Research Article, J Spine Neurosurg Vol: 13 Issue: 1
Lumbar Interbody Spinal Fusions Using a Novel Cellular Bone Matrix Allograft
John David Dorchak1, Miller Gantt2, and J. Kenneth Burkus1*
1Department of Orthopedic Surgery, Jack Hughston Memorial University Hospital, Phenix City, Alabama, United State of America
2Department of Orthopedic Surgery, Mercer University School of Medicine, Avenue, Columbus, Georgia
*Corresponding Author: J. Kenneth Burkus,
Department of Orthopedic Surgery,
Jack Hughston Memorial University Hospital, Phenix City, Alabama, United State
of America
E-mail: jkb66@knology.net
Received date: 12 January, 2024, Manuscript No. JSNS-24-124991;
Editor assigned date: 15 January, 2024, PreQC No. JSNS-24-124991 (PQ);
Reviewed date: 29 January, 2024, QC No. JSNS-24-124991;
Revised date: 07 February, 2024, Manuscript No. JSNS-24-124991 (R);
Published date: 14 February, 2024, DOI: 10.4172/2325-9701.1000184
Citation: Dorchak JD, Gantt M, Burkus JK (2024) Lumbar Interbody Spinal Fusions Using a Novel Cellular Bone Matrix Allograft. J Spine Neurosurg 13:1.
Abstract
In lumbar spinal arthrodesis surgery, bone graft substitutes are used to avoid the complications and limitations associated with autologous Iliac Crest Bone Grafts (ICBGs). Cellular Allogeneic Bone Matrices (CBMs) are used as a biologic replacement for iliac crest bone grafts in lumbar spinal fusions. Advanced cryopreservation techniques utilized during allograft processing have been used to retain native viable Mesenchymal Stem Cells (MSCs) and other osteoprogenitor cells available for transplantation. Our goal was to evaluate the clinical efficacy of the application of an advanced CBM implant used as a bone graft replacement in patients with degenerative lumbar disc disease undergoing lumbar interbody fusion surgery.
A consecutive series of fourteen patients (eight males and six females) with a mean age of 60 years (range 40 to 78 years) who underwent single or two-level Lumbar Interbody Fusion (LIF) using a cellular allogeneic bone matrix as the sole bone graft material were retrospectively reviewed. All patients were followed for a minimum of twelve months, and preoperative, three, six and twelve-month postoperative data were assessed. Lumbar fusion status was evaluated by plane and dynamic radiographs.
All patients showed successful radiographic fusion at six months after surgery with evidence of bridging trabecular bone across the interspace. There was no deterioration in fusion status between six and twelve months; all patients remained fused. There was no evidence of implant migration or subsidence. No adverse events were identified at surgery or during the course of follow-up. Improvement in back and leg pain symptoms was identified in all patients; there was no documented loss of neurological function.
The use of a cellular allogeneic bone matrix provided sufficient biological support for successful lumbar spinal interbody fusions.
Keywords
Lumbar interbody fusion; Cellular bone matrix; Cellular allogeneic bone matrix; Cellular bone scaffold; Degenerative lumbar disc disease
Introduction
Spinal fusion surgery is a commonly used procedure for treating degenerative conditions of the lumbar spine [1,2]. Minimally invasive surgical techniques and spinal instrumentation have evolved to restore disc space height and sagittal alignment and provide segmental stabilization of the disc space to facilitate de novo bone growth across the interspace [3]. Successful interbody fusion requires the addition of grafting materials containing osteogenic cells or that provide osteoinductive signals to form bone across the intervertebral interspace. Autologous bone from the iliac crest has been widely used in bone graft procedures because of these osteogenic properties. While Iliac Crest Bone Grafts (ICBG) are effective, they have been associated with a number of complications, including pain and infection at the donor site [4-8]. Alternatives to ICBGs include local bone, allografts, synthetics, recombinant proteins, and bone marrow aspirate. These options are used individually or in combination [9-14].
