Review Article, J Nephrol Ren Dis Vol: 4 Issue: 1
Paradigm Shift to Tolerance from Conventional Immunosuppression in Renal Transplant: A Basic Strategy through Lymphocyte Depletion
SM Suhail* and KT Woo
Department of Renal Medicine, Singapore General Hospital, DUKE-NUS Graduate Medical School, Academia L3, 20 College Road, 169856, Singapore
*Corresponding Author : Suhail SM
Department of Renal Medicine, Singapore
General Hospital and Adjunct Asst Professor, DUKE-NUS Graduate Medical
School, Academia L3, 20 College Road, 169856, Singapore
Tel: +6597254761;
E-mail: grmsms@sgh.com.sg
Received Date: April 16, 2020; Accepted Date: April 26, 2020; Published Date: May 04, 2020
Citation: Suhail SM, Woo KT (2020) Paradigm Shift to Tolerance from Conventional Immunosuppression in Renal Transplant: A Basic Strategy through Lymphocyte Depletion. J Nephrol Ren Dis 4:1.
Abstract
The current immunosuppressive protocols for renal allograft management, targets mainly the resident and host lymphocytes that come into play to reject the grafted tissue. This is analogous to use of antibiotics against invading microorganisms. This strategy is incomplete as the allograft under goes acute rejection episodes, and long term chronic rejection along with many of the adverse events associated with immunosuppressive medicines.
On the other hand, transplant tolerance is defined as a state of donor-specific unresponsiveness to the allograft without a need for ongoing pharmacologic immunosuppression or with a minimal use of single or dual agents. This is a paradigm shift in the allograft management. Even though impossible to achieve currently, anecdotal reports showing complete or partial tolerance do appear in the literature. In summary, that happens through lymphocyte depletion to a degree that provides tolerance.
In theory and practice, there are three strategies of achieving tolerance: (a) achieving a state of hematological chimera, which can induce complete tolerance, (b) more recent, prope or near tolerance where immune-reactive T-cells are eliminated or inhibited using monoclonal antibodies, and (c) most recent, research-based Chimeric Antigen Receptor for T-regulatory (CAR-T) cell therapy using genetically engineered T-reg cells, targeting specific T-Cell Receptors (TCR) for attenuation of Tcell immune-reactivity, which induces energy.
Safe and reliable strategies for the induction of full tolerance have not yet been achieved. However, in the light of currently available anecdotal and prospective randomized studies, a plausible paradigm shift to tolerance could be anticipated in near future.
This review article would address the possible strategies, indications and applicability of inducing tolerance by lymphocyte depletion.
Keywords: Renal allograft; B and T lymphocytes; Tolerance; Immunosuppressive medicines
Introduction
Immunologic tolerance is a state of immune unresponsiveness specific to a particular antigen or set of antigens induced by previous exposure to that antigen or set of antigens.
Tolerance is generally accepted to be an active process and, in essence, learning experience for T-lymphocytes (T-cells). Induction of immunologic tolerance has been achieved, and studied in many laboratory animal models, but it remains an elusive goal in clinical organ transplantation [1].
Transplant tolerance is defined as a state of donor-specific unresponsiveness without a need for ongoing pharmacologic immunosuppression. This could eliminate the adverse events associated with consumption of immunosuppressive medicines. Even though, safe and reliable strategies for complete tolerance have not yet been developed, various prospective studies showed reduced need of conventional immunosuppressive agents after tolerance induction. Furthermore, this reduction of medications is also observed in anecdotal case-reports and major studies targeting T and B lymphocyte ablation [2].
In this review, we will propose a paradigm shift from conventional immunosuppressive protocols to a protocol of reduced immunosuppression by pre-emptive lymphocyte depletion in living related renal allograft recipients with low immunologic risks.
Prologue
Illustration of clinical tolerance
At the prologue of clinical tolerance in renal transplant, a form of acquired complete tolerance was achieved anecdotally in cases of Multiple Myeloma (MM) patients where total bone marrow and lymphocyte ablation were done by whole body irradiation and chemotherapy; followed by donor specific bone marrow transfusion to repopulate the recipient ’s marrow and lymphoid tissue. The same patients under went renal transplantation from the same donors, later. The renal allografts were maintained without any immunosuppression subsequently [3]. This tolerance was achieved due to hematological chimera that developed during the course of treatment of MM through allogenic bone marrow transplant after bone marrow and lymphoid tissue ablation.
