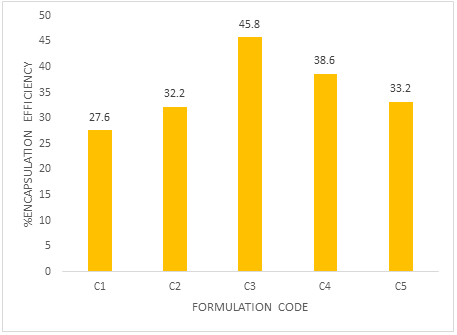Research Article, J Pharm Drug Deliv Res Vol: 14 Issue: 1
Preparation, Characterization and Evaluation of Microencapsulation of Nebivolol Hydrochloride Microbeads by Ionotropic Gelation Method
Tejasdhanawade S and Nagalakshmi S*
Department of Pharmaceutics, Sri Ramachandra Institute of Higher Education and
Research (DU), Chennai, Tamil Nadu, India
*Corresponding Author:Nagalakshmi S
Department of Pharmaceutics, Sri Ramachandra Institute of Higher Education and
Research (DU), Chennai, Tamil Nadu, India
E-mail: nagalakshmi.s@sriramachandra.edu.in
Received date: 02 September, 2023, Manuscript No. JPDDR-23-112254;
Editor assigned date: 04 September, 2023, PreQC No JPDDR-23-112254 (PQ);
Reviewed date: 18 September, 2023, QC No. JPDDR-23-112254;
Revised date: 15 January, 2025, Manuscript No. JPDDR-23-112254 (R);
Published date: 22 January, 2025, DOI: 10.4172/2325-9604.1000316
Citation:Tejasdhanawade S, Nagalakshmi S (2025) Preparation, Characterization and Evaluation of Microencapsulation of Nebivolol Hydrochloride Microbeads by Ionotropic Gelation Method. J Pharm Drug Deliv Res 14:1.
Abstract
The research focuses on the meticulous process of preparing, characterizing, and evaluating microencapsulated Nebivolol Hydrochloride (NH) microbeads through the ionotropic gelation method, employing sodium alginate as the polymer matrix. This study represents a vital advancement in drug delivery systems due to Nebivolol's effectiveness in treating cardiovascular conditions.
The preparation stage involves the formation of NH-loaded microbeads through ionotropic gelation. Nebivolol Hydrochloride is encapsulated within a sodium alginate polymer matrix, primarily through the interaction between calcium ions and sodium alginate. This process is meticulously controlled to ensure uniform encapsulation and drug distribution.
Characterization of the microbeads is critical to understanding their physical and chemical properties. Various techniques, including Scanning Electron Microscopy (SEM), Fourier- Transform Infrared Spectroscopy (FTIR), and Differential Scanning Calorimetry (DSC), are employed to assess the microbeads' morphology, chemical structure, and thermal behavior.
The evaluation phase entails testing the microencapsulated NH microbeads for drug release kinetics, stability, and bioavailability. Dissolution studies reveal the sustained release profile of NH from the microbeads, a vital factor in drug delivery systems. Stability tests ensure the long-term viability of the microencapsulated drug, while bioavailability studies assess its effectiveness.
In summary, this research demonstrates the successful preparation of NH microbeads using sodium alginate via ionotropic gelation. Characterization techniques provide valuable insights into the microbeads' properties, and the evaluation phase determines their suitability as a drug delivery system for nebivolol hydrochloride, potentially enhancing its therapeutic efficacy in cardiovascular treatment.
Keywords: Nebivolol hydrochloride; Microbeads; Iontropic gelation; Encapsulation; Cardiovascular treatment
Introduction
Micro-beads
Micro-beads are tiny plastic particles that are less than 5 millimeters in size. They are commonly used as exfoliates in personal care products like facial scrubs, body washes, and toothpaste. Micro beads are made of different types of plastic, including polyethylene, polypropylene, and polystyrene.
Micro-beads are designed to be washed down the drain after use, but they are too small to be filtered out by wastewater treatment plants [1]. As a result, they end up in waterways and oceans, where they can be ingested by aquatic animals and even enter the food chain. Micro beads are not biodegradable, so they persist in the environment for hundreds of years.
