Research Article, J Pharm Drug Deliv Res Vol: 12 Issue: 5
Green Synthetic Overture to Carbon based Nanomaterials from Biosources
Christma Eunice Sherina P*, Francis Tisha N, Shalini J, Rinita J, Riya Jose and NS Nirmala Jothi
Department of Physics, Loyola College, Chennai, India
*Corresponding Author: Christma Eunice Sherina P
Department of Physics, Loyola College, Chennai, India
E-mail: chrisherinaphy@gmail.com
Received date: 27 April, 2023, Manuscript No. JPDDR-23-97272;
Editor assigned date: 02 May, 2023, PreQC No. JPDDR-23-97272 (PQ);
Reviewed date: 16 May, 2023, QC No. JPDDR-23-97272;
Revised date: 27 June, 2023, Manuscript No. JPDDR-23-97272 (R);
Published date: 04 July, 2023, DOI: 10.36648/2325-9604.100242
Citation: Sherina CEP, Tisha FN, Shalini J, Rinita J, Jose R, et al. (2023) Green Synthetic Overture to Carbon based Nanomaterials from Biosources. J Pharm Drug Deliv Res 12:5.
Abstract
Nanomaterials are consistently conquering modern society and are uncovering ways to sneak into a variety of uses aimed at enhancing individual standards of everyday life. Carbon serves as an anchor for everything that exists on earth and carbon based materials are essential in the evolution of material science. During the past few decades, quite a few numbers of carbon based nanomaterials have been progressed by dint of diversified synthetic process. Fresh green approaches and innovations are pertinent to carbon based nanomaterials posed by researchers in every corner of the world have widened massive opportunities towards the green nanotechnology revolution. This paper deals with experimental and the characterization procedures of the as synthesized carbonbased nanomaterials. Herein, we report carbon based nanomaterials green synthesized via bottom up approach by utilizing carrot (Daucus carota) and ginger (Zingiber officinale) as sources. The experimental employs the extracts of both the sources being exposed to high temperature and a limited period. The characterization studies are examined by means of SEM-EDAX analysis, FTIR analysis, XRD analysis, UV-vis spectroscopic analysis, PL analysis, DLS and zeta potential analysis for both the samples is discussed furthermore.
Keywords: Green synthesis; Green sources; Biomass derived carbon; Hydrothermal synthesis; Carbon based nanomaterials; Carrot; Ginger
Introduction
Nanomaterials have flourished as an exhilarating class of materials in high demand, for a broad myriad of advantageous uses. Nanomaterials consist of a diverse range of examples with at least one dimension ranging from 1 nm to 100 nm [1]. Nano sized materials featuring symmetrical morphology have sparked the curiosity of several scientists due to their role in core understanding of science and conceivable uses. The emergence of nanotechnology may also lead to fresh and unforeseen ecological concerns, such as new classes of toxic particles and relevant environmental hazards. We currently have a multitude of nanotechnology products that are available or in development that are putting the finishing touches [2]. We have many products over the next handful of years that retain promising future for revolutionizing and prevent illness, but we remain unaware of the environmental consequences of nanotechnology linked with rapid changes in technology. Whilst still implementing this new technology for health, environmental, and sustainability benefits, scientific researchers should indeed continue investigating the environmental and health repercussions [3-7]. Carbon, in its myriad guises, has been used in technology and human life since ancient times. Carbon based materials such as graphite, charcoal, and carbon black were originally developed for both writing and artistic supplies since the dawn of time. The aforementioned substances have been conveniently fabricated into carbon-based nanomaterials such as carbon nanotubes, graphene oxide, graphene quantum dots, nanodiamonds, fullerenes, carbon nano onions, and furthermore attributed to their superior chemical, mechanical, electrical, and thermal properties. Carbon based nanomaterials are now becoming incredibly common in the scientific and technological fields. These contribute significantly in the evolvement of material science, as these are well chosen to be biocompatible, ultra light weight and are chemically inert. Since the early 1990’s, when C-based nanomaterials were first explored, a series of methods have been developed. Hydrothermal synthesis is a best method to synthesize carbon based nanomaterials from the category of bottom up approaches. It is a technique which depends upon the formation of the material in high temperature and pressure. Environmental technology plays a chief role in redefining the presentday science field. An environmental favorable green process is now the most essential and has become popular amongst the puzzles of the world associating with the poisoning of the environment. In more recent times, recyclables, specifically bio wastes, have been repurposed into the most amusing carbon sources for carbon based nanomaterials manufacturing [8]. Although bio wastes tend to be economical and environmentally friendly, they are becoming ever more common. Researchers have thus begun implementing renewable, affordable, and plentiful carbon based nanomaterials for sustainable development in a situation like this. Through hydrothermal approach of carbon-based nanomaterial using peculiar sustainable carbon sources has provided immense progress due to its ease and good quantum yield. For example, carbon based nanomaterials were well prepared by various natural resources together with rose heart radish extract Liu, et al. walnut shells Cheng, et al. watermelon peels Zhou, et al. apple juice Mehta, et al. orange juice Sahu, et al. milk Wang, et al. honey Mandani, et al. so on and so forth.
