Review Article, J Womens Health Issues Care Vol: 6 Issue: 6
Gamete, Fertilization and Embryo Compatible Lubricants: The New FDA Product Code ‘PEB’ Updates the Standard of Care for Trying-to-Conceive Couples
Ellington JE* and Clifton GD
BioOrigyn, LLC, Valleyford, WA, USA
*Corresponding Author : JE Ellington
BioOrigyn, LLC, Valleyford, WA 99036, USA
Tel: 5099545164
E-mail: jellington@bioorigyn.com
Received: September 26, 2017 Accepted: November 14, 2017 Published: November 20, 2017
Citation: Ellington JE, Clifton GD (2017) Gamete, Fertilization and Embryo Compatible Lubricants: The New FDA Product Code ‘PEB’ Updates the Standard of Care for Trying-to-Conceive Couples. J Womens Health, Issues Care 6:6. doi: 10.4172/2325-9795.1000294
Abstract
Recently, the U.S. Food and Drug Administration created a unique Product Code (“PEB”) for personal lubricants that are “gamete, fertilization and embryo compatible” for use by couples who are trying to conceive and by health care providers during fertility interventions. This designation falls under the Obstetrical and Gynecological Therapeutic Devices portion of the Code of Federal Regulations Title 21. These Class 2 lubricant devices differ from other patient and personal lubricants (e.g. not specifically gamete, fertilization and embryo compatible) with regards to the required biocompatibility and toxicology testing; lot release specifications; quality monitoring; and premarket FDA review process for device clearance. The PEB lubricants undergo premarket testing and ongoing monitoring more similar to Reproductive Media and Supplements, than to traditional lubricants. Inspite of the testing and regulatory requirements for this Product Code, many consumers, physicians, and medical-specialty societies remain unaware of this specific labeling, which can simplify optimal product selection for a unique patient group. This review briefly discusses: how PEB lubricants (e.g. “Fertility Lubricants”) differ from other types of lubricants; why product choice in lubricants matters for couples who are trying to conceive; and general formula differences between current PEB Lubricants. The awareness of a new regulatory code, which identifies lubricants that are gamete, fertilization and embryo compatible, remains lacking amongst healthcare providers and consumers. Public and reproductive health is better served if intended uses of lubricant products are read, understood and explained to patients.
Keywords: Patient lubricant; Personal lubricant; Fertility lubricant; Embryo; Oocyte; Sperm; Trying to conceive; Fertility; Reproduction; Vaginal lubricant; Gamete; Fertilization
Introduction
Recently, the U.S. Food and Drug Administration (FDA) created a unique Product Code for personal lubricants that are “gamete, fer tilization and embryo compatible” for use by couples who are trying to conceive and by health care providers during fertility interventions [1]. This designation falls under the Obstetrical and Gynecological Therapeutic Devices (Subpart F) portion of the Code of Federal Regulations (CFR) Title 21 [2]. These Class 2 lubricant devices, regulated under the new Product Code “PEB”, differ from other patient and personal lubricants (not specifically gamete, fertilization and embryo compatible) with regards to required biocompatibility and toxicology testing; lot release specification; quality monitoring; and premarket FDA review process for device clearance. The PEB lubricants undergo premarket testing and ongoing monitoring more similar to Reproductive Media and Supplements, than to traditional lubricants [3,4].
Inspite of the testing and regulatory requirements for this Product Code, many consumers, physicians, and medical-specialty societies remain unaware of this specific labeling, which can simplify optimal product selection, for the unique trying-to-conceive (TTC) patient group. This review will briefly discuss: how PEB lubricants (e.g. “Fertility Lubricants”) differ from other types of lubricants; why product choice in lubricants matters for couples who are TTC; and general formula differences between current PEB Lubricants. Public Health is better served if intended uses of lubricant products are read and understood by providers and then explained to patients.
Lubricant Device Classification
In general, lubricants are considered by the FDA as medical devices that fall into several product classes and codes, based on their intended use and the regulatory review process they undergo. Examples of a few products in each code, as well as common production, safety and biocompatibility testing for each code are provided in Tables 1 and 2.
| Class 1# | Class 2 | Class 2 |
|---|---|---|
| Patient Lubricant (KMJ) | Personal Lubricant (NUC) | Gamete, Fertilization, And Embryo Compatible Personal Lubricants (PEB0 |
| pH | pH | pH |
| Viscosity | Viscosity | Viscosity |
| Preservative effectiveness | Preservative effectiveness | Osmolality |
| Microbial count (bioburden) | Microbial count (bioburden) | Preservative effectiveness |
| Shelf-life testing | Shelf-life testing | Microbial count (bioburden) |
| Cytotoxicity | Cytotoxicity | Endotoxin testing |
| Skin irritation | Skin irritation | Shelf-life testing |
| Skin sensitization | Skin sensitization | Cytotoxicity |
| Vaginal irritation | Skin irritation | |
| Acute systemic toxicity | Skin sensitization | |
| Condom compatibility* | Vaginal irritation | |
| Acute systemic toxicity | ||
| Impact on sperm motility computer assisted & manual | ||
| Impact on sperm DNA/chromatin integrity | ||
| Impact on sperm cervical mucus penetration | ||
| Impact on sperm penetration into lubricant | ||
| Animal in-vitro fertilization and subsequent embryo development | ||
| Mouse embryo assay | ||
| Condom compatibility* |
Table 1: Testing Generally Expected for Each Product Code of Lubricant [1,4,6,115].
| Patient Lubricant Code: KMJ Class: 1 | Personal Lubricant Code: NUC Class: 2 | Gamete, Fertilization, & Embryo Compatible Personal Lubricants |
|---|---|---|
| E-Z Lubricating Jelly | Durex Silicone | Astroglide TTC Fertility Friendly |
| K-Y Lubricating Jelly | Good Clean Love | BabyDance Fertility Lubricant |
| PDI Sterile Lubricating Jelly | K-Y Brand Yours + Mine | Conceive Plus |
| Aquagel Lubricating Jelly | Replens Long-Lasting | Pre-Seed* Vaginal Lubricant |
| Sterile Saline Solution | Walgreen’s Personal Lubricating Jelly | Pre’ Vaginal Lubricant |
Table 2: Examples of Products in Each Lubricant Product Code and Device Class [116].
Specific lubricant classes and codes consist of the following.
Class 1 patient lubricants
These lubricants are General Hospital Products (Part 880 of the CFR), considered medical devices intended solely to lubricate a body orifice (e.g. vagina, urethra, rectum, etc.), in order to facilitate entry of a diagnostic or therapeutic device (e.g. catheters, thermometers, enemas, douches, tampons, etc.) [5]. These lubricants are not indicated for use with condoms, nor are they labeled for use to enhance the comfort of intimate sexual activity or to supplement the body’s natural lubrication. They are also not indicated for use during fertility procedures or by TTC couples. Class 1 lubricants can be sold without premarket notification and without an FDA safety data review [6]. However, their production and sale must comply with good manufacturing practices; labeling requirements; and device registration and listing [7]. Most Class 1 lubricants carry the Product Code designation of KMJ. The Class 1 Product Code of MMS covers a single product (i.e. “Exam Room Astroglide”) [8].