Real world experience (RWE)
A similar kind of hematological chimera with a specific renal donor to a specific renal recipient is not practicable during the pre-transplant period. This is neither medically safe, nor ethically amenable to be practiced. However, we observed a precedence of near tolerance in a renal transplant, done few years ago with a living renal donor. The graft was maintained on conventional three immunosuppressive drugs. Initial two acute cellular rejection (AR, BANFF-1A) episodes were treated by conventional antirejection therapy with pulse Methylprednisolone each time. Following the episodes of AR, the triple immunosuppressive therapy was escalated to Cyclosporine-A (CyA), Mycophelolatemofetil (MMF) and Prednisolone in standard dosage, with a stable target CyA trough level of 150 μG/l and a peak level of 800 μG/l.
The serum creatinine was stable at 130 μmol/l on average. After some years of transplant, non-Hodgkin lymphoma (Post-transplant lymphoproliferative Disorder, PTLD) developed, and anti-lymphoma chemotherapies were given in cycles at 3 weeks interval for subsequent five cycles. Each cycle of chemotherapy included IV Rituximab followed by Cyclophosphamide, Doxorubicin, Vincristine, and Prednisolone (R-CHOP). With this large amount of lymphocyte depletion therapy, the graft immunosuppression was curtailed to half dose CyA with a peak level of 300 μG/L and a trough of 50 μG/l, his Prednisolone dosage was reduced to 5 mg daily, and MMF was discontinued. Renal graft function had been stable at 90 μmol/l since then with low dose of dual immunosuppressive medicines.
This management provided the prologue of prope tolerance in this review; that was achieved, enabling minimal immunosuppression for maintaining stable graft life.
Key point
In this review, the keypoint to be discussed for achieving tolerance in renal allograft, is the way to getting maximally attainable lymphocyte (B and T-cell) depletion in contrast to ablation, in a medically safe and ethically approved strategy in living related renal transplantation.
Current Concepts and Practices in Achieving Tolerance in Renal Allograft
Mechanisms of rejection and tolerance
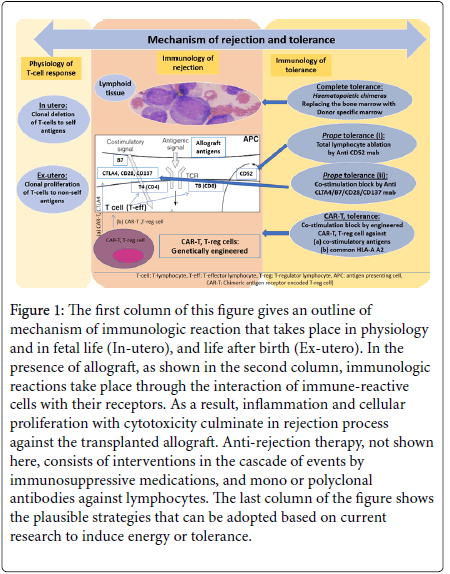
An outline of mechanism of immunologic reaction that takes place in physiology in fetal life, and life after birth is presented in the Figure 1, first column. The second column shows immunologic reactions that take place through the interaction of immune-reactive cells with their receptors in the presence of allograft. As a result, inflammation and cellular proliferation with cytotoxicity culminate in rejection process. Anti-rejection therapy by immunosuppressive medications, and monoclonal or polyclonal antibodies against lymphocytes, constitutes the intervention protocols in the cascade of these events. The last column of the Figure 1 shows the plausible strategies that can be adopted based on current research to induce energy or tolerance.
Figure 1: The first column of this figure gives an outline of mechanism of immunologic reaction that takes place in physiology and in fetal life (In-utero), and life after birth (Ex-utero). In the presence of allograft, as shown in the second column, immunologic reactions take place through the interaction of immune-reactive cells with their receptors. As a result, inflammation and cellular proliferation with cytotoxicity culminate in rejection process against the transplanted allograft. Anti-rejection therapy, not shown here, consists of interventions in the cascade of events by immunosuppressive medications, and mono or polyclonal antibodies against lymphocytes. The last column of the figure shows the plausible strategies that can be adopted based on current research to induce energy or tolerance.