In recent years, the use of micro-beads has come under scrutiny due to their negative impact on the environment. Many countries, including the United States, Canada, and the United Kingdom, have banned the use of micro-beads in personal care products [2]. Some companies have also voluntarily phased out the use of micro-beads and replaced them with natural alternatives like ground nuts and seeds, or biodegradable materials.
Preparation of micro-beads by different techniques
In recent years, the use of micro-beads has come under scrutiny due to their negative impact on the environment. Many countries, including the United States, Canada, and the United Kingdom, have banned the use of micro-beads in personal care products [3].
Some companies have also voluntarily phased out the use of micro beads and replaced them with natural alternatives like ground nuts and seeds, or biodegradable materials
The goal of any drug delivery system is to provide a therapeutic amount of drug to the proper site in the body to achieve promptly and then maintain the desired concentration. That is drug delivery system should deliver the drug at a rate dictated by needs of the body over a specific period of treatment.
The design of effective drug delivery systems has recently become an integral part of the development of new medicines. Hence, research continuously keeps on searching for ways to deliver drugs over an extended period of time, with a well-controlled release profile [4].
Oral drug delivery is the most desirable and preferred method of administering therapeutic agents for their systemic effects. In addition, the oral medication is generally considered as the first avenue investigated in the discovery and acceptance, convenience, and cost effective development of new drug entities and pharmaceutical formulations, mainly because of patient manufacturing process.
For many decades treatments of an acute disease or a chronic illness has been mostly accomplished by delivery of drugs to patients using various conventional pharmaceutical dosage like tablets, capsules, pills, suppositories, creams, ointments, liquids, aerosols and injectables as drug carriers.
This type of drug delivery system is known to provide a prompt release of drug. So to achieve and maintain the drug concentration within therapeutically effective range needed for treatment, it is often essential to take this type of drug delivery system several times a day which results in a significant fluctuation in drug levels.
For many drug substances, conventional immediate release formulations provide clinically effective therapy while maintaining the required balance of pharmacokinetic and pharmacodynamic profiles with acceptable level of safety to the patient [5].
Multiple unit dosage form includes
Micro-granules/spheroids: Drug wet granulated alone or incorporated into inert granules, and then coated to control the release pattern [6].
Pellets: Pellets are prepared by coating inert drug pellets with film forming polymers. The release depends upon coating composition of polymer and amount of coatings.
Microcapsules: Microcapsules are prepared by applying relatively thin coating to small particles of solids, droplet of liquid and dispersion.
Beads: Micro-beads, as the name suggests they are nearly spherical, small with diameter of 0.5-1000 μm in size, solid and free flowing particulate carriers containing dispersed drug particles either in solution or crystalline form that allow a sustained release or multiple release profiles of treatment with various active agents without major side effects.
Additionally, the beads maintain functionality under physiological conditions, can incorporate drug to deliver locally at high concentration ensuring that therapeutic levels are reached at the target site while reducing the side effects by keeping systemic concentration low.
The micro-beads are produced from several polymers such as cationic polymers e.g. chitosan, anionic polymers e.g. sodium alginate, and binding components e.g. gelatin, chondroitin sulfate, avidin in predetermined ratio [7].
The techniques which are used for formulation of sustained release beads are as follows:
Iono-tropic gelation method
It involves simply the interaction of an ionic polymer with oppositely charge ion to initiate cross linking. Unlike simple monomeric ions, the interaction of polyanion with cations cannot be completely explained by the electro-neutrality principle [8].
The three dimensional structure and presence of other groups influence the ability of cations to conjugate with anionic functionalities or vice-versa. There are two sub-methods by which beads can be generated using iono-tropic gelation technique.
The methods differ from each other in the source of the cross linking ion. In one of the methods, the cross-linker ion is positioned externally, whereas in the other method, the cross-linker ion is incorporated within the polymer solution in inactive form [9].