Allogeneic bone grafts that contain living cells, including Mesenchymal Stem Cells (MSCs), have the potential to implant lineages capable of osteoblastic activity to the surgical fusion site [15-17]. These advanced allografts, known as Cellular Bone Matrices (CBMs), combine the inductivity of a Demineralized Bone Matrix (DBM) with a cellular component of cancellous bone containing MSCs. Through aseptic tissue, processing and advanced cryopreservation techniques, native cells, including MSCs and other osteoprogenitor cells, are retained [18]. This optimized CBM provides a minimum of 750,000 cells per cc within an osteoconductive matrix [13]. This viable tissue matrix provides the three components necessary for osteogenesis and may improve the rates of new bone formation and subsequent fusion in spinal surgeries. These data evaluate the clinical efficacy of an advanced CBM allograft in patients with degenerative lumbar disc disease undergoing Lumbar Interbody Fusion (LIF) surgery.
Materials and Methods
A single investigator reviewed fourteen patients who had been treated at a single site between January 2022 and May 2022. Consecutive patients diagnosed with degenerative lumbar disc disease between L1 and the sacrum were not randomized; all underwent instrumented interbody fusion and received the optimized CBM (VIA Form+, Vivex Biologics, Inc., Atlanta, Georgia) as a stand-alone bone graft replacement when used with an interbody device. This study received IRB approval (HIRB-2023-13).
Stand-alone bone graft substitute
In this clinical study, the optimized CBM was used without any autogenous grafts, including local bone or bone marrow aspirate. The CBM contains three key components that are considered essential for bone formation: An osteoconductive matrix from cancellous bone chips (100-300 μm), an osteoinductive potential achieved from demineralized cortical bone and an endogenous cellular content with potential for osteogenic differentiation [11-14,16]. Through aseptic processing, donor bone is separated into two components: Viable cellrich cancellous bone and cortical bone that is demineralized to accentuate growth factors. Cells attached to cancellous bone chips express markers linked with those identified as mesenchymal stem cells and osteoblasts [19]. These cell-rich grafts are frozen and protected using a proprietary non-DMSO cryoprotectant [18]. This novel processing results in a 92% viability of cells with expanded cells demonstrating osteogenic potential that was confirmed by in vitro assessment of alkaline phosphatase activity [20].
Inclusion criteria
All patients had clinical complaints of low back pain and radiating leg pain that was unresponsive to a minimum of eight weeks of nonoperative treatment that included immobilization, traction, modalities, medications, and physical therapy. In addition to recurrent or persistent complaints of pain, all patients had an objective neurologic deficit that included one or more of the following: An asymmetric deep tendon reflex, a sensory deficit in a dermatomal pattern, or motor weakness. All patients had a correlative neuroradiographic study. Smoking status and the presence of diabetes, osteoporosis, and obesity were recorded for all patients. Patients were not treated surgically if they had a chronic medical condition that required medication, such as steroids or nonsteroidal antiinflammatory medications that could interfere with fusion.
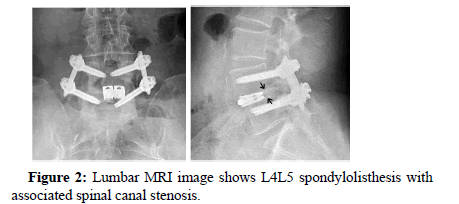
Patients included in this study required plain radiographic findings documenting single or two-level degenerative lumbar disc disease, and they had undergone an additional confirmatory MRI scan. Patients with up to a Grade one spondylolisthesis were also included.
Surgical technique
The CBM was used exclusively as a bone graft replacement. The CBM was placed in the disc space and in the intradiscal implant. Pedicle screws were inserted through a minimally invasive percutaneous technique. Following surgery, all patients were encouraged to ambulate immediately after surgery, and physical activities were advanced at the discretion of the attending surgeon. An external lumbar orthosis was used at the discretion of the attending surgeon.