In embryogenesis, as shown in the first column of Figure 1, mechanism of T-cell response is the clonal deletion of auto reactive Tcells in the thymus to the fetal antigens so that the organism is rendered self-tolerant to self-antigens. In fetal life, there is also clonal expansion of T-cells, not exposed to exogenous antigen, and not reactive to endogenous fetal antigens [4]. In ex-utero life, the process of deletion is reversed to the state of proliferation on exposure to exogenous antigens whether organ or organism. Against microorganisms, survival of the individual depends on antimicrobial agents, and protection depends on vaccination. For allograft, we need immunosuppression unless a state of tolerance is achieved. As shown in the second column of Figure 1, the whole process of immune identification and rejection is carried out by hematological immune cells; the principle guide being the immune reactive T-cells, the suppression of this mechanism is the hall mark of graft maintenance and possible tolerance [5].
In theory and practice
There are three strategies of achieving tolerance: a) achieving a state of hematological chimeras, which can be termed as complete tolerance, b) more recent, prope or near tolerance where immune-reactive T-cells are eliminated or inhibited, and c) most recent, research -based Chimeric Antigen Receptor for regulatory T-cells (CAR-T) therapy using genetically engineered T-regulatory (T-reg) cells, expressing receptors for T-cell co-stimulation, signal-2, for blocking orablating Tcell immune-reactivity, inducing anergy.
Complete tolerance
Transplant between monozygotic twins is an example of complete tolerance as there is no immune-reactivity between the twins because of genetic similarities [6]. Acquired complete tolerance was achieved anecdotally in multiple myeloma cases where, total bone marrow and lymphoid ablation was obtained by whole body irradiation and chemotherapy, followed by donor specific bone marrow transfusion to repopulate the recipients marrow and lymphoid tissue before renal transplantation-a form of hematological chimera [3]. As the immunogenic T-cells belong to the renal donor, complete tolerance was achieved, even though, not in reality yet.
Prope tolerance
• Pioneered initially by Dr. Calne with use of Campath-1H (Cam University Path) in clinical organ transplantation, it showed achieving a partial tolerance where allograft could be maintained with minimal immunosuppression. Campath-1H is a humanized Chimeric rabbit Monoclonal Antibody (MAb) against CD52 lymphocyte receptor that is present in all lymphocytes. Two administrations of 20 mg at 3 months interval, achieve depletion of lymphocytes for long time, allowing the recipients lymphoid tissue to repopulate with lymphocytes, naïve to the allograft. Thus achieving immune tolerance [7]. INTAC, 3C and similar studies showed promising results for minimizing long-term immunosuppression [8]
• More recently, with newer unique monoclonal antibodies against receptors for T-cell co-stimulation (CTLA-4, CD28, B7, CD137), signal-2 co-stimulation is blocked, inducing T-cell anergy. BENEFIT study used Belatacept, a selective co-stimulation blocker which is a human fusion protein combining extracellular portion of cytotoxic T-lymphocyte associated antigen 4 (CTLA-4) with Fc of human IgG1, to show this prope tolerance in phase 3 trial [9]. Unfortunately it did not show positive result
Chimeric antigen receptor for T-regulatory (CAR-T) cell therapy
Most recently research on CAR-T therapy targeting T-reg cells has been pioneered, aiming to block T-effector cell up-regulation. CAR-T cell are genetically engineered T-reg cells expressing antigenic component for CTLA-4 as described above, has shown to block costimulation by blocking CTLA-4 co-stimulatory receptor on the CD-3 T-cells; thus induces T-cell anergy for CD-3, CD-4 and CD-8 Tlymphocytes. This is an example of induction of tolerance in organ transplantation. This has been shown to be effective in Skin and islet cell graft, and has been speculated to be effective in inducing tolerance in renal and liver allograft [10,11].