Herein, et al. we report the preparation of carbon based nanomaterials using carrot and ginger juices as raw materials. Carrot, an archetype of the Apiaceae family Zaccari, et al. includes the genus Daucus and species Carota, and serves as one of the most prominent root crops cultivated all over the world, for its fleshy edible roots; and it is used for human and animal consumption. Carrot roots are indeed conical in form. The root comes in peculiar shade, which would include orange, yellow, purple, red, and white. The different colors are caused by differing pigment contents [9]. Carrots can be used to make extensive new byproducts. Carrot juice and its extracts are among the most frequently consumed beverages. The quantity and quality of root vegetable juice extracted by pressing are altered by pre-treatment factors that include pH, temperature, and time. Ginger is an element of the Zingiberaceae plant family, which includes the genus Zingiber. Since olden days, ginger is consumed as traditional medicines and herbal, and it is prevalently used for seasoning or as condiment Mbaveng, et al. till date [10]. In this paper, the extracts of carrot (Daucus carota) and ginger (Zingiber officinale) are used as duet sources in the green synthesis of carbon based nanomaterials via the hydrothermal method at a short duration of time without the need for any chemicals, just deionized water. This process has the substantial benefit of not necessitating a strong acid solvent, a surface passivation reagent, or a laborious post-treatment procedure [11].
Materials and Methods
Pure distilled water was purchased from Merck. Fresh tenders of carrot (Daucus carota) and ginger (Zingiber officinale) were purchased from the regional markets. These were well pulverized for their extracts individually with the addition of double distilled water and filtered to obtain the required extract [12].
Hydrothermal synthesis from carrot extract
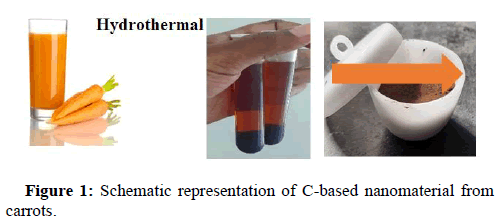
250 grams of carrots were diced and processed for its extract. It was further treated hydrothermally around 150°C to 200°C, as we identified the stability condition under the specified reaction time [13]. The carbonized black solution was cooled down to ambient temperature. The solution was then centrifuged at 5000 rpm speed for 15 mins to obtain black precipitate containing C-based material. It was then left to dry at 180°C and finely powdered (Figure 1).
Hydrothermal synthesis from ginger extract
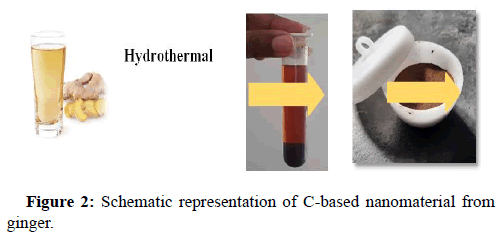
The fresh tender ginger was diced and processed after its skin was scraped. To completely eradicate solid residues, 20 mL of extracted ginger juice was heated and then centrifuged for about 10 minutes at 5000 rpm speed. The extract (supernatant) was then adjusted to pH 7 using KOH. Finally, the supernatant was filtered to clear every other remaining solid residue (Figure 2). The extracted juice was hydrothermally treated. Under the hydrothermal treatment around 150°C to 200°C, we identified the stability condition under a specified reaction time [14]. The black carbonized solution that developed was left to dry at room temperature. The reacted solution begot was centrifuged at 5000 rpm speed to get the precipitate comprising C-based material. The prepared precipitate is further dried at 180°C and finely powdered.
