Asia. J Pak Med Assoc 68:85-89. [Crossref] [Google Scholar]
- Ross MK, Wei W, Ohno-Machado L (2014) “Big data” and the electronic health record. Yearb Med Inform 9: 97-104. [Crossref] [Google Scholar]
- Ali A, Iqbal F, Taj A, Iqbal Z, Amin MJ, Iqbal QZ (2013) Prevalence of microvascular complications in newly diagnosed patients with type 2 diabetes. Pak J Med Sci 29: 899-902. [Crossref] [Google Scholar]
- Iftikhar M, Hussain A, Rizvi A (2014) Frequency of peripheral neuropathy in patients with diabetes mellitus. J ayub med coll abbottabad 26:584–586. [Crossref] [Google Scholar]
- Abraham A, Barnett C, Katzberg HD, Lovblom LE, Perkins BA, Bril V (2018) Sex differences in neuropathic pain intensity in diabetes. J Neurol Sci 388: 103-106. [Crossref] [Google Scholar]
- Jaiswal M, Divers J, Dabelea D, Isom S, Bell RA, et al. (2017) Prevalence of and risk factors for diabetic peripheral neuropathy in youth with type 1 and type 2 diabetes: SEARCH for Diabetes in Youth Study. Diabetes care 40: 1226-1232. [Crossref] [Google Scholar]
- Nisar MU, Asad A, Waqas A, Ali N, Nisar A, et al. (2015) Association of diabetic neuropathy with duration of type 2 diabetes and glycemic control. Cureus 7(8). [Crossref] [Google Scholar]
- Callaghan BC, Gao L, Li Y, Zhou X, Reynolds E, et al. (2018) Diabetes and obesity are the main metabolic drivers of peripheral neuropathy. Ann Clin Transl Neuro 5: 397-405. [Crossref] [Google Scholar]
- Juster-Switlyk K, Smith AG (2016). Updates in diabetic peripheral neuropathy. F1000Research
- Gibbons CH, Freeman R (2014) Treatment-induced neuropathy of diabetes: an acute, iatrogenic complication of diabetes. Brain 138(1):43-52. [Crossref] [Google Scholar]
 Spanish
Spanish  Chinese
Chinese  Russian
Russian  German
German  French
French  Japanese
Japanese  Portuguese
Portuguese  Hindi
Hindi 