Host Factors and Graft Factors that Increases Alloreactivity Compromising Tolerance
Highly sensitized recipients
Recipients with preformed antibodies against donor antigens require pre-transplant desensitization to reduce the load of preformed antibodies in order to have a rejection free or reduced rejection incident, post-transplant survival [12]. They usually need a higher immunosuppressive protocol for graft survival. These recipients sometimes include cases of repeat transplant, multiple blood transfusion recipients, multigravida, ABO incompatible recipient and recipient with donor specific antibodies. These recipients show higher amount of Panel Reactive antibodies (PRA), positive B-cell and T-cell cross-match [13]. Obviously, they are not the appropriate candidates for achieving tolerance.
Donor factors
Donor factors that increase the incidences of rejection, and reduced graft survival with the need for increased immunosuppression, may not be considered for protocols of tolerance. Because of higher immunogenic potentials of the donor organ, there would be an increased and continuous requirement for highly potent immunosuppressive protocol. This is in addition to the desensitization protocol, if any, that could be required pre-transplant or immediate post-transplant. These desensitization protocols include various combinations of monoclonal or polyclonal anti-lymphocyte antibodies, IV immunoglobulin, immuno-adsorption technique and plasmapheresis. Highly immunogenic allograft could include diseased donor kidney with prolonged cold ischemia time, HLA mismatch, and ABO incompatible donor kidney [14,15].
Current practice for highly sensitized and highly immunogenic donor kidney
Protocols for highly sensitized recipients and highly immunogenic allograft, are available, and practiced in targeted situations. These protocols aim at reducing the humoral and cellular response to the grafted organ with an aim to prevent acute rejection episodes, and to prolong graft half-life with minimal subsequent acute and chronic rejection process. In most situations they need continued consumption of maximized highly potent and toxic triple immunosuppressive therapy. Evidently, the scope of achieving tolerance in this situationis limited [16].
Paradigm shift to tolerance
Hence paradigm shift from conventional immunosuppression to tolerance needs to be considered preferably in minimally to normally sensitized recipients who would be receiving a low risk donor kidney. The aim of this shift is to achieve a rejection free stable normal allograft survival with minimal immunosuppression by use of one or two immunosuppressive medicine at lower dose. This would ensure minimum drug related toxicity including PTDM, PTLD, chronic infections, metabolic syndrome and malignancies.
Donor and recipient relationship and selection
Selection for tolerance achievement protocol would require prioritization to living donation with ABO compatible, better HLA matching recipients, preferably with closer family relationship. Matching physiognomic parameters might also be a consideration. Exclusion of highly sensitized recipients as described above is an important requirement for this paradigm shift protocol.
Current trials in the prope tolerance
As described above, the protocols of current trials for prope tolerance, begins with lymphocyte depletion prior to or immediately with transplantation. INTAC, 3C and similar studies on Campath-1H showed promising results for minimizing long-term immunosuppression [7,8]. However, the protocols have not been generalized for common use in transplantation. Limiting factors are higher incidences of sepsis and malignancy. This could be anticipated by the profound B and T lymphocyte depletion induced by CAMPATH-1H. In addition, newer unique monoclonal antibodies against TCR CTLA-4, CD28, B7, CD137 (the receptors for T-cell costimulation) that block signal-2, as shown in BENEFIT study with Belatacept, could produce tolerance by inducing T-cell anergy. As these studies had limitations, a concrete protocol for this T-cell anergy is also lacking [9]. Most recent, prope tolerance induction by CAR-T therapy against TCR is also in infancy for renal transplantation [10,11].
The paradigm shift protocol
The significant point to note at the prologue was the profound lymphocyte depletion during the six cycles of R-CHOP therapies during the course of PTLD treatment. These resulted in neutropenia and lymphopenia with counts dropping to less than 0.9 × 10(9)/l and 0.5 × 10(9)/l, respectively, necessitating withdrawal of MMF, and maintaining the graft with a low dose of CyA, and small dose of Prednisolone, mimicking prope tolerance in Figure 2.