Instrumentation
The surface morphology and particle size of the obtained samples was analyzed using a FEI-quanta FEG 200 F high resolution scanning electron microscope with an accelerating voltage of -200 V to 30 kV, the elemental configuration of the sample was confirmed by Energy Dispersive X-Ray diffraction (EDAX) analysis. The dried powder of C-based material was submitted to FTIR spectroscopic studies. FTIR was recorded on a PerkinElmer spectrum system-one instrument at a resolution of 1 cm−1 in KBr pellet. X-ray powder diffraction was used to catch on the crystalline phase and the purity of the as prepared samples (XRD) using Bruker USA D8 advance with a Cu Kα radiation source (λ=0.15406 nm) [15]. UV-Visible absorption spectrum was monitored in PerkinElmer lambda 950 UV-VIS-NIR spectrophotometer operated at ≤ 0.05 nm resolutions. The photoluminescence excitation and emission spectra were obtained on a JY fluorolog-3-11 spectrofluorometer. The zeta potential and size distribution of the C-based nanomaterial was analysed using Nanotrac Wave II, Microtrac, Inc. USA.
Results and Discussion
Scanning Electron Microscopy (SEM) and Energy Dispersive X-Ray Analysis (EDAX)
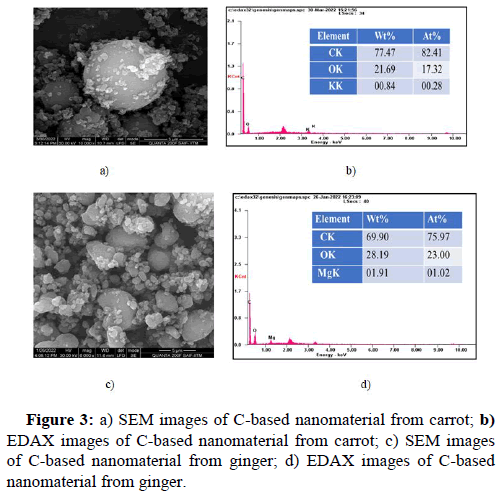
The SEM morphological analysis of carrot mediated C-based nanomaterial indicated that the particle was observed to be in spherical shape Liu, et al.; Mehta, et al., with uniformity and well separated from each other, whereas, the ginger mediated C-based nanomaterial confirmed the presence of spherical nanoparticle Mehta, et al.; Shen, et al., and varied to more irregular shapes, with the particles being agglomerated into nanostructures of much larger sizes. Figure 3 shows the morphology of the carrot and ginger mediated C-based nanomaterial respectively [16].
For further study, EDAX was preferred to analyse the elemental composition in the nanomaterial. Figure 3b and Figure 3d indicates the EDAX spectrum of carrot and ginger mediated C-based nanomaterial, respectively. The EDAX analysis of the carrot mediated carbon based nanoparticle confirms the presence of carbon, oxygen and potassium [17]. On the other hand, ginger mediated carbon based nanoparticle confirms the presence of carbon, oxygen and magnesium from the emission of energies of these elements which is plotted as a spectrum between x-ray counts and energy (keV) respectively.
Fourier Transform Infrared Spectroscopy (FTIR) analysis
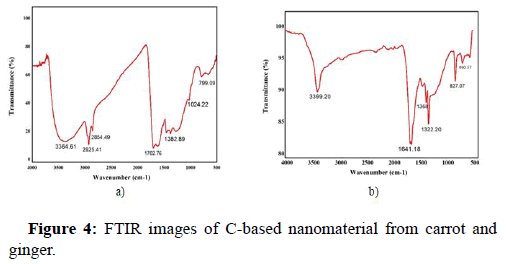
The major constituents and the structure of the carrot and ginger mediated carbon based nanoparticle are figure out with the aid of FTIR analysis. Figure 4a and Figure 4b indicate the FTIR spectrum of the carrot and ginger mediated C-based nanomaterial, respectively [18]. The FTIR spectrum of the carrot mediated and ginger mediated C-based nanoparticle exhibits characteristic absorption bands which are tabulated below in Table 1.
| Green source used | Wavenumber | Bonding assignment |
|---|---|---|
| Carrot | 3364 cm-1 | O−H/N−H stretching vibrations |
| 2925.41 cm-1 | −CH stretching vibrations | |
| 1382.39 cm-1 | C−OH vibrations | |
| Ginger | 3399.20 cm-1 | O−H/N−H stretching vibrations |
| 2924.90 cm-1 | C−H vibrations | |
| 1641.98 cm-1 | C=C vibrations |
Table 1: FTIR absorption bands for carrot mediated and ginger mediated carbon based nanomaterials.