Class 2 personal lubricants
These lubricants are Obstetrical and Gynecological Therapeutic Devices (Part 884 of the CFR), as a subset of the Section covering Condoms [9]. Personal Lubricants are marketed for direct application to the penis and/or vagina with intended use to “moisturize and lubricate, to enhance the ease and comfort of intimate sexual activity, and supplement the body’s natural lubrication” [4]. These lubricants may or may not be tested for compatibility with condoms made from various materials (e.g. latex, polyisoprene, and/or polyurethane). No aspect of gamete, fertilization or embryo compatibility is reviewed for these products, nor do their uses include mention of fertility patients or TTC couples. The expanded indications of use for Personal Lubricants (over Patient Lubricants) represent a higher patient risk, and as such, they are subject to agency premarket notification and monitoring before and during commercial availability [10]. Most Personal Lubricants are classified in the NUC Product Code. This Product Code also more recently includes products commercially referred to as “Vaginal Moisturizers”.
Class 2 gamete, fertilization, and embryo compatible personal lubricants
These lubricants are also Obstetrical and Gynecological Therapeutic Devices (Part 884 of the CFR), under the recently established Product Code PEB [1]. These lubricants are specifically reviewed for their biocompatibility with gametes (sperm and oocytes), as well as their potential impact on fertilization and embryo development. The PEB Fertility Lubricants carry similar indications of use to the Personal Lubricants, for sexual activity, but they further have intended use claims of “compatible with sperm, oocytes, and embryos;” “can be used by couples trying to conceive” and for “fertility interventions to facilitate entry of diagnostic and therapeutic devices”. These expanded indications of use arise from a cadre of required testing that mimic, and even exceed, Special Control testing for Reproductive Media and Supplements [3]. Manufacturers of Fertility Lubricants are also required to develop lot release and stability specifications to confirm that each lot has, and retains over time, properties that support gamete and embryo function (e.g. distinct pH, osmolality, and endotoxin levels). Similar to Reproductive Media, PEB manufacturers, upon request, should provide a Certificate of Analysis for each lot to assure specifications and quality for clinical use. Additionally, Fertility Lubricant labeling requires unique language to help guide TTC women in product use (e.g. instructing women to contact physician if they do not become pregnant after six months of product use). At the time of this writing, five lubricants have PEB clearance with indications of use during fertility interventions and by TTC couples [11-15].
Overall, premarket review of the Class 2 NUC and PEB Lubricants by the FDA includes assessment of: the proposed labeling; indications for use; substantial equivalence in comparison to a previously cleared predicate device within the new product’s Class and Code; biocompatibility data consistent with the product’s labeled uses; shelf-life stability; and antimicrobial effectiveness. Manufacturers of Class 2 Lubricants are also subject to routine and on-going inspection and oversight by the FDA to assure compliance with device regulations and current good manufacturing practices. Lubricants that are cleared through the 510 (k) processes are not allowed to advertise that they are FDA “approved” [10]. Although progress has been made to reduce the use of live-animals (e.g. rabbits, mice, guinea pigs) to demonstrate safety and lack of toxicity, currently, all Class 2 Lubricants are currently required to undergo some such studies [16].
Unique Biocompatibility Testing of Fertility Lubricants
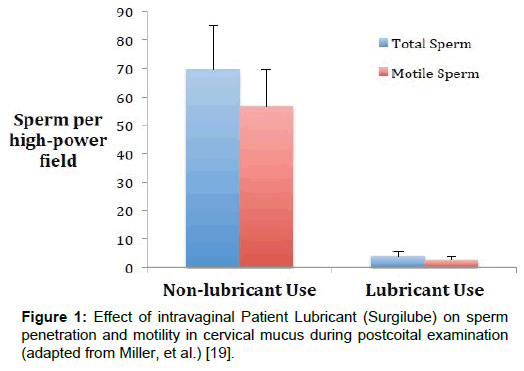
The specific biocompatibility testing requirements for the Fertility Lubricants have evolved over the past decade. Initially, concerns were expressed to the FDA that Patient and Personal Lubricants not containing chemical spermicides where being labeled as “non-spermicidal”, and actively marketed as safe to use when trying to conceive or for clinical fertility interventions [17]. These claims were not supported by numerous publications (dating from the 1970s), showing that such lubricants caused significant harm to sperm in vitro (equivalent to contraceptive gels); as well as in vivo (Figure 1) [18-25]. The sperm-toxic nature of these common Patient and Personal Lubricants was proposed to be due to very low pH levels (~pH 3), very high osmolality (>3,000 mOsm/kg); and/or the presence of specific membrane penetrating chemicals (e.g. glycerol), which although useful in sperm cryopreservation, appear to have toxic effects at room temperature and higher [23,26-31].
Figure 1: Effect of intravaginal Patient Lubricant (Surgilube) on sperm penetration and motility in cervical mucus during postcoital examination (adapted from Miller, et al.) [19].
The first clearance of a lubricant carrying indications of use for fertility interventions occurred in 2006 [15], with another as “safe for use by trying-to-conceive couples” in 2008 [14]. These clearances established the concept of lubricant products designed specifically to not harm sperm, oocytes, fertilization or embryos when conception was desired; as well as created a pathway for Fertility Lubricant testing and review. However, it has not been until recently that the unique PEB Product Code came into use [1].
A key aspect of the PEB Lubricants is that they must have physical properties in a narrow range (e.g. mimicking physiologic solutions of semen and fertile cervical mucus), to prevent osmotic and/ or pH shock and damage to gametes or embryos [31,32]. Specifically, these products are in the range of pH neutrality (e.g. pH 7) and isoosmolality/ isotonicity (e.g. 300 mOsmo/kg). Additional unique testing for Fertility Lubricants includes assessing the impact of the product on: human sperm motility and survival (manual and computer assisted); sperm chromatin integrity; the ability of sperm to penetrate into the lubricant; the ability of sperm to penetrate into cervical mucus; mouse embryo development; and in vitro fertilization and subsequent embryo development in an animal model [31,33-41]. Finally, endotoxin screening in the PEB Lubricants at production and over time, are required due to the deleterious impact of even very low levels of endotoxin on gamete and embryo function [26,42-47].
Common Misbranding of Lubricants Impacting Fertility Patients
The recent PEB Product Code offers improved safety and information for consumers and providers when choosing products that support an individual’s reproductive goals. Despite this clarification, many illegal and/or misbranded lubricant products targeted at TTC individuals remain for sale in the United States. These products may impact vulnerable fertility patients where inflated, false claims or harmful product properties can be particularly deleterious. Health care providers offer a first line of defense in protecting patients through notification of local FDA authorities when illegal products are identified. The regulatory status of any lubricant can quickly be assessed utilizing the FDA 510(k) searchable database at https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfpmn/pmn.cfm
Misbranding and/or illegal sale of lubricants often occurs based on consumer desire for certain types of product (e.g. natural, organic, no animal testing). However, natural products can be contaminated with high endotoxins, heavy metals and even bacteria, all of which can profoundly impair gamete and embryo function [26,48,49]. Consumers also often wrongly believe that if a product is available for purchase in the U.S., it has been “approved” by the FDA and must be safe [50]. Misbranded and/or illegal lubricants are unfortunately available in the U.S., both on retail shelves and through the Internet. Common concerns with misbranded lubricants that may impact fertility patients include:
• Products that are being illegally imported for sale into the U.S. through Internet channels. This includes many “organic” and “natural” products. Gamete biocompatibility, preservative effectiveness, product stability and endotoxin levels have not been reviewed and are not reported for these products. Nor are these products under FDA monitoring for cGMP. As of this writing, no “organic” lubricants have a PEB clearance.