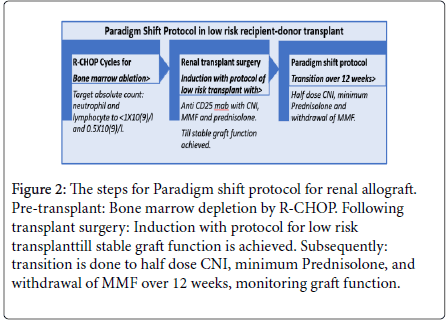
Figure 2: The steps for Paradigm shift protocol for renal allograft. Pre-transplant: Bone marrow depletion by R-CHOP. Following transplant surgery: Induction with protocol for low risk transplanttill stable graft function is achieved. Subsequently: transition is done to half dose CNI, minimum Prednisolone, and withdrawal of MMF over 12 weeks, monitoring graft function.
The donor and recipient need to be of low immunologic risk category as described above. Figure 2 shows the steps as follows:
• Step-1: Pre-transplant R-CHOP Cycles for Bone marrow depletion. Target neutrophil and lymphocyte counts are 1 × 10(9)/l and 0.5 × 10(9)/l, respectively. This is to be achieved by use of IV Rituximab 375 mg/m 7 days prior to CHOP regimen, that includes IV Cyclophosphamide 750 mg/m (day 1), IV Doxorubicin 50 mg/m IV (day 1), IV Vincristine 1.4 mg/m (day 1) and Prednisolone 50 mg for 5 days from day 3. This cycle to be repeated 3 to 6 times till the desired lymphocyte depletion is achieved
• Step-2: Induction with protocol of low risk transplant after renal transplant surgery with Anti CD25 monoclonal antibody along with CNI, MMF and Prednisolone at standard dose till stable graft function is achieved
• Step-3: Half dose CNI, minimum Prednisolone and withdrawal of MMF. This transition is done subsequently over 12 weeks, monitoring the graft function
The rational of this paradigm shift protocol
The rationale of R-CHOP therapy prior to transplantation is to achieve predominant B-lymphocyte depletion. Subsequently, the new B-lymphocytes that are released from bone marrow and lymphoid tissues are naïve to the renal allograft. As such they would fail to display humoral rejection to the graft.
The rational of using CNI and MMF during the induction period, is to target the host and resident T-lymphocytes at the engraftment period in order to avoid acute cell mediated rejection episodes [17]. As the host and resident T-lymphocytes get depleted through the use of CNI and MMF, newer T-lymphocytes are released from bone marrow and lymphoid tissues that are relatively naïve to the allograft; thus they take it as self, and also do not mount rejection response.
With this sequential B- and T-lymphocyte depletion during the peritransplant period, the host acquires a state of prope tolerance to the renal allograft as shown at the prologue. This helps to maintain the renal allograft with low dose dual immunosuppression avoiding complications of conventional immunosuppressive drugs, both shortterm and long-term.
Epilogue
The short-term and long-term side effects of current immunosuppressive regimens with more toxic agents, newer monoclonal antibodies, and desensitization procedures in high risk recipients and donors, could draw a disastrous epilogue for any transplant programme. Nonetheless, considering renal transplantation as the best modality of treatment for dead kidneys, the current guideline-based immunosuppressive regimens needs to continue for a sustainable transplant programme. Similarly, need for high-risk transplant in diseased donor, highly sensitized recipients, HLA mismatch and ABO incompatible recipients cannot be ignored either, considering the increased waiting list for transplantation.
However, in low risk donor-recipient relationship, a paradigm shift protocol as described above can prove applicable as shown in the real world experience scenario at the prologue. Induction with T-cell depletion with monoclonal antibodies in low risk group is not yet protocol, and is associated with increased incidences of sepsis and malignancies. In addition, B-cells are not ablated with these protocols; as such, risk of acute and chronic humoral rejection continues with early culmination of graft life. Rituximab is used in current transplant patients, but for antibody mediated rejection episodes only.
Conclusion
We present the real world experience scenario as a unique model of achieving prope tolerance in low risk transplant group requiring a low dose dual immunosuppression to avoid long term metabolic, septic and malignant complications. We agree that a prospective large cohort-based observational study is required for validation of this RWE paradigm shift protocol for achieving tolerance in low risk renal allograft.
Acknowledgement
The author sincerely thanks all staffs of renal department and patient for their involvement in the management of the case. The plan, writing, opinion and conclusion expressed here are those of the author only, and do not necessarily reflects those of the individual involved in the management of the patient.
Conflict of Interests
This study was done as a part of routine clinical management; and hence funding was not mandated. As it was not a clinical trial, and as it was a clinical assessment only, ethical considerations were not mandated as well.