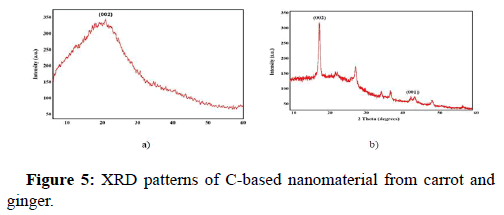
X-Ray Diffraction (XRD) analysis
The Figures 5a and Figure 5b showcases the XRD patterns of C-based nanomaterials derived using carrot and ginger. Figure 5a depicts the XRD pattern of carrot mediated C-based material, thereby confirming itself to have frail crystalline nature of carbon. The single diffraction peak is displayed broad and intense centered at 2θ=19.6° corresponding to the interlayer distance of ~4.524 A° which is practically higher than the graphitic interplanar spacing of 0.33 nm. This *diffraction peak is assigned to (002) diffraction plane of graphitic carbon (JCPDS 26-1076). According to Scherrer’s formula, the predicted particle size is 18.67 nm. Secondly, Figure 5b depicts the XRD pattern of ginger-mediated C-based material shows a narrow sharp peak at 2θ=18.02° that can be ascribed to the crystallite structure of carbon. It accredits to the interplanar spacing of ~4.9 A°. The weak, broad band at 2θ=43.3° is due to the partial graphitization of the C-based material. The diffraction planes are noticed to be (002) and (001) respectively. With all peaks considered, the XRD patterns of the sample shows sharp diffraction peaks centered at 18.02°, 28.3° and 43.3°, 47.8° and owe to the disordered carbon atoms and its crystallographic nature. According to Scherrer's formula, the predicted crystallite (grain) size is 15.14 nm.
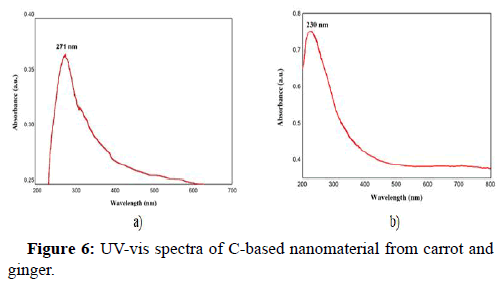
UV-visible spectroscopic analysis
UV-vis spectroscopy is probably the most beneficial tool for determining the optical properties of C-based nanomaterials. Figure 6a shows the UV-vis spectra of the D. carota extract mediated C-based nanomaterial and Figure 6b shows the UV-vis spectra of the Z. officinale extract mediated C-based nanomaterial [19]. In the Figure 6a, the absorption maxima is seen at 271 nm and matches π−π* transition of C=C (sp2) bonds Arul, et al.; Sharma, et al., whereas, in Figure 6b, the absorption maxima is seen at 230 nm and it is assigned to the π–π* transition of C=C bonds of aromatic rings.
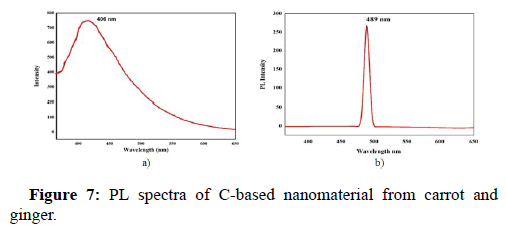
Photoluminescence (PL) analysis
The PL behaviour dependent on excitation is most prominent in the carbon based fluorescent materials. PL analysis was employed to examine the photoluminescence properties of the obtained product. The PL spectra of D. carota extract mediated C-based nanomaterial is depicted in Figure 7a and the PL spectra of Z. officinale mediated C-based nanomaterial is depicted in Figure 7b. The C-based nanoparticle synthesized using carrot as green source exhibited fluorescence at an excitation wavelength range of 300 to 600 nm. The emission peak of the fluorescent C-based nanoparticle is 406 nm with an input of 365 nm as excitation wavelength. Au contrarie, the C-based nanoparticle green synthesized with ginger displayed fluorescence at an excitation wavelength of 300 to 600 nm. The nanoparticle exhibits maximum excitation at 365 nm and the emission peak is found at 489 nm. It is also evident that Atchudan, et al.; Atchudan, et al., synthesized multicolor emitting carbon dots from malus floribunda via a one-step hydrothermal method. With 406 nm as the maximum emission peak possessing exemplary stability, the as-synthesized N-CDs were used as a fluorescent probe for the imaging of bio-cells. Thus, we can presume that carbon based nanomaterials having good optical property and supreme stability will pertain as efficient imaging probes.