• Products that are cleared as Class 2 lubricants but that advertise “no animal testing”. As of this writing, no PEB lubricants have been cleared without some live animal safety testing.
• Products making fertility or gamete safety claims, but that are not cleared as PEB. Products stating they are sperm, oocyte or embryo compatible or are safe for use when trying to conceive, without PEB clearance, are misbranded.
• Products making claims of increased conception or fertility rates.
Although Fertility Lubricants have been helpful for many fertility patients, especially those with inadequate natural secretions, there are currently no products with indications of use to improve conception rates. Such statements likely represent drug claims and would require agency approval of a New Drug Application [51]. Companies manufacturing and marketing drug products without an approved application are in violation of the Food and Drug Administration Act. The FDA has numerous remedies for enforcement including requesting voluntary compliance, issuing a Warning Letter, or initiating a seizure, injunction, or other legal proceedings [51].
Lubricant Choice in Fertility Patients
The PEB Product Code identifies products that have undergone regulatory review and instituted ongoing product testing to screen for gamete and embryo toxicity. As such, these products offer a regulated, standard of care lubricant choice for TTC couples and fertility patients. Unfortunately, many consumers and even health care providers remain unclear about the potential impact of lubricant choice on gamete and reproductive health. Even professional guidance documents are not up-to-date in discussions on this lubricant classification. Most recently a reproductive physician guideline for TTC couples underscores a potential impact of Personal Lubricants on fertility [52]. However, recommendations include, in addition to a PEB Lubricant, oils found to be harmful to gamete and vaginal health [27,53-61]; and a product recalled years prior to the guideline’s publication [62].
The impact on clinical birth outcomes for healthy and/or subfertile individuals using different classes or codes of lubricants is unknown. Clinical, in-vivo research has demonstrated that Patient Lubricant used midcycle prior to coitus, profoundly interferes with sperm penetration into cervical mucus (Figure 1) [19]. Conversely, a recent secondary-analysis of data, with 32 subjects who occasionally or regularly used lubricant during their fertile window, versus 94 women who never used lubricant, found no impact of lubricants on fecundability [63]. However, this study used calendar day from menses to assign the fertile window; categorized users as those using lubricant at least once during an extended fertile window from 5 days before to 3 days after presumed ovulation; and combined Fertility Lubricant users with non-lubricant users in the analysis. Despite the preliminary nature of this data, the results have been shared in reproductive medicine reviews, without adequate discussion of the study limitations, or the counterbalancing risks represented by the potential gamete toxicity of non-PEB lubricants [52,64,65]. In particular, this risk includes increased sperm chromatin damage and/or reduced embryo development following 30-minute sperm exposure to 10% concentrations of oils or a Personal Lubricant, exposures likely for post-ejaculated sperm in the vagina [27,31,58]. Given the longstanding data showing in vitro sperm and embryo damage following contact with Patient and Personal Lubricants, it is unlikely there is adequate Public Health benefit to recommend such lubricants to fertility patients, outside of their stated intended uses.
Use of Oils in Fertility Patients
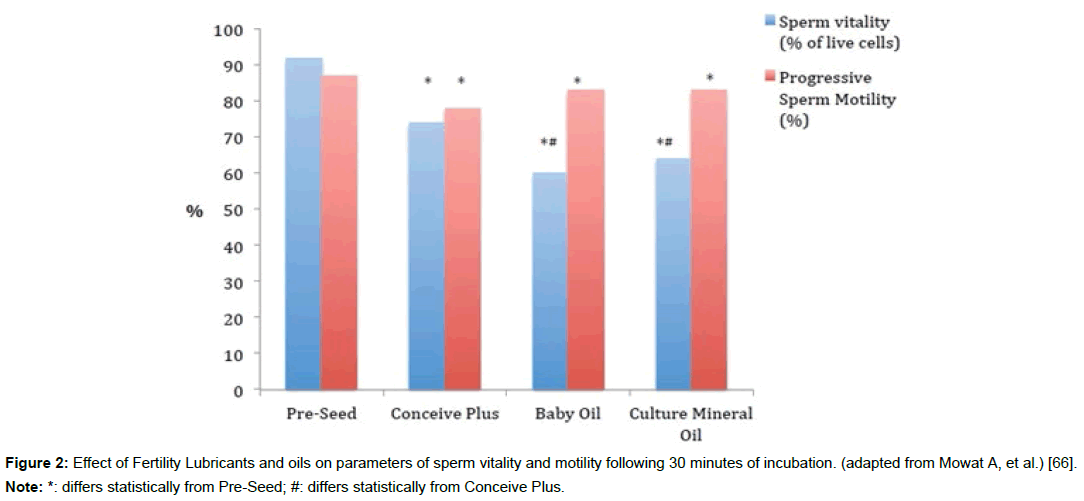
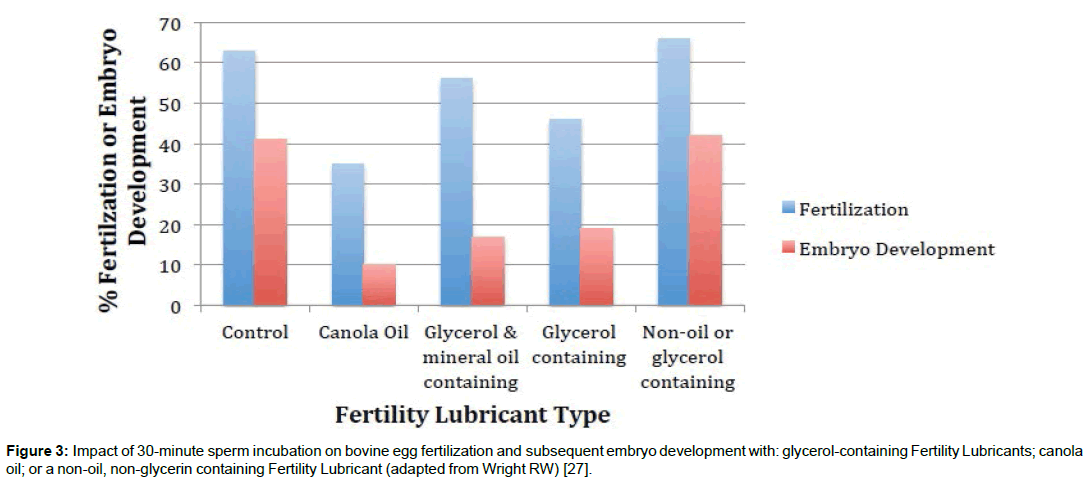
In part, because of confusion on which lubricants are safe to use when TTC, many consumers and providers look to oils (mineral or natural) as alternatives. However, oils can deleteriously impact sperm and developing embryos, and interfere with normal vaginal function. Specifically, oil use in the vagina is associated with an eight-fold increase in vaginal infection rates [53]. Other studies have shown gamete and/or embryo toxicity after contact with oils (e.g. baby, canola, olive, mineral), including apparent sub-lethal sperm damage that interferes with subsequent embryo development in an animal model (Figures 2 and 3) [20,25,27,58-61,66]. This toxicity is likely due to the peroxides and pro-inflammatory chemicals that form in natural and synthetic oils over time [54-57,60]. These compounds can alter gamete, mucosal and bacterial ecosystem physiology [54,55]. The levels of peroxides in oil products differ between types of oil and even between lots and individual containers of oils within the same type of oil, based on: manufacturing process, storage exposure to light and room temperature, and purity [54-57]. Added vitamin E in oils can act as either a pro- or antioxidant depending on production and storage conditions [56].