Patient demographics
Patients with single or two-level degenerative disc disease of the lumbar spine and associated radiculopathy were treated by a Posterior Lumbar Interbody Fusion (PLIF) (fourteen levels) or Extreme Lateral Interbody Fusion (XLIF) (three levels) using a titanium interbody implant, pedicle screw fixation and the advanced CBM allograft bone graft substitute. Patient age, sex, smoking status, and comorbidities were assessed.
Clinical and radiographic follow-up
All patients were examined at three, six, and twelve months; four patients were followed for eighteen months. All clinical outcomes were assessed by the attending physician; however, clinical outcomes were not assessed through patient-derived questionnaires. Neurological physical examination was conducted at each follow-up clinical visit. Neurological success was defined as maintenance or improvement in three objective clinical findings: Sensory, motor, and reflex testing.
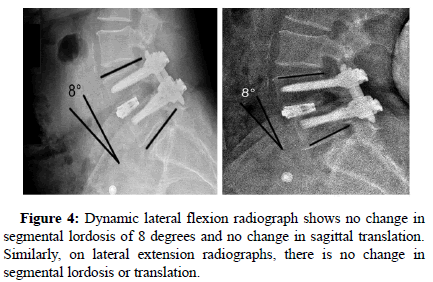
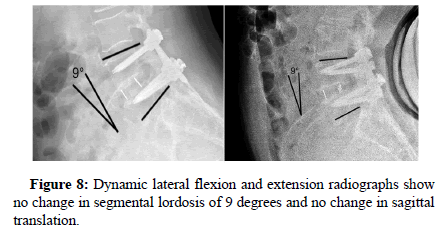
Neutral anteroposterior and lateral radiographs were obtained at each visit. Dynamic flexion-extension lateral radiographs were taken at six, twelve, and eighteen months. Sagittal plane angulation was measured on neutral lateral radiographs and determined by Cobb’s criteria. Intradiscal distraction and subsidence were measured by assessing the vertical distance between the midpoints of the adjacent vertebral endplates. Intradiscal motion and implant migration were assessed on dynamic radiographs. All radiographs were reviewed by an independent physician. Successful fusion was defined by three criteria. The first was uninterrupted bridging bone across the instrumented disc space through either the interbody implants or around the implants. At the host bone implant interface, there were no radiolucent lines around more than 50% of either implant. In addition, on dynamic flexion extension radiographs, there was less than 5° of angular motion and less than 3 mm of translation.
Adverse events
All patients were monitored for the presence of adverse events during the surgical procedure and during routine or unanticipated office visits. All complications were documented, including additional surgical procedures, spinal injections, and hospital readmissions.
Results
All patients in the study had a minimum follow-up of 12 months; no patients were lost to follow-up. The fourteen-patient cohort included six females and eight males with an average age of 60 years ranging from 40 to 78 (Table 1). Four patients (4/14; 28%) smoked or used tobacco products; three (3/14; 21%) were obese with a BMI of greater than 30; three (3/14; 21%) were diabetic. The choice of implant was not correlated with comorbidities or the number of levels treated. There were eleven single-level and three 2-level fusions between L1 and L5 (Tables 2 and 3).
| Patient Demographics | Male | Female |
|---|---|---|
| No. of Patient | 8/14 (57%) | 6/14 (43%) |
| Age | 40-78 (Average 60) | |
| Smokers | 4/14 (28%) | |
| Diabetes | 3/14 (21%) | |
| Obesity (BMI>30) | 3/14 (21%) | |
| Osteoporosis (T-Score>2.5) | None | |
Table 1: Information about Patient Demographics.
| Levels treated 17 |
|---|
| L12: 1 |
| L23: 1 |
| L34: 1 |
| L45: 8 |
| L5S1: 6 |
Table 2: Lumbar Spinal Levels Treated.
| Surgical Procedures |
|---|
| PLIF (single level) 8/14 (57%) |
| PLIF (two level) 3/14 (21%) |
| XLIF (single level) 3/14 (21%) |
Table 3: Information of surgical procedures.