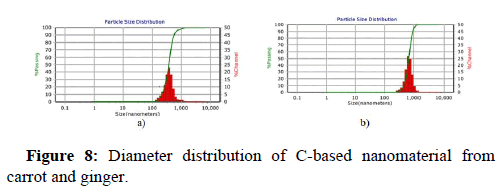
DLS and zeta potential analysis
DLS (Dynamic Light Scattering) and zeta potential analyses have expanded in prominence as swift, simple, and consistent techniques to determine particle size and surface charge. Observations mostly in drug delivery literature label NP-dispersions with zeta potential values of 0-10 mV, 10-20 mV, 20-30 mV, and >30 mV as very unstable, fairly stable, moderately stable, and highly stable, accordingly. Yifang Yuan and his colleagues Yuan, et al. synthesized carbon dots through the hydro thermolysis of milk, thereafter the carbon dots were applied for anticancer drug delivery using DOX as a chemotherapeutic drug. It is observed that the zeta potential value of -7.38 mV contributed to the electrostatic interaction of DOX to the surface of the carbon dots. Herein we report, the zeta potential study of carrot mediated C-based nanomaterial in aqueous solution is seen to be -43.8 mV and ginger synthesized C-based nanomaterial in aqueous solution is -41.7 mV. Both the synthesized nanomaterials showcased negative zeta potentials and the existence of carboxyl and hydroxyl around surface of the carbon-based nanomaterial is confirmed. These negative values also indicate that these C-based nanomaterials are colloid ally stable in biological environments. Figure 8a and Figure 8b depicts the diameter distribution of C-based nanomaterial of carrot mediated and ginger mediated respectively. The polydispersity index of the carrot mediated C-based nanomaterial and ginger synthesized C-based nanomaterial is noted to be 0.0923 and 0.0639, respectively.
Conclusion
In this study, the extracts of fresh tender carrots and ginger have been used to synthesize carbon based nanomaterials at high temperature for a specific time period, hydrothermally. The SEM-EDAX analysis indicates both the samples showed spherical morphology with the confirmation of Potassium in the sample synthesized with carrot extract and Magnesium in the sample synthesized via ginger extract. FTIR showed similar absorption bands in both the samples. The XRD analysis of carrot synthesized C-based nanomaterial confirmed poor crystalline nature with a broad and intense peak at 19.6° corresponding to the interlayer distance of ~4.524 A° depicting (002) diffraction planes. On the other side of the coin, it is noted that ginger mediated C-based material showed a narrow sharp peak at 18.02° confirming the crystalline nature of carbon, attributing to the (002) diffraction planes. The UV-Vis spectra of the carrot synthesized C-based nanomaterial showed the absorption maxima at 271 nm and ginger synthesized C-based nanomaterial showed the absorption at 230 nm. The fluorescence spectra of carrot synthesized C-based nanomaterial is found at 406 nm and at 489 nm the fluorescence spectra of ginger synthesized C-based nanomaterial is found. Both the synthesized nanomaterials showcased negative zeta potentials. Thus, C-based nanomaterial adopting carrot and ginger are synthesized and its properties are hereby discussed.
Disclosure Statement
No potential conflict of interest was reported by the authors.
References
- Anzar N, Hasan R, Tyagi M, Yadav N, Narang J (2020) Carbon nanotube-A review on synthesis, properties and plethora of applications in the field of biomedical science. Sens Int 1:100003
- Arul V, Sethuraman MG (2019) Hydrothermally green synthesized nitrogen doped carbon dots from Phyllanthus emblica and their catalytic ability in the detoxification of textile effluents. ACS Omega 4(2):3449-3457.
[Crossref] [Google Scholar] [PubMed]
- Atchudan R, Edison TN, Perumal S, Vinodh R, Lee YR (2020) Multicolor emitting carbon dots from Malus floribunda and their interaction with Caenorhabditis elegans. Materials Letters 261:127153.
