Case Report, J Surg Clin Pract Vol: 2 Issue: 1
Pneumothorax Ex Vacuo Following Thoracentesis for Persistent Pleural Effusion
Katherine Florecki1*, Jordan Anaokar2, Mark Katlic1 and Yvonne Carter1
1Department of Surgery, Sinai Hospital, Baltimore, USA
2Division of Diagnostic Imagaing, Fox Chase Cancer Center, Philadelphia, USA
*Corresponding Author : Katherine Florecki
Department of Surgery, Sinai Hospital of Baltimore, 2401 W Belvedere Ave, Baltimore, USA
Tel: 630- 802-5983
Fax: 410- 601-8442
E-mail: kfloreck@lifebridgehealth.org
Received: December 05, 2017 Accepted: November 26, 2017 Published: January 02, 2018
Citation: Florecki K, Anaokar J, Katlic M, Carter Y (2017) Pneumothorax Ex Vacuo Following Thoracentesis for Persistent Pleural Effusion. J Surg Clin Pract 2:1
Abstract
Pneumothorax ex vacuo (PEV) refers to a localized pneumothorax adjacent to a collapsed lung. Underlying causes of PEV include chronic atelectasis, endobronchial obstruction and visceral pleural restriction secondary to an inflammatory or malignant process. Recognition of PEV is crucial in directing appropriate treatment and refraining from unnecessary procedures, for example thoracosotomy tube placement. We present the case of 28 yo male who had a persistent pneumothorax following thoracentesis despite adequate thoracostomy tube drainage. Patient was subsequently diagnosed with a PEV secondary to bronchogenic adenocarcinoma. Treatment consisted of chemotherapy for underlying carcinoma and not thoracostomy tube.
Keywords: Pneumothorax ex vacuo; Thoracentesis
Case Report
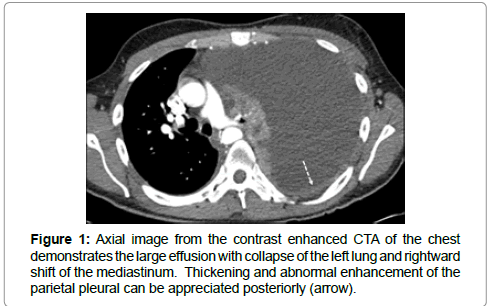
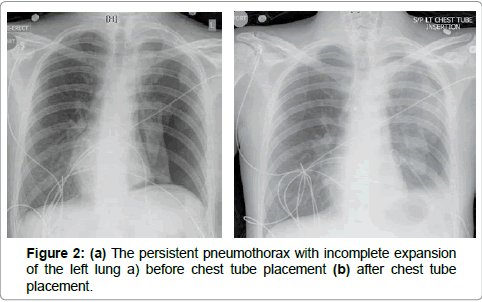
A 28 year-old gentleman presenting with 1-month history of dry cough and dyspnea was found to have a complete opacification of the left hemithorax. A subsequent computed tomography (CT) chest scan demonstrated a large left pleural effusion with complete collapse of the left lung, abnormal thickening and enhancement of the posterior parietal pleura, and mediastinal shift (Figure 1). Two large volume thoracentesis lead to resolution of the effusion and development of a large pneumothorax managed with a percutaneous drain (Figures 2a and b).
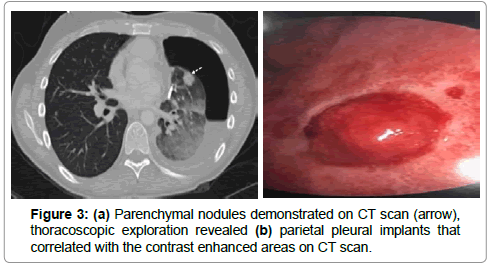
Persistent pneumothorax after thoracostomy tube drainage lead to a thoracic surgery consult. The patient had no supplemental oxygen requirement, chest x-ray revealed partial lung re-expansion, and neither an air leak nor respiratory variation was appreciated in the chest tube collection chamber. A repeat CT chest confirmed incomplete expansion of the left lung with a hydropneumothorax, thickened visceral and parietal pleurae and left upper lobe parenchymal nodules (Figure 3a). After no endobronchial lesion was found on diagnostic bronchoscopy, the patient underwent video assisted thoracoscopic surgery (VATS) with biopsy of parietal pleural implants found on exploration (Figure 3b). Histologic analysis of the pleural implant biopsies confirmed a diagnosis of bronchogenic adenocarcinoma with an ALK mutation. The chest tube was removed on postoperative day one, and the patient remained asymptomatic on follow up 2 weeks later with a persistent fluid filled pleural space.