Clinical outcomes
At the last follow-up examination, all patients had sustained improvement in back and leg pain symptoms when compared to their preoperative status. No standardized patient-reported outcome questionnaires or numeric rating scales were used in these assessments. Neurological success was also observed in all study patients, with none showing a loss in neurological functioning for the duration of the study.
Radiographic outcomes
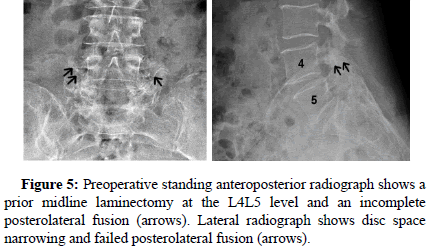
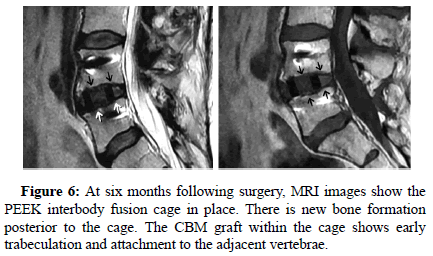
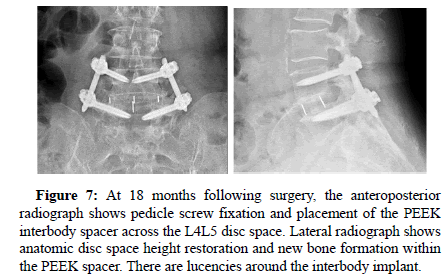
All patients showed successful radiographic fusion at six months after surgery with evidence of bridging trabecular bone across the interspace, forming a continuous bony connection from the superior vertebral body to the inferior vertebral body (Figures 1-4). In addition, there was no evidence of radiolucency involving more than 25% of the superior or inferior implant-vertebral interface. No patients had deterioration in their fusion status between the six and twelve-month follow-up reviews; all patients remained fused. There was no evidence of implant migration or subsidence at any disc level studied. There were no radiographic fusion differences in patients treated with one or two-level fusion surgeries. Similarly, there were no differences in fusion outcomes between the PLIF and XLIF (Figures 5-8) surgical approaches.
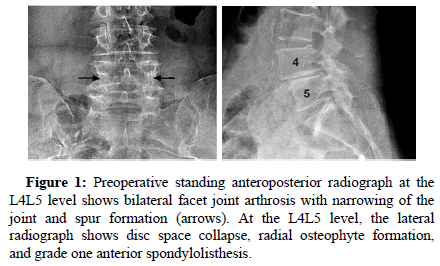
Figure 1: Preoperative standing anteroposterior radiograph at the L4L5 level shows bilateral facet joint arthrosis with narrowing of the joint and spur formation (arrows). At the L4L5 level, the lateral radiograph shows disc space collapse, radial osteophyte formation, and grade one anterior spondylolisthesis.
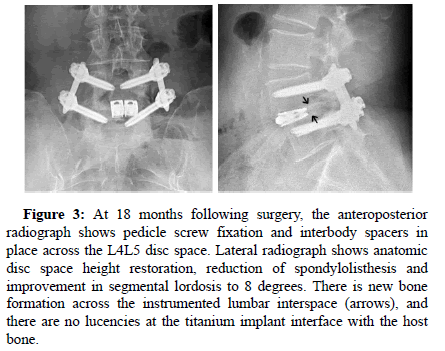
Figure 3: At 18 months following surgery, the anteroposterior radiograph shows pedicle screw fixation and interbody spacers in place across the L4L5 disc space. Lateral radiograph shows anatomic disc space height restoration, reduction of spondylolisthesis and improvement in segmental lordosis to 8 degrees. There is new bone formation across the instrumented lumbar interspace (arrows), and there are no lucencies at the titanium implant interface with the host bone.