Iono-tropic gelation method is classified into two types:
External gelation method: The external gelation method involves the use of a metal ion solution as a source of the cross linking ion. The polymer solution containing drug is extruded through a needle into this solution with mild agitation. As soon as the polymeric drop comes in contact with the metal ion solution, instant gelation occurs, resulting into self-sustained bead formation. The beads are cured for a specified time period into the gelation medium following which, they are removed and dried. The external gelation occurs as a result of rapid diffusion of the cross-linker ions into the partially gelled beads.
Internal gelation method: The internal gelation method involves the generation of the cross-linker ion ‘in situ’. This method involves the use of an insoluble metal salt (such as calcium carbonate and barium carbonate) as a source of cross inking cation. The cation is released, in situ, by lowering the pH of the solution, thereby solubilizing the metal salt and releasing the metal ion (Figure 1).
Figure 1: Graphical abstract
Materials and Methods
Calibration curve: 10 mg of the drug was precisely measured and transferred into a 10 ml volumetric flask. pH 7.4 was added to adjust the volume to 10 ml, resulting in a concentration of 100 μg/ml (designated as stock solution A). From stock solution A, 1 ml was extracted and placed into another 10 ml volumetric flask. PH 7.4 was added to adjust the volume to 10 ml, yielding a concentration of 100 μg/ml (labeled as Stock Solution B). To prepare serial dilutions with concentrations of 20, 40, 60, 80, and 100 μg/ml, 0.2, 0.4, 0.6, 0.8, and 1 ml of stock solution B were respectively transferred into separate 10 ml volumetric flasks, and the volume was adjusted to 10 ml using pH 7.4. The absorbance of each resulting solution was measured at 282 nm using a UV-visible spectrophotometer, and the standard calibration curve was constructed using Microsoft Excel 2021 program.
Pre-formulation study
FT-IR: FTIR (Fourier Transform Infrared Spectroscopy) is a technique used for the analysis of chemical compounds. It works by measuring the absorption of infrared radiation by a sample, which produces a unique fingerprint spectrum that can be used to identify the sample.
The FTIR procedure typically involves the following steps:
• Sample preparation: The sample to be analyzed is prepared by placing it in a sample holder, which can be a thin film or a pellet. The sample should be homogeneous and free of impurities that could interfere with the measurement.
• Instrument setup: The FTIR instrument is set up according to the manufacturer’s instructions. This includes setting the appropriate parameters for the measurement, such as the wavelength range, resolution, and number of scans.
Background measurement: A background measurement is taken with an empty sample holder or a blank sample to account for any interference from the instrument or the environment.
• Sample measurement: The sample is placed in the sample holder, and the instrument measures the infrared radiation that passes through the sample. The absorption of the infrared radiation by the sample is measured over a range of wavelengths, typically from 4000 to 400 cm.
• Data analysis: The FTIR instrument produces a spectrum that shows the absorption of the infrared radiation by the sample as a function of wavelength. The spectrum is then analyzed using software to identify the functional groups present in the sample and to compare it to reference spectra in databases.
Overall, FTIR is a powerful analytical technique that provides information about the chemical structure and composition of materials. Its widespread use in fields such as chemistry, biology, and materials science reflects its versatility and reliability.
• FTIR study was carried out for drug (Nebivolol hydrochloride, sodium alginate and carrageenan)
• Inference: There were no interactions found between drug and polymers.
DSC: DSC stands for "Differential Scanning Calorimetry." It is a powerful analytical technique used to study the thermal behavior of materials, particularly pharmaceutical compounds. DSC is widely employed in the pharmaceutical industry and research laboratories to investigate the stability, purity, and thermal properties of drug substances and drug products
Here are some key details about Differential Scanning Calorimetry (DSC) in pharmaceuticals:
Principle: DSC measures the heat flow associated with physical and chemical changes that occur in a sample as it is subjected to controlled temperature changes. The technique compares the heat flow to a reference material while both are subjected to the same temperature program. Any thermal events, such as phase transitions, crystallization, melting, or degradation, result in characteristic peaks on the DSC thermogram.
Applications in pharmaceuticals
Melting point determination: DSC is commonly used to identify the melting points of pure drug substances, which is crucial for quality control and formulation development.