Figure 2: Effect of Fertility Lubricants and oils on parameters of sperm vitality and motility following 30 minutes of incubation. (adapted from Mowat A, et al.) [66].
Note: *: differs statistically from Pre-Seed; #: differs statistically from Conceive Plus.
Figure 3: Impact of 30-minute sperm incubation on bovine egg fertilization and subsequent embryo development with: glycerol-containing Fertility Lubricants; canola oil; or a non-oil, non-glycerin containing Fertility Lubricant (adapted from Wright RW) [27].
In studies on gamete safety using medical-grade mineral oil, even relatively low levels of peroxidation have been found to cause damage [58,60]. This is due, in part, to sperm cells’ high sensitivity to oxidative stress with limited ability to repair resulting damage. Such oxidative damage of sperm can cause DNA (chromatin) breakage impacting fertilization, subsequent embryo development and even offspring health [67,68]. Natural oils (e.g. almond, canola, olive) are also not screened for gamete safety and highly prone to oxidative breakdown during routine storage conditions. Virgin coconut oil is less prone to oxidative damage and has become popular for topical application. However, coconut oil has significant antimicrobial activity, and its impact on the vaginal microbiome is unknown [54]. Coconut oil is also one of the most effective oils in decreasing water movement across epithelial surfaces [54,69]. Cervical mucin particle hydration is a key factor in normal vaginal physiology, especially during the formation of the “egg white” fertile cervical mucus that allows sperm transport during conception [70,71]. It is unknown how coating of the vaginal mucosa with oils might change cervical mucus formation and function.
Unfortunately, consumers and providers have no readily available way of determining the level of oxidation and peroxide chemical content in any given unit of an oil-containing product. Nor can these toxic compounds be screened for at home after opening, even though their levels have been found to increase during routine storage [56].
Comparison of Excipients Used in Fertility Lubricants
Excipients are the chemical ingredients used in formulations to give a product its desired characteristics. In general, lubricants contain combinations of the following common classes of excipients: solvents, thickeners/gel formers, buffers, preservatives, moisturizers/ humectants, osmolality adjusters, buffers, and pH modifiers. Excipient choice is most often based on functionality and previous use in vaginal formulations. The unique physical characteristics of Fertility Lubricants with regards to pH, osmolality and endotoxin levels also dictate aspects of their formulation, as compared to other lubricants. The ingredients for currently marketed PEB Fertility Lubricants are shown in Table 3. In addition to the common lubricant excipients in these products, each of the Fertility Lubricants contains one or more product-unique ingredient. These unique ingredients include simple sugars, divalent cations, bioactive polymers and botanical products.
| Astroglide TTC Fertility Friendly Personal Lubricant | BabyDance Fertility Lubricant | Conceive Plus | Pre-Seed Vaginal Lubricant | |
|---|---|---|---|---|
| Common Excipients | Purified water | Purified water | Water | Purified water |
| Propylene glycol | Cetyl-hydroxyethylcellulose | Hypromellose | Hydroxylethylcellulose | |
| Hydroxylethylcellulose | Hypromellose | Sodium phosphate | Pluronic | |
| Potassium phosphate | Carbomer homopolymer type b | Sodium dihydrogen phosphate | Sodium chloride | |
| Sodium phosphate | Sodium phosphate | Potassium chloride | Sodium phosphate | |
| Methylparaben | Potassium phosphate | Sodium chloride | Carbomer | |
| Propylparaben | Sodium chloride | Glycerol | Methylparaben | |
| Sodium hydroxide. | Sodium hydroxide | Methylparaben | Sodium hydroxide | |
| Phenethyl alcohol | Potassium phosphate | |||
| Caprylyl glycol | Propylparaben | |||
| Product-Unique Ingredients | Fructose | Salvia sclarea (clary sage) Xylose | Magnesium chloride | Arabinogalactan |
| Galactose | Calcium chloride |
Table 3: Common Excipients & Product-Unique Ingredients in Currently Marketed Fertility Lubricants [117-119].
Some “inactive” excipients modify vaginal function
All Class 2 Lubricants, including the PEB Fertility Lubricants undergo routine safety testing using established assays (Table 1), to confirm nontoxicity and biocompatibility with skin and vaginal tissues. However, in recent years, more sensitive cell-culture and molecular assays have demonstrated that common vaginal products and “inactive” excipients, at levels previously considered safe, interfere with vaginal ecosystem function [53,72-78]. Cervicovaginal epithelial integrity, cellular cytokine production, and lactobacillus survival are negatively impacted by certain formulas, and/or excipients at common concentrations [72-78]. Such disruption of the protective vaginal barrier and microbiome has been associated with dysbiosis, increased rates of sexually transmitted diseases and even possibly increased cancer incidence [74,79]. The science around design and development of vaginal products is rapidly expanding, and likely will offer products of greater safety and less irritation potential in the future. Interestingly, inter-individual variability in sensitivity to these chemicals has been noted, with tissues from some women being unaffected, while samples from other women showing epithelial fracture after excipient contact [77]. Several authors surmise that these compounds are “generally safe at clinically relevant concentrations”; however, patient factors such as cervicovaginal mucus quantity, frequency of use, hormonal status and underlying genital inflammation could all impact how such chemicals affect vaginal ecosystem and barrier function [76,77].
In particular, the solvent cell-permeating molecules of glycerol (glycerin) and propylene glycol used in some Fertility Lubricants have been found to impact cells of the vaginal ecosystem. These compounds can decrease human cervicovaginal tissue integrity and/ or viability [76,77]. They can also increase cytokine production (an early marker of subtoxicity), which triggers inflammation [77]. Cellpermeating molecules are osmotically active, like salts, but they move freely across cell membranes without active transport. For this reason, they are often used in cryopreservation of gametes and embryos, but they can also disrupt cell function at higher temperatures [23,28-30]. Similar to the above data showing an impact of such chemicals on vaginal epithelial cells, in the only direct PEB comparison studies done to date, results suggest that gamete function may also be somewhat impacted after exposure to glycerol-containing versus glycerolfree Fertility Lubricants (Figures 2 and 3) [27,66].
Preservative choice is critical in fertility lubricants
A critical aspect in design and review of PEB Lubricants is the simultaneous accumulation of data addressing both formula’s gamete and embryo toxicity, as well as the product’s preservative effectiveness and endotoxin levels. No sterile PEB Lubricants are currently available. Therefore, these lubricants require preservatives to protect against bacterial, fungal and yeast contamination during manufacturing, long-term storage and ongoing use. Many preservatives can damage sperm at concentrations commonly used in topical products [26]. Sperm are, after all, one-cell organisms not unlike the bacteria the preservatives are designed to harm. Also, even low levels of endotoxin, which can come from formula ingredients; water used during manufacturing; or exposure of the lubricant to mixing and packaging equipment can negatively impact gamete function [26,42-46]. Endotoxin limits accepted for Reproductive Media are commonly used for Fertility Lubricants [47]. The regulatory review and ongoing monitoring of PEB Lubricants offers an independent pathway to ensure that the required goals of gamete compatibility, antimicrobial effectiveness, and low endotoxin levels are met for commercial Fertility Lubricants. The lack of such oversight is of paramount concern with lubricants illegally sold into the U.S. (especially natural or organic products), or misbranded Patient and Personal Lubricants being sold as safe to use when trying to conceive.