Figure 7: At 18 months following surgery, the anteroposterior radiograph shows pedicle screw fixation and placement of the PEEK interbody spacer across the L4L5 disc space. Lateral radiograph shows anatomic disc space height restoration and new bone formation within the PEEK spacer. There are lucencies around the interbody implant.
Adverse events
In the present study, there were no complications associated with the use of the CBM as a bone graft substitute. No adverse events were identified at surgery or during the course of follow-up. No patients sustained any loss of neurological function. No patients underwent any additional surgical procedures; none had revision of their supplemental posterior fixation. No patients had additional spinal injections.
Discussion
Successful interbody fusion requires the addition of either osteogenic cells or osteoinductive signals to form bone across the vertebral interspace [1-3]. Autologous bone from the iliac crest is an established standard for many types of bone graft procedures because of its unique osteogenic properties. While autogenous bone grafting is effective, it is associated with a number of problems, such as pain and infection at the donor site [4-8]. Additionally, there is often a limited amount of graft available. A cellular bone allograft that contains viable MSCs closely resembles the natural cellular and biochemical profile of ICBG, containing osteogenic cell precursors and cytokines. CBMs can be utilized without the associated morbidity of bone graft harvesting. Additionally, there have been no reported limitations or disadvantages associated with the use of viable cellular allograft matrices. The cell content offers the potential advantages of long-term cell proliferation, self-renewal capabilities, and multipotent differentiation. Prior clinical and laboratory studies on CBMs have shown variable success [10-14]. This inconsistency may be related to the allograft harvesting technique, the type of allograft (particulate vs fiber), the percentage of the product that is fully demineralized, the use of additional carriers and the chosen cryoprotectant. Advanced preservation of allograft stem cells is essential to enable the retention of regenerative potential for CBMs [18].
In this study, all patients showed radiographic evidence of fusion at six months after surgery. There was no evidence of implant migration or hardware failure on postoperative radiographs in any patient throughout the duration of the study. Patients improved with their clinical symptoms and back and leg pain. None of the patients underwent any additional surgical procedures. This retrospective study demonstrates that advanced CBMs can promote consistent fusion across the lumbar interspace. In addition, the deleterious effects of smoking, diabetes, osteoporosis, and obesity on the rates of fusion may be overcome by utilizing this grafting material.
There are several limitations associated with this retrospective study. The study was not randomized or controlled by comparing operative patients with patients treated nonoperatively or with a control arm (fusion without cellular allograft). Clinical and radiographic outcomes were assessed for only twelve months. Longer follow-ups are necessary to identify potential treatment failures. Clinical outcomes were not assessed through established patient-derived questionnaires. Radiographic assessment of lumbar spinal fusion with standing and flexion-extension radiographs may not identify all pseudarthroses. Thin-cut CT scans of the interbody fusions were not utilized.
Conclusion
Cellular bone matrices contain a heterogeneous population of cells that have the capacity for self-renewal and osteogenic differentiation. These cellular allografts represent a promising alternative to autologous ICBG because they offer osteogenic, osteoinductive, and osteoconductive properties when used as a stand-alone bone replacement in the surgical treatment of degenerative lumbar disc disease. Autogenous bone grafting and the morbidities and complications associated with this second surgery may be eliminated with this advanced allograft technology.
Author Contributions
JDD and JKB participated in the conception and design of the study, and all three authors participated in the acquisition, analysis, or interpretation of the data for the work. Additionally, all three authors participated in drafting the article or reviewing it critically for important intellectual content. Finally, all three authors gave the final approval of the version to be published and are in agreement to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Acknowledgements
The authors would like to thank the Hughston Foundation for their assistance in medical illustrations and manuscript editing.
References
- Lee CK, Vessa P, Lee JK (1995) Chronic disabling low back pain syndrome caused by internal disc derangements. The results of disc excision and posterior lumbar interbody fusion. Spine 20: 356-361.
- Gill K, Blumenthal SL (1993) Posterior lumbar interbody fusion: A 2 year follow-up of 238 patients. Acta Orthop Scand 251: 108-110.