Discussion
Pneumothorax after thoracentesis is rare, occurring in about 6% of cases, with roughly one-third requiring chest tube insertion [1]. Etiologies include introduction of room air into the pleural space during the procedure or laceration of the lung with escape of alveolar air into the pleural space. In a small subset of these patients, the pneumothorax will persist despite chest tube insertion [2]. In these cases, one must consider a third etiology, a pneumothorax ex vacuo (PEV). This term was originally described in children with bronchial obstruction and lobar atelectasis, who developed a localized pneumothorax adjacent to the collapsed lobe thought to be from gas drawn to the pleural space from a vacuum effect [3]. It later has been applied to cases in which the lung fails to expand due to underlying restrictive pleural disease, or “trapped lung”.
A malignant pleural effusion, even in the absence of obvious pleural tumor, can induce a fibrous visceral peel on the pleura. These patients often have restrictive pleural disease or non-compliant lung parenchyma due to malignancy and/or loss of surfactant that inhibit lung re-expansion. The pneumothorax that develops following removal of the pleural fluid is not related to puncture of the visceral pleura, but rather is the result of an ex vacuo effect in which the failure of the lung to re-expand and the negative intrapleural pressures introduced as the effusion is removed creates a vacuum in which gas from the pulmonary venules is drawn into the pleural space [4]. The clinical examination following thoracentesis may be the best indicator as to whether the pneumothorax is related to traumatic laceration of the lung or an ex vacuo cause. Patients with an iatrogenic pneumothorax from injury to the visceral pleural can develop shortness of breath, fatigue and even signs of hemodynamic instability due to a continued air leak; while PEV is often asymptomatic. Factors surrounding the procedure should also be considered. For example, the overall pneumothorax rate is extremely low for ultrasound guided procedures [1], and it would be unusual to cause a traumatic laceration of the visceral pleural of the lung that was completely collapsed due to a large pleural effusion during an imaging guided procedure. Although this does not apply in our case, a new pneumothorax in an asymptomatic patient who has undergone multiple thoracentesis may be indicative of a restrictive pleural disease.
In asymptomatic patients with a pneumothorax following thoracentesis, one should consider a physical examination and repeat CT for further evaluation prior to consideration of chest tube placement. As Boland, et al. and Ponrartana, et al. reported, there has been no proven benefit of catheter drainage in patients with PEV and patients often remain asymptomatic despite reaccumulation of the fluid [2,5].
For patients with large pleural effusions and a finding of a pneumothorax on post-procedural chest radiograph, it can be difficult to distinguish post-traumatic from ex-vacuo etiologies. For those with small to moderate volume effusions, the configuration of the air within the pleural space can sometimes be similar to the pre-procedural radiograph, which could help signal the presence of “trapped lung” from restrictive pleural disease with a resultant ex vacuo pneumothorax. Cross sectional imaging with CT is more helpful in evaluating a suspected ex vacuo pneumothorax, as it can detect the presence of an endobronchial obstruction and provide better detail of the pleural surfaces. Restrictive pleural disease from either malignant or inflammatory causes can cause thickening of the visceral pleural surface, often with concomitant parietal pleural thickening, which is much better appreciated with CT. One may also appreciate infiltrative tumor in the underexpanded lung, or rounded atelectasis as an indicator or chronic pleural disease [6].
Conclusion
Our case highlights the significance of differentiating PEV from standard pneumothorax, and considering malignancy as the underlying etiology. Our patient remained asymptomatic and was subsequently enrolled in a clinical trial for his stage IV lung cancer. Underlying causes of PEV include chronic atelectasis, endobronchial obstruction and visceral pleural restriction secondary to an inflammatory or malignant process. Recognition of PEV is crucial in directing appropriate treatment and refraining from unnecessary procedures [7].
References
- Gordon CE, Feller-Kopman D, Balk EM, Smetana GW (2010) Penumthorax Following Thoracentesis: Systemic Review and Meta-analysis. Arch Intern Med 170: 332-333.
- Boland GW, Gazelle GS, Girard MJ, Muller PR (1998) Asymptomatic hydropneumothorax after therapeutic thoracentesis for malignant pleural effusions. AJR Am J Roentgenol 170: 943-946.
- Berdon WE, Dee G, Abramson S, Altman R, Wung J (1984) Localized Pneumothorax Adjacent to a Collapsed Lobe: A Sign of Bronchial Obstruction. Radiology 150: 691-694.
- Chang YC, Patz EF, Goodma PC (1996) Pneumothorax after small-bore catheter placement for malignant pleural effusions. AJR Am J Roentgenol 166: 1049-1051.
- Ponratana S, le-Berge JM, Kerlan RK, Wilson MW, Gordon RL (2005) Management of Patiehts with “ExVacuo” Pneumothorax After thoracentesis. AJR 12: 980-986.
- Webb R, Higgins C (2005) Thoracic Imaging: Pulmonary and Cardiovascular Radiology. Lippincott Williams & Wilkins, Philadelphia, USA.
- Bhardwaj M, Ragupathi L (2015) Pnemothorax ex vacuo in a Patient with Malignant Pleural Effusion after PleurX Catheter Placement. Medicine Forum 16: 20.
 Spanish
Spanish  Chinese
Chinese  Russian
Russian  German
German  French
French  Japanese
Japanese  Portuguese
Portuguese  Hindi
Hindi