Declaration of Disclaimer
This RWE protocol proposed here is open for research, study, use, and citation in any scope of renal transplantation. No permission from the corresponding author is required.
References
- Vignali DA, Collison LW, Workman CJ (2008) How regulatory T cells work. Nat Rev Immunol 8: 523-532.
- Calne R, Moffatt SD, Friend PJ, Jamieson NV, Bradley JA, et al. (1999) Campath 1H allows low-dose cyclosporine monotherapy in 31 cadaveric renal allograft recipients. Transplantation 68: 1613-1616.
- Bühler LH, Spitzer TR, Sykes M, Sachs DH, Delmonico FL, et al. (2002) Induction of kidney allograft tolerance after transient lymphohematopoietic chimerism in patients with multiple myeloma and end-stage renal disease. Transplantation 74: 1405-1409.
- Ander SE, Michael SD, Coyne CB (2019) Immune response at maternal-fetal interface. Sci Immunol 4: 6114.
- Ciancio G, Burke GW, Gaynor J, Carreno MR, Cirocco RE, et al. (2005) A randomized trial of three renal transplant induction antibodies: Early comparison of tacrolimus, mycophenylate mofetil, and steroid dosing, and newer immunosuppressive monitoring. Transplantation 80: 457-465.
- Brown JB (1937) Homografting of skin with report of success in identical twins. Surgery 102: 558.
- Hanaway MJ, Woodle ES, Mulgaonkar S, Peddi RV, Kaufman DB, et al. (2011) Alemtuzumab Induction in renal transplantation. For the INTAC Study Group. N Engl J Med 364: 1909-1919.
- F Vincenti, B Charpentier, Y Vanrenterghem, L Rostaing, B Bresnahan, et al. (2010) A phase III study of belatacept‐based immunosuppression regimens versus cyclosporine in renal transplant recipients (BENEFIT Study). Am J Transpl 10: 535-546.
- Boardman DA, Philippeos C, Fruhwirth GO, Ibrahim MAA, Hannen RF, et al. (2017) Expression of a chimeric antigen receptor specific fordonor HLA class I enhances the potency of human regulatory T cells inpreventing human skin transplant rejection. Am J Transpl 17: 931-943.
- Benoit S, Jeffrey Ab (2001) Complexities of CD28/B7: CTLA-4 co-stimulatory pathways in autoimmunity and transplantation. Annu Rev Immunol 19: 225-252.
- Amico P, Hönger G, Mayr M, Steiger J, Hopfer H, et al. (2009) Clinical relevance of pre-transplant donor-specific antibodies detected by single antigen flow beads. Transplantation 87: 1681-1688.
- Couzi L, Araujo C, Guidicelli G, Bachelet T, Moreau K, et al. (2011) Interpretation of positive flow cytometric crossmatch in the era of single-antigen bead assay. Transplantation 91: 527-535.
- Stuart F (2000) Overview of living and deceased organ donors, immune suppression and outcomes. Organ transplantation. Georgetown: Landes Bioscience, pp: 52-53.
- Meshari KA, Pall A, Chaballot A, Gamal H, Mana H, et al. (2013) Outcome of desensitization in HLA and ABO incompatible living donor kidney transplantation: A single centre experience. Transplant Proc 45: 1423-1426.
- Marfo k, Amy L, Akalin E (2011) Dsensitisation protocol and their outcome. Clin J Am Soc Nephrol 6: 922-936.
- Halloran P, Mathew T, Tomlanovich S, C Groth, L Hooftman, et al. (1997) Mycophenolate mofetil in renal allograft recipients: A pooled efficacy analysis of three randomized, double-blind, clinical studies in prevention of rejection. Renal Transplant Study Groups. Transplantation 63: 39-47.
- Vincenti F, Ramos E, Brattstrom C, Cho S, Ekberg H, et al. (2001) Multicenter trial exploring calcineurin inhibitors avoidance in renal transplantation. Transplantation, pp: 1282-1287.
 Spanish
Spanish  Chinese
Chinese  Russian
Russian  German
German  French
French  Japanese
Japanese  Portuguese
Portuguese  Hindi
Hindi