- Pimenta L, Tohmeh A, Jones D, Amaral R, Marchi L, et al. (2018) Rational decision making in a wide scenario of different minimally invasive lumbar interbody fusion approaches and devices. J Spine Surg 4: 142-155.
- Banwart JC, Asher MA, Hassanein RS (1995) Iliac crest bone graft harvest donor site morbidity. A statistical evaluation. Spine 20: 1055.
- Fernyhough JC, Schimandle JJ, Weigel MC, Edwards C, Levine AM (1992) Chronic donor site pain complicating bone graft harvesting from the posterior iliac crest for spinal fusion. Spine 17: 1474.
- Younger EM, Chapman MW (1983) Morbidity at bone graft donor sites. J Orthop Trauma 3: 192-195.
- Kim DH, Rhim R, Li L, Martha J, Swaim BH, et al. (2009) Prospective study of iliac crest bone graft harvest site pain and morbidity. Spine J 9: 886-892.
- Schwartz CE, Martha JF, Kowalski P, Wang DA, Bode R, et al. (2009) Prospective evaluation of chronic pain associated with posterior autologous iliac crest bone graft harvest and its effect on postoperative outcome. Health Qual Life Outcomes 7: 49.
- Waldrop RP, Rehak CR, Burkus JK (2020) Posterior lumbar interbody fusion using a porous PEEK implant and bone marrow concentrate. J Spine Neurosurg 9: 4.
- Gao Y, Li J, Cui H, Zhang F, Sun Y, et al. (2019) Comparison of intervertebral fusion rates of different bone graft materials in extreme lateral interbody fusion. Medicine (Baltimore) 98: e17685.
- Kerr EJ, Jawahar A, Wooten T, Kay S, Cavanaugh DA, et al. (2011) The use of osteo-conductive stem-cells allograft in lumbar interbody fusion procedures: an alternative to recombinant human bone morphogenetic protein. J Surg Orthop Adv 20: 193-197.
- Lee DD, Kim JY (2017) A comparison of radiographic and clinical outcomes of anterior lumbar interbody fusion performed with either a cellular bone allograft containing multipotent adult progenitor cells or recombinant human bone morphogenetic protein-2. J Orthop Surg Res 12: 126.
- Tally WC, Temple HT, Subhawong T, Ganey T (2018) Transforaminal lumbar interbody fusion with viable allograft: 75 consecutive cases at 12-month follow-up. Int J Spine Surg 12: 76-84.
- Tohmeh AG, Watson B, Tohmeh M, Zielinski XJ (2012) Allograft cellular bone matrix in extreme lateral interbody fusion: Preliminary radiographic and clinical outcomes. ScientificWorldJournal 2012: 263637.
- Majors AK, Boehm CA, Nitto H, Midura RJ, Muschler GF (1997) Characterization of human bone marrow stromal cells with respect to osteoblastic differentiation. J Orthop Res 15: 546-557.
- Brodano BG, Mazzoni E, Tognon M, Griffoni C, Manfrini M (2012) Human mesenchymal stem cells and biomaterials interaction: A promising synergy to improve spine fusion. Eur Spine J 21: S3-9.
- LoGuidice A, Houlihan A, Deans R (2016) Multipotent adult progenitor cells on an allograft scaffold facilitate the bone repair process. J Tissue Eng 7: 2041731416656148.
- Martin WB, Sicard R, Namin SM, Ganey T (2019) Methods of cryoprotectant preservation: Allogeneic cellular bone grafts and potential effects. Biomed Res Int 2019: 5025398.
- Malinin TI, Carpenter EM, Temple HT (2007) Particulate bone allograft incorporation in regeneration of osseous defects; importance of particle sizes. Open Orthop J 18: 19-24.
- Data on File. (2021) VIVEX BIOLOGICS, INC.
 Spanish
Spanish  Chinese
Chinese  Russian
Russian  German
German  French
French  Japanese
Japanese  Portuguese
Portuguese  Hindi
Hindi