Polymorphism analysis: It helps to detect and characterize different polymorphic forms of a drug, which can have significant implications on the drug's solubility, dissolution, and stability.
Solid-state characterization: DSC can identify and quantify amorphous and crystalline fractions of a sample, providing valuable insights into drug stability and processing.
Compatibility studies: DSC is used to study drug-excipient compatibility, ensuring that the components of a formulation do not interact negatively, leading to degradation or loss of efficacy.
Stability studies: It can assess the thermal stability of drugs and formulations, aiding in the determination of shelf life and storage conditions.
Instrumentation: DSC instruments consist of a sample cell and a reference cell, both containing identical amounts of an inert material (usually an empty aluminum pan). The sample cell contains the drug sample or formulation, and both cells are placed in a controlled temperature environment. As the temperature is increased or decreased, the heat flow between the sample and reference cells is measured, and a thermo-gram is generated.
Interpretation: DSC thermo-grams show peaks that correspond to specific thermal events. For example, a sharp endothermic peak usually represents the melting of a crystalline substance, while an exothermic peak could indicate drug degradation. The onset, peak temperature, and area under the peaks provide valuable information about the sample's thermal behavior.
Advantages: DSC is a non-destructive technique that requires minimal sample preparation. It is rapid, accurate, and reproducible, making it an essential tool in pharmaceutical research and development.
Overall, Differential Scanning Calorimetry (DSC) plays a crucial role in the pharmaceutical industry, aiding in the characterization and quality assessment of drug substances and formulations, as well as supporting stability studies and ensuring the safety and efficacy of pharmaceutical products
Preparation of microbeads
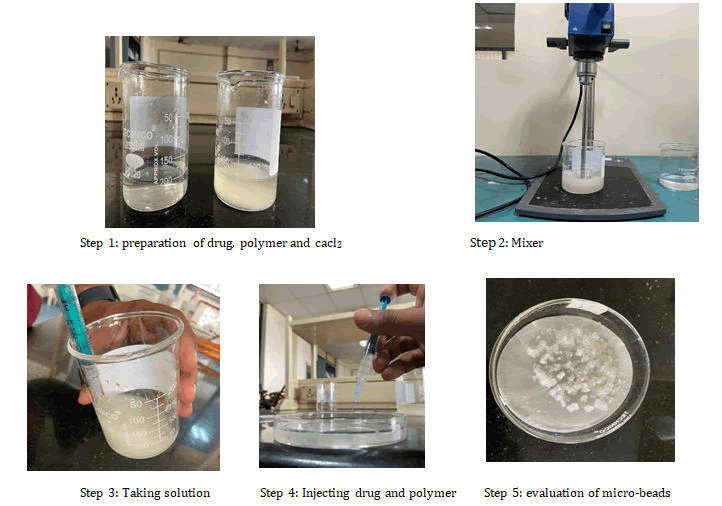
Ionotropic gelation method: Micro-beads containing nebivolol Hydrochloride were prepared by ionotropic gelation technique. The sodium alginate solution was prepared by dispersing the weighed quantity of sodium alginate in deionized water. Accurately weighed quantity (1 g) of nebivolol hydrochloride was added to 100 ml polymeric solution of Sodium alginate and drug were thoroughly mixed with help of homogenizer at 1500 rpm to get a homogenous drug-polymeric mixture. The formed mixture allowed standing for 1 hr to make it bubble free. By following the same procedure the alginate beads of different ratios of drug: Polymer were prepared. The resulted homogenous dispersion was extruded into 100 ml of 6% cross-linker solution (CaCl2) through hypodermic syringe with flat tip needle (18 g) and stirred at 100 rpm. The formed micro-beads were allowed to cure for 30 min in the cross-linker solution to complete the gelation. The beads were removed after the gelation period and washed with ethanol to harden the beads surface and finally with distilled water repeatedly to make free from un-reacted ion. The micro-beads were then filtered and dried in hot air oven at 400â?? for 18 h.