Preservatives have also been studied for subtoxic effects on the vaginal ecosystem with the newer assays [76,77]. Several preservatives used in Patient and Personal Lubricants were found to be highly disruptive to vaginal cell integrity and/or cytokine production (e.g. sorbic acid and benzalkonium chloride). Lower molecular weight parabens (e.g. methyl and propyl) found in most Fertility Lubricants have some limited impact on vaginal cell permeability, but do not consistently trigger high cytokine release [76,77]. Parabens are another chemical class that has variable individual response in terms of sensitivity of vaginal epithelium [77]. In spite of consumer concerns about these preservatives, the low molecular weight parabens, appear to maintain product safety without negatively impacting gamete and embryo function. However, a new Fertility Lubricant has been recently cleared that is not manufactured with parabens (Table 3). Many consumers and providers alike will be interested in a “parabenfree” alternative.
Product-unique fertility lubricant ingredients
An overview of function is provided for each of the productunique ingredients currently in Fertility Lubricants (Table 3).
The divalent cations, calcium and magnesium are included in one PEB product. Both are present in human semen, primarily from the prostate gland, resulting in high levels in the first fraction of the ejaculate (e.g. mean calcium 130mg/dl and mean magnesium 51 mg/ dl; versus serum levels around 10 and 2 mg/dl) [80]. Any consistent correlation between semen calcium and magnesium levels and sperm motility, or time to pregnancy, has not been shown [80-83]. Intracellular calcium levels regulate sperm capacitation, and extracellular calcium levels in solution enhance capacitation through multiple ion channels [84]. However, timing and location of sperm capacitation is critical for fertilization. Sperm physiologically encounter lowered calcium levels in the vagina at ovulation in the presence of fertile cervical mucus [85]. Specifically, vaginal fluid volume is inversely related to calcium levels in the vagina [85,86]. Even small calcium elevations in solutions rapidly impact the hydration of secreted cervical mucus granules. This is especially critical during the formation of the copious, slippery cervical mucus found at ovulation. Such “fertile” cervical mucus is formed by rapid absorption of water and swelling of the highly condensed mucin granules, which expand 1,000-fold over their intracellular volume [86]. Cervical mucus hydration at ovulation is dependent on lowering calcium and hydrogen ions in the vaginal canal (as cervical mucus becomes more alkaline) [71]. Elevations of calcium in solutions have been found to decrease the velocity with which the mucus granules hydrate and may impact the viscoelastic nature of fertile cervical mucus and sperm transport [86].
The biopolymer arabinogalactan is listed as an ingredient in two PEB lubricant products. Arabinogalactan is a large, naturally sourced polysaccharide present in many plant gums produced in high purity for medical use [87-90]. Bioactivity includes strong antioxidant properties [87-90]. Arabinogalactan is not immunogenic and is well tolerated. It has been used as a food additive, as a humectant in cosmetics, and in pharmaceutical applications for over 50 years [88]. Numerous studies over the past decade have shown the gamete, embryo, and vaginal mucosal safety of arabinogalactan-containing Fertility Lubricants [26,31,61,66,74,91,92]. Additionally, high concentrations of arabinogalactans have been used in reproductive medicine in density gradient washes for sperm preparation; as well as embryo freezing [93,94]. An arabinogalactan-containing, glycerol-free Fertility Lubricant supports superior human sperm motility (Figure 2); and improved fertilization and embryo development (animal model) as compared to glycerol- containing Fertility Lubricants and oil products (Figure 3) [27,66].
Another PEB Fertility Lubricant contains a combination of the simple sugars, fructose and galactose. Fructose in human seminal plasma serves as an energy substrate for sperm [95]. Addition of fructose to freezing extenders improves sperm motility [96]. However, fructose levels are higher in semen from subfertile and overweight men, than in fertile, healthy men [97]. Also, fructose levels in semen are not associated with improved sperm motility rates. The addition of fructose to reproductive media decreases sperm binding to Fallopian tube cells; zona binding; and oocyte penetration in animal model and human sperm assays (e.g. hamster egg penetration decrease from 61% to 21%) [98-100]. Fructose is not a sugar usually found in human cervical mucus [101]. Galactose, in contrast, is a major sugar of cervical mucus (e.g. up to 20% of sugar content) and is only found in varying levels in seminal plasma [95,102]. Galactose concentrations are elevated in semen from azoospermic men [95]. Pretreatment of sperm with galactose dramatically decreases zona binding and hamster egg penetration of human sperm (e.g. from 61% to 2%) [100,102,103]. No difference in human sperm motility is found when galactose is added to sperm media [104].
A PEB containing botanical extracts of raspberry xylose and Clary sage (Salvia sclarea) essential oil has recently been introduced. This Fertility Lubricant is the first cleared without any added parabens [11]. Although the product contains alternative preservatives often used in topical products, Clary sage also provides some antimicrobial activity against vaginal pathogens, while mostly sparing lactobacillus species [105,106]. Clary sage essential oil is a reported anxiolytic; decreasing cortisol levels, respiratory rates and blood pressure in human and animal studies, likely through dopamine level modulation [107-109]. Previous studies on the impact of Clary sage on gamete function are limited. However, Clary sage has antioxidant activity, via a hydrogen peroxide scavenging effect equivalent to alpha-tocopherol (e.g. ~50% reduction in peroxide levels) [106]. Another sage species (S. officinalis) has shown an antioxidant effect for sperm during cryopreservation [110].
Xylose is a critical sugar in glycosaminoglycan biosynthesis, helping to regulate cell hydration. Xylose is present in the sperm glycocalyx that regulates sperm transport and selection in the female, as well as being found in human semen and cervical mucus in trace levels [111]. Xylosyltransferases catalyze the transfer of xylose to a proteoglycan core as a rate-limiting step in glycosaminoglycan biosynthesis to form chondroitin sulfate, heparin sulfate, and dermatan sulfate, found in cervical mucus [112]. Subfertile men have half of the seminal xylosyltransferase of fertile men, although the impact of this on fertility is not known [113]. Addition of xylose to freezing extenders improves sperm motility and intact acrosomes after cryopreservation in human and animal models [96,114].
Conclusion
The new PEB Fertility Lubricant Product Code provides standardized design, review and regulation pathways, as well as clear labeling for lubricants to be used by trying-to-conceive couples. Understanding ingredient differences in Fertility Lubricants can help providers select the best product for individual patients. To better understand these novel products, physicians are encouraged to request data from PEB product manufacturers to evaluate the subject numbers; experimental design; statistical analysis and andrology laboratory setting used during product development and testing. Standard of care for the fertility patient and trying-to-conceive couples should include healthcare-provider initiated discussions about lubricant choice, as well as education about labeling to aid increased informed product selection.
Disclosure
This article represents the opinions of JE Ellington and GD Clifton who have decades of experience in device, personal care and fertility product development. The article is not legal advice. The authors have received compensation from numerous companies, including Glyciome, Church and Dwight, Fairhaven Health, and BioOrigyn, related to multiple products, and patents for such, mentioned in this paper.