- Bhattacharjee S (2016) DLS and zeta potential what they are and what they are not? J Control Release 235:337-351.
[Crossref] [Google Scholar] [PubMed]
- Chandrasekaran P, Arul V, Sethuraman MG (2020) Ecofriendly synthesis of fluorescent nitrogen-doped carbon dots from Coccinia grandis and its efficient catalytic application in the reduction of methyl orange. J Fluoresc 30(1):103-112.
[Crossref] [Google Scholar] [PubMed]
- Cheng C, Shi Y, Li M, Xing M, Wu Q (2017) Carbon quantum dots from carbonized walnut shells: Structural evolution, fluorescence characteristics, and intracellular bioimaging. Mater Sci Eng C Mater Biol Appl 79:473-480.
[Crossref] [Google Scholar] [PubMed]
- D’souza SL, Chettiar SS, Koduru JR, Kailasa SK (2018) Synthesis of fluorescent carbon dots using Daucus carota subsp. sativus roots for mitomycin drug delivery. Optik 158:893-900.
- de B, Karak N (2013) A green and facile approach for the synthesis of water soluble fluorescent carbon dots from banana juice. RSC Advances 3:8286-8290.
- Huang G, Lu CH, Yang HH (2019) Magnetic nanomaterials for magnetic bioanalysis. Novel Nano Bio Environ Energy App 89-109.
- Jin H, Gui R, Wang Y, Sun J (2017) Carrot derived carbon dots modified with polyethyleneimine and nile blue for ratiometric two photon fluorescence turn-on sensing of sulfide anion in biological fluids. Talanta 169:141-148.
[Crossref] [Google Scholar] [PubMed]
- Liu W, Diao H, Chang H, Wang H, Li T, et al. (2017) Green synthesis of carbon dots from rose heart radish and application for Fe3+ detection and cell imaging. Sensors and Actuators B: Chem 241:190-198.
- Ma T, Tian C, Luo J, Sun X, Quan M, et al. (2015) Influence of technical processing units on the α-carotene, β-carotene and lutein contents of carrot (Daucus carrot L.) juice. J Funct Food 16:104-113.
- Ma X, Li S, Hessel V, Lin L, Meskers S, et al. (2019) Synthesis of luminescent carbon quantum dots by microplasma process. Chem Eng Process Process Intensi 140:29-35.
- Mehta VN, Jha S, Kailasa SK (2014) One pot green synthesis of carbon dots by using Saccharum officinarum juice for fluorescent imaging of bacteria (Escherichia coli) and yeast (Saccharomyces cerevisiae) cells. Mater Sci Eng C Mater Biol Appl 38:20-27.
[Crossref] [Google Scholar] [PubMed]
- Murugan N, Prakash M, Jayakumar M, Sundaramurthy A, Sundramoorthy AK (2019) Green synthesis of fluorescent carbon quantum dots from Eleusine coracana and their application as a fluorescence ‘turn off’sensor probe for selective detection of Cu2+. Appl Surf Sci 476:468-480.
- Muthukumar T, Chamundeeswari M, Prabhavathi S, Gurunathan B, Chandhuru J, et al. (2014) Carbon nanoparticle from a natural source fabricated for folate receptor targeting, imaging and drug delivery application in A549 lung cancer cells. Eur J Pharm Biopharm 88:730-736.
[Crossref] [Google Scholar] [PubMed]
- Sahu S, Behera B, Maiti TK, Mohapatra S (2012) Simple one step synthesis of highly luminescent carbon dots from orange juice: Application as excellent bio-imaging agents. Chem Commun (Camb) 48:8835-8837.
[Crossref] [Google Scholar] [PubMed]
- Shaikh AF, Tamboli MS, Patil RH, Bhan A, Ambekar JD, et al. (2019) Bioinspired carbon quantum dots: An antibiofilm agents. J Nanosci Nanotechnol 19:2339-2345.
[Crossref] [Google Scholar] [PubMed]
- Sharma N, Das GS, Yun K (2020) Green synthesis of multipurpose carbon quantum dots from red cabbage and estimation of their antioxidant potential and bio-labeling activity. Appl Microbiol Biotechnol 104:7187-7200.
[Crossref] [Google Scholar] [PubMed]
 Spanish
Spanish  Chinese
Chinese  Russian
Russian  German
German  French
French  Japanese
Japanese  Portuguese
Portuguese  Hindi
Hindi