Characterization studies
Particle size analysis: Particle size of microbeads was determined by using an optical microscope under regular polarized light, and the mean particle size was calculated by measuring 100 particles with the help of a calibrated ocular micrometer.
Swelling index: The swelling index of the micro-beads is an indication of the capacity of the microbeads to imbibe water and swell. For estimating swelling index, the micro-beads (50 mg) were weighed initially then suspended in 25 ml of phosphate buffer pH 7.4. The beads were taken out at different time intervals using stainless steel grid and blotted carefully without pressing hard to remove the excess surface liquid. The swollen beads were weighed using electronic microbalance. The studies were performed in triplicate and average values were taken in data analysis.
Swelling Index=Weight of wet micro-beads/Weight of dry micro beads
Determination of encapsulation efficiency: The amount of nebivolol hydrochloride present in the micro-beads was determined. The powdered micro-beads were extracted in to 50 ml of phosphate buffer (pH 7.4) by magnetic stirring for a period of 2 h. The solution was filtered through Whatman filter paper no.5, suitably diluted and estimated for drug content spectrophotometrically at 282 nm using UV-Visible spectrophotometer (UV-1601). Encapsulation efficiency was calculated by the following formula.
Encapsulations efficiency (%)=Experimental drug content × 100/ Theoretical drug content
Drug content estimation: Different batches of micro-beads were checked for drug content uniformity. Accurately weighed (50 mg) amount of dried micro-beads were taken in a pestle and mortar and powdered. The powdered micro-beads were then separately dissolved in adequate quantity of 0.1 N HCl and 7.4 pH phosphate buffer and kept for 24 h. the solution was then filtered, scanned for absorbance was noted down at 282 nm using UV spectrophotometer (Shimadzu Model process was repeated in triplicate and average was calculated).
In-vitro drug release studies: The in vitro drug release studies were performed using dissolution test apparatus. The dissolution medium was hydrochloric acid buffer (pH 1.2) for first 2 h and 7.4) for subsequent h. The microbeads were efficiency was calculated by the following 1601, Japan). Each double-sided carbon adhesive tapze and the scanning electron phosphate buffer (pH allowed to sink in the vessel containing 900 ml of dissolution medium and the release of nebivolol hydrochloride was investigated at about 50 rpm at temp 37°C ± 0.5°C. During dissolution 10 ml aliquot was withdrawn at interval of 1 h and same was replaced with equal volume of fresh medium The withdrawn samples were filtered through Whatmann filter paper no.42 and diluted with the same buffer to 10 ml. Absorbance was measured at 282 nm using UV-visible spectrophotometer. Cumulative percent drug released was found out at each time interval and graph was plotted between cumulative % drug release v/s time.
Morphology
SEM: Surface morphology of microbeads was investigated by Scanning Electron Microscopy (SEM) using JSM 6380A (JOEL, Japan). The microbeads, coated with Platinum by ion Auto fine coater JFC-1600 (JOEL, Japan), for 20 s at 1.1 V under argon atmosphere were mounted onto metal stubs using double micrographs were taken.
Results and Discussion
Formulation of microbeads by ionotropic gelation method
Microbeads were prepared by ionotropic gelation method by the following composition (Table 1).
| S. no. | Batch code | Drug (%w/v) | Polymer (% w/v) | Cross-linker (%w/v) |
| 1 | C1 | 1 | 2 | 3% |
| 2 | C2 | 1 | 2.5 | 3% |
| 3 | C3 | 1 | 3 | 3% |
| 4 | C4 | 1 | 3.5 | 3% |
| 5 | C5 | 1 | 4 | 3% |
Table 1: Composition of micro-beads formulation of iono-tropic gelation method batch code drug (%w/v) polymer (% w/v) cross-linker (%w/v).