References
- United States Food and Drug Administration (2017) Product classification: lubricant, personal, gamete, fertilization, and embryo compatible.
- United States Food and Drug Administration (2017) CFR - Code of federal regulations title 21.
- United States Food and Drug Administration (1997) Devices used for in vitro fertilization and related assisted reproduction procedures.
- United States Food and Drug Administration (2017) Device product classification: lubricant, personal.
- United States Food and Drug Administration (2001) Code of federal regulations title 21. General hospital and personal use devices.
- United States Food and Drug Administration (2017) Device product classification: lubricant, patient.
- United States Food and Drug Administration (2017) Class I / II exemptions.
- United States Food and Drug Administration (2017) 510(k) Pre-marketed notification, lubricant, vaginal, patient.
- United States Food and Drug Administration (2017) Code of Federal Regulations Title 21. Obstetrical and gynecological therapeutic devices: condom.
- United States Food and Drug Administration (2015) What is the difference between FDA-listed, 510(k) exempt, cleared and approved medical devices?
- United States Food and Drug Administration (2017) 510(k) Premarket notification: k162319, babydance fertility lubricant.
- United States Food and Drug Administration (2017) 510(k) Premarket notification: K141132, astroglide TTC fertility friendly personal lubricant.
- United States Food and Drug Administration. 510(k) Premarket notification: K131355, conceive plus.
- United States Food and Drug Administration. 510(k) Premarket notification: K072741, Pre-Va vaginal lubricant.
- United States Food and Drug Administration (2017) 510(k) Premarket notification: K0514361, pre' vaginal lubricant.
- United States Food and Drug Administration (2016) Use of international standard ISO 10993-1, Biological evaluation of medical devices - Part 1: Evaluation and testing within a risk management process.
- Ellington J, Clifton G (2004) Need for improved labeling for use of vaginal lubricants by trying-to-conceive couples. U.S. Food and Drug Administration.
- Boyers SP, Corrales MD, Huszar G, DeCherney AH (1987) The effects of Lubrin on sperm motility in vitro. Fertil Steril 47: 882-884.
- Miller B, Klein TA, Opsahl MS (1994) The effect of a surgical lubricant on in vivo sperm penetration of cervical mucus. Fertil and steril 61: 1171-1173.
- Anderson L, Lewis SE, McClure N (1998) The effects of coital lubricants on sperm motility in vitro. Hum Reprod 13: 3351-3356.
- Tagatz GE, Okagaki T, Sciarra JJ (1972) The effect of vaginal lubricants on sperm motility and viability in vitro. Am J Obstet Gynecol 113: 88-90.
- Goldenberg RL, White R (1975) The effect of vaginal lubricants on sperm motility in vitro. Fertil Steril 26: 872-873.
- Tulandi T, McInnes RA (1984) Vaginal lubricants: effect of glycerin and egg white on sperm motility and progression in vitro. Fertil Steril 41: 151-153.
- Frishman GN, Luciano AA, Maier DB (1992) Evaluation of Astroglide, a new vaginal lubricant: effects of length of exposure and concentration on sperm motility. Fertil Steril 58: 630-632.
- Kutteh WH, Chao CH, Ritter JO, Byrd W (1996) Vaginal lubricants for the infertile couple: effect on sperm activity. Int J Fertil Menopausal Stud 41: 400-404.
- Mortimer D, Barratt CL, Bjorndahl L, de Jager C, Jequier AM, et al. (2013) What should it take to describe a substance or product as 'sperm- safe'. Hum Reprod Update 19: i1-i45.
- Wright RW (2010) Impact of glycerol-free versus glycerol-containing fertility lubricants on sperm function, including ability to fertilize and support embryo development in an in vitro model. Fertil Steril 94: S209-S210.
- Critser JK, Huse-Benda AR, Aaker DV, Arneson BW, Ball GD (1988) Cryopreservation of human spermatozoa. III. The effect of cryoprotectants on motility. Fertil Steril 50: 314-320.
- Gilmore JA, McGann LE, Liu J, Gao DY, Peter AT, et al. (1995). Effect of cryoprotectant solutes on water permeability of human spermatozoa. Biol Reprod 53: 985-995.
- Gao DY, Liu J, Liu C, McGann LE, Watson PF, et al. (1995) Prevention of osmotic injury to human spermatozoa during addition and removal of glycerol. Hum Reprod 10: 1109-1122.
- Agarwal A, Deepinder F, Cocuzza M, Short RA, Evenson DP (2008) Effect of vaginal lubricants on sperm motility and chromatin integrity: a prospective comparative study. Fertil Steril 89: 375-379.
- WHO/UNFPA/FHI (2012) Use and procurement of additional lubricants for male and female condoms: WHO/UNFPA/FHI, Geneva, Switzerland.
- Gardner DK, Reed L, Linck D, Sheehan C, Lane M (2005) Quality control in human in vitro fertilization. Semin Reprod Med 23: 319-324.
- Cooper TG, Noonan E, von Eckardstein S, Auger J, Baker HW, et al. (2010) World Health Organization reference values for human semen characteristics. Hum Reprod Update 16: 231-245.
- De Geyter C, Bals-Pratsch M, Doeren M, Yeung CH, Grunert JH, et al. (1988) Human and bovine cervical mucus penetration as a test of sperm function for in-vitro fertilization. Human reproduction 3: 948-954.
- Evenson DP, Larson KL, Jost LK (2002) Sperm chromatin structure assay: its clinical use for detecting sperm DNA fragmentation in male infertility and comparisons with other techniques. J Androl 23: 25-43.
- van den Bergh M, Baszo I, Biramane J, Bertrand E, Devreker F, et al. (1996) Quality control in IVF with mouse bioassays: a four years' experience. J Assist Reprod Genet 13: 733-738.
- Mortimer D, Aitken RJ, Mortimer ST, Pacey AA (1995) Workshop report: clinical CASA--the quest for consensus. Reprod Fertil Dev 7: 951-959.
- Keel BA, Schalue TK (2000) Correlation of the bovine cervical mucus penetration test with human sperm characteristics in 1406 ejaculates. Arch Androl 44: 109-115.
- World Health Organization (WHO) (2010) Laboratory manual for the examination and processing of human semen (5th edtn) WHO Press, Switzerland.
- Santos RR, Schoevers EJ, Roelen BA (2014) Usefulness of bovine and porcine IVM/IVF models for reproductive toxicology. Reprod Biol Endocrinol 12: 117.
- Dumoulin JC, Menheere PP, Evers JL, Kleukers AP, Pieters MH, et al. (1991) The effects of endotoxins on gametes and preimplantation embryos cultured in vitro. Hum Reprod 6: 730-734.
- Snyman E, Van der Merwe JV (1986) Endotoxin-polluted medium in a human in vitro fertilization program. Fertil Steril 46: 273-276.
- Urata K, Narahara H, Tanaka Y, Egashira T, Takayama F, et al. (2001) Effect of endotoxin-induced reactive oxygen species on sperm motility. Fertil Steril 76: 163-166.
- Fishel S, Jackson P, Webster J, Faratian B (1988) Endotoxins in culture medium for human in vitro fertilization. Fertil Steril 49: 108-111.
- Montoro L, Subias E, Young P, Baccaro M, Swanson J, et al. (1990) Detection of endotoxin in human in vitro fertilization by the zona-free mouse embryo assay. Fertil Steril 54: 109-112.
- The United States Pharmacopeial Convention (2016) USP-Bacterial endotoxins test. USP/NF-The official compendia of standards. Baltimore, USA.
- Antignac E, Nohynek GJ, Re T, Clouzeau J, Toutain H (2011) Safety of botanical ingredients in personal care products/cosmetics. Food Chem Toxicol 49: 324-341.
- Pawar RS, Tamta H, Ma J, Krynitsky AJ, Grundel E, et al. (2013) Updates on chemical and biological research on botanical ingredients in dietary supplements. Anal Bioanal Chem 405: 4373-4384.
- Erekson EA, Martin DK, Brousseau EC, Yip SO, Fried TR (2014) Over-the-counter treatments and perineal hygiene in postmenopausal women. Menopause 21: 281-285.
- United States Food and Drug Administration (2016) Inspections, compliance, enforcement, and criminal investigations: human drugs.
- American Society for Reproductive Medicine (2017) Optimizing natural fertility: a committee opinion. Fertil Steril 107:52-58.
- Brown JM, Hess KL, Brown S, Murphy C, Waldman AL, et al. (2013) Intravaginal practices and risk of bacterial vaginosis and candidiasis infection among a cohort of women in the United States. Obstet Gynecol 121: 773- 780.
- Verallo-Rowell VM, Katalbas SS, Pangasinan JP (2016) Natural (mineral, vegetable, coconut, essential) oils and contact dermatitis. Curr Allergy Asthma Rep 16: 51.
- Zarate J, Goicoechea E, Pascual J, Echevarria E, Guillen MD (2009) A study of the toxic effect of oxidized sunflower oil containing 4-hydroperoxy-2-nonenal and 4- hydroxy-2-nonenal on cortical TrkA receptor expression in rats. Nutr Neurosci 12: 249-259.
- Auezova L, Saliba C, Hajj-Moussa E, Hosry LE, Yammine S, et al. (2012) A methodological approach to study almond oil stability in relation to α-tocopherol supplementation. Food and Nutrition Sciences 3: 1710-1715.
- Lee J, Choe E (2009) Effects of phosphatidylcholine and phosphatidylethanolamine on the photooxidation of canola oil. J Food Sci 74: C481-C486.
- Otsuki J, Nagai Y, Chiba K (2007) Peroxidation of mineral oil used in droplet culture is detrimental to fertilization and embryo development. Fertil Steril 88: 741-743.
- Otsuki J, Nagai Y, Chiba K (2009) Damage of embryo development caused by peroxidized mineral oil and its association with albumin in culture. Fertil Steril 91: 1745-1749.
- Morbeck DE, Khan Z, Barnidge DR, Walker DL (2010) Washing mineral oil reduces contaminants and embryotoxicity. Fertil Steril 94: 2747-2752.
- Sandhu RS, Wong TH, Kling CA, Chohan KR (2014) In vitro effects of coital lubricants and synthetic and natural oils on sperm motility. Fertil Steril 101: 941-944.
- United States Food and Drug Administration (2009) Class 2 device recall: ConceivEase.
- Steiner AZ, Long DL, Tanner C, Herring AH (2012) Effect of vaginal lubricants on natural fertility. Obstet Gynecol 120: 44-51.
- Mesen TB, Steiner AZ (2014) Effect of vaginal lubricants on natural fertility. Curr Opin Obstet Gynecol 26: 186-192.
- Ellington J (2012) Effect of vaginal lubricants on natural fertility. Obstet Gynecol 120: 1208-1209.
- Mowat A, Newton C, Boothroyd C, Demmers K, Fleming S (2014) The effects of vaginal lubricants on sperm function: an in vitro analysis. J Assist Reprod Genet 31: 333-339.
- Evenson DP, Jost LK, Marshall D, Zinaman MJ, Clegg E, et al. (1999) Utility of the sperm chromatin structure assay as a diagnostic and prognostic tool in the human fertility clinic. Hum Reprod 14: 1039-1049.
- Whittington K, Harrison SC, Williams KM, Day JL, McLaughlin EA, et al. (1999) Reactive oxygen species (ROS) production and the outcome of diagnostic tests of sperm function. Int J Androl 22: 236-242.
- Noor NM, Khan AA, Hasham R, Talib A, Sarmidi MR, et al. (2016) Empty nano and micro-structured lipid carriers of virgin coconut oil for skin moisturisation. IET Nanobiotechnology 10: 195-199.
- Nunn KL, Wang YY, Harit D, Humphrys MS, Ma B, et al. (2015) Enhanced trapping of HIV-1 by human cervicovaginal mucus is associated with lactobacillus crispatus-dominant microbiota. mBio 6: e01084-15.
- Nakano R, Leao R, Esteves S (2015) Insights into the role of cervical mucus and vaginal pH in unexplained infertility. MedicalExpress 2: 1-8.
- Adriaens E, Remon JP (2008) Mucosal irritation potential of personal lubricants relates to product osmolality as detected by the slug mucosal irritation assay. Sex Transm Dis 35: 512-516.
- Cunha AR, Machado RM, Palmeira-de-Oliveira A, Martinez-de-Oliveira J, das Neves J, et al. (2014) Characterization of commercially available vaginal lubricants: a safety perspective. Pharmaceutics 6: 530-542.
- Dezzutti CS, Brown ER, Moncla B, Russo J, Cost M, et al. (2012) Is wetter better? An evaluation of over-the-counter personal lubricants for safety and anti- HIV-1 activity. PLoS One 7: e48328.
- Fashemi B, Delaney ML, Onderdonk AB, Fichorova RN (2013) Effects of feminine hygiene products on the vaginal mucosal biome. Microb Ecol Health Dis 24.
- Gali Y, Delezay O, Brouwers J, Addad N, Augustijns P, et al. (2010) In vitro evaluation of viability, integrity, and inflammation in genital epithelia upon exposure to pharmaceutical excipients and candidate microbicides. Antimicrob Agents Chemother 54: 5105-5114.
- Hu M, Zhou T, Dezzutti CS, Rohan LC (2016) The effect of commonly used excipients on the epithelial integrity of human cervicovaginal tissue. AIDS Res Hum Retroviruses 32: 992-1004.
- Fichorova RN, Mendonca K, Yamamoto HS, Murray R, Chandra N, et al. (2015) A quantitative multiplex nuclease protection assay reveals immunotoxicity gene expression profiles in the rabbit model for vaginal drug safety evaluation. Toxicol Appl Pharmacol 285: 198-206.
- Onderdonk AB, Delaney ML, Fichorova RN (2016) The human microbiome during bacterial vaginosis. Clin Microbiol Rev 29: 223-238.
- Valsa J, Skandhan KP, Khan PS, Sumangala B, Gondalia M (2012) Split ejaculation study: semen parameters and calcium and magnesium in seminal plasma. Cent European J Urol 65: 216-218.
- Sorensen MB, Bergdahl IA, Hjollund NH, Bonde JP, Stoltenberg M, et al. (1999) Zinc, magnesium and calcium in human seminal fluid: relations to other semen parameters and fertility. Mol Hum Reprod 5: 331-337.
- Wong WY, Flik G, Groenen PM, Swinkels DW, Thomas CM, et al. (2001) The impact of calcium, magnesium, zinc, and copper in blood and seminal plasma on semen parameters in men. Reprod Toxicol 15: 131-136.