Microbeads containing Nebivolol hydrochloride were prepared by ionotropic gelation technique. The sodium alginate solution was prepared by dispersing the weighed quantity of sodium alginate in deionized water. Accurately weighed quantity (1 g) of Nebivolol Hydrochloride was added to 100 ml polymeric solution of sodium alginate and drug were thoroughly mixed with help of homogenizer at 1500 rpm to get a homogenous drug-polymeric mixture. The formed mixture allowed standing for 1 h to make it bubble free. By following the same procedure the alginate beads of different ratios of drug: Polymer were prepared. The resulted homogenous dispersion was extruded into 100 ml of 6% cross-linker solution (CaCl2) through hypodermic syringe with flat tip needle (18 g) and stirred at 100 rpm. The formed micro-beads were allowed to cure for 30 min in the cross linker solution to complete the gelation. The beads were removed after the gelation period and washed with ethanol to harden the beads surface and finally with distilled water repeatedly to make free from un-reacted ion. The micro-beads were then filtered and dried in hot air oven at 400â?? for 18 h (Table 2 and Figure 2). The following graphical abstract represents the method adopted.
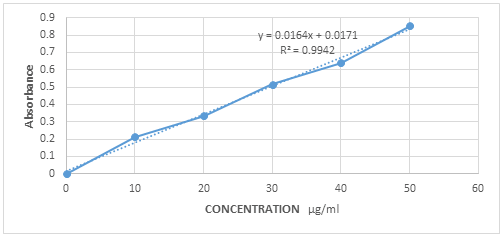
| Concentration μg/ml | Absorbance |
| 10 | 0.2125 |
| 20 | 0.3356 |
| 30 | 0.5154 |
| 40 | 0.6398 |
| 50 | 0.8523 |
Table 2: Calibration curve.
The standard calibration curve obtained was straight line which proved that the drug in the concentration range 10-50 μg/ml obeyed the Beer-Lambert's law. There was an increase in absorbance with respect to increase in concentration. The calibration curve was found to be linear (Figure 2).
Figure 2: Calibration curve.
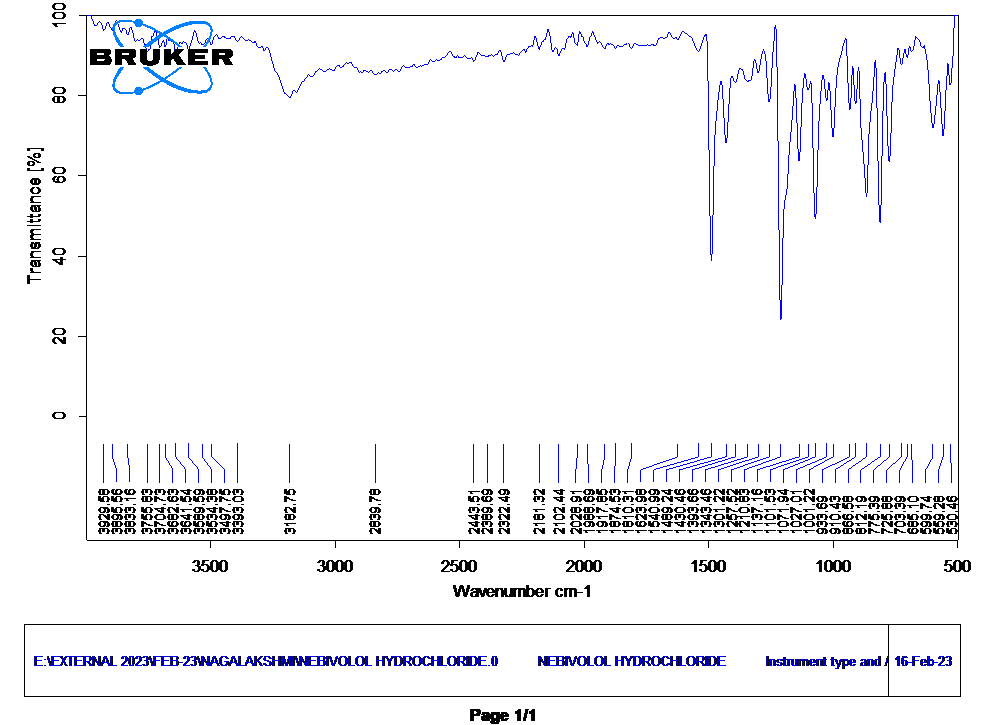