- Banjoko SO, Adeseolu FO (2013) Seminal plasma pH, inorganic phosphate, total and ionized calcium concentrations in the assessment of human spermatozoa function. J Clin Diagn Res 7: 2483-2486.
- Cisneros-Mejorado A, Hernandez-Soberanis L, Islas-Carbajal MC, Sanchez D (2014) Capacitation and Ca(2+) influx in spermatozoa: role of CNG channels and protein kinase G. Andrology 2: 145-154.
- Wagner G, Levin RJ (1980) Electrolytes in vaginal fluid during the menstrual cycle of coitally active and inactive women. J Reprod Fertil 60: 17-27.
- Espinosa M, Noe G, Troncoso C, Ho SB, Villalon M (2002) Acidic pH and increasing [Ca(2+)] reduce the swelling of mucins in primary cultures of human cervical cells. Hum Reprod 17: 1964-1972.
- Moschini R, Gini F, Cappiello M, Balestri F, Falcone G, et al. (2014) Interaction of arabinogalactan with mucins. Int J Biol Macromol 67: 446-451.
- Lopez-Franco Y, Higuera-Ciapara I, Goycoolea F, Wang W, Konjac A (2009) Other exudates: tragancanth, karays, mesquite gum and larchwook arabinogalactans. Handbook of Hydrocolloids (2nd Edtn) CRC Press, USA.
- Kolesnikova LI, Karpova EA, Vlasov BY, Sukhov BG, Mov BA (2015) Lipid peroxidation-antioxidant defense system during toxic liver damage and its correction with a composite substance containing selenium and arabinogalactan. Bulletin of experimental biology and medicine159: 225-228.
- Burgalassi S, Nicosia N, Monti D, Falcone G, Boldrini E, et al. (2007) Larch arabinogalactan for dry eye protection and treatment of corneal lesions: investigations in rabbits. J Ocul Pharmacol Ther 23: 541-550.
- Agarwal A, Malvezzi H, Sharma R (2013) Effect of an isotonic lubricant on sperm collection and sperm quality. Fertil Steril 99: 1581-1586.
- Vargas J, Crausaz M, Senn A, Germond M (2011) Sperm toxicity of "nonspermicidal" lubricant and ultrasound gels used in reproductive medicine. Fertil Steril 95: 835-836.
- Khan I, Urich M, Shmoury MR, Sharara HM, Gill I, et al. (2000) A three-year study on density gradient medium “arabinogalactan” for sperm separation. Fertil Steril 74: S198.
- Phillips P, Jahnke M (2016) Embryo transfer (techniques, donors, and recipients). Veterinary clinics of North America: food animal practice: bovine theriogenology. Elsevier, Philadelphia, USA.
- Nissen HP, Heinze I, Kreysel HW, Schirren C (1979) Neutral sugar composition of proteins of human seminal plasma from different andrological diagnoses. Andrologia 11: 470-474.
- Yildiz C, Kaya A, Aksoy M, Tekeli T (2000) Influence of sugar supplementation of the extender on motility, viability and acrosomal integrity of dog spermatozoa during freezing. Theriogenology 54: 579-585.
- Said L, Galeraud-Denis I, Carreau S, Saad A (2009) Relationship between semen quality and seminal plasma components: alpha-glucosidase, fructose and citrate in infertile men compared with a normospermic population of Tunisian men. Andrologia 41: 150-156.
- Mori K, Daitoh T, Kamada M, Maeda N, Maegawa M, et al. (1993) Fertilization and early embryology: Blocking of human fertilization by carbohydrates. Hum Reprod 8: 1729-1732.
- Dobrinski I, Ignotz GG, Thomas PG, Ball BA (1996) Role of carbohydrates in the attachment of equine spermatozoa to uterine tubal (oviductal) epithelial cells in vitro. Am J Vet Res 57: 1635-1639.
- Rogers BJ, Perreault SD (1990) Importance of glycolysable substrates for in vitro capacitation of human spermatozoa. Biol Reprod 43: 1064-1069.
- Povoa H Jr., Bastos JJ, Silva ME, Ariza A, Moraes MI, et al. (1986) Glucose in human semen. Biomed Biochim Acta 45: 685-686.
- Carlstedt I, Lindgren H, Sheehan JK, Ulmsten U, Wingerup L (1983) Isolation and characterization of human cervical-mucus glycoproteins. Biochem J 211: 13-22.
- Miranda PV, Gonzalez-Echeverria F, Marin-Briggiler CI, Brandelli A, Blaquier JA, et al. (1997) Glycosidic residues involved in human sperm-zona pellucida binding in vitro. Mol Hum Reprod 3: 399-404.
- Williams AC, Ford WC (2001) The role of glucose in supporting motility and capacitation in human spermatozoa. J Androl 22: 680-695.
- Schwiertz A, Duttke C, Hild J, Muller HJ (2006) In vitro activity of essential oils on microorganisms isolated from vaginal infections. International Journal of Aromatherapy 16: 169-174.
- Gulcin Ü, Uguz MT, Oktay M, Beydemir S, Küfrevioglu O (2004) Evaluation of the antioxidant and antimicrobial activities of clary sage (Salvia sclarea L.). Turk J Agric For 28: 25-33.
- Lee KB, Cho E, Kang YS (2014) Changes in 5-hydroxytryptamine and cortisol plasma levels in menopausal women after inhalation of clary sage oil. Phytother Res 28: 1599-1605.
- Seol GH, Shim HS, Kim PJ, Moon HK, Lee KH, et al. (2010) Antidepressant- like effect of Salvia sclarea is explained by modulation of dopamine activities in rats. J Ethnopharmacol 130: 187-190.
- Seol GH, Lee YH, Kang P, You JH, Park M, et al. (2013) Randomized controlled trial for Salvia sclarea or Lavandula angustifolia: differential effects on blood pressure in female patients with urinary incontinence undergoing urodynamic examination. J Altern Complement Med 19: 664-670.
- Monton A, Gil L, Malo C, Olaciregui M, Gonzalez N, et al. (2015) Sage (Salvia officinalis) and fennel (Foeniculum vulgare) improve cryopreserved boar epididymal semen quality study. Cryo letters 36: 83-90.
- Tecle E, Gagneux P (2015) Sugar-coated sperm: Unraveling the functions of the mammalian sperm glycocalyx. Mol Reprod Dev 82: 635-650.
- Gotting C, Kuhn J, Kleesiek K (2007) Human xylosyltransferases in health and disease. Cell Mol Life Sci 64: 1498-1517.
- Gotting C, Kuhn J, Brinkmann T, Kleesiek K (2002) Xylosyltransferase activity in seminal plasma of infertile men. Clin Chim Acta 317: 199-202.
- Farrant J (1980) General principles of cell preservation. Frozen human semen. Springer Science & Business Media, London, UK.
- United States Food and Drug Administration (2017) 510(k) Premarket notification database.
- BioFilm IP LLC (2017) Astroglide TTC. Frequently asked questions: What is in TTC?
- Church & Dwight Co., Inc. (2017) Pre-seed fertility-friendly lubricant.
- Sasmar Pharmaceuticals (2017) Conceive plus package insert.
- Fairhaven Health, LLC (2017) BabyDance fertility lubricant: What are the ingredients in BabyDance.
 Spanish
Spanish  Chinese
Chinese  Russian
Russian  German
German  French
French  Japanese
Japanese  Portuguese
Portuguese  Hindi
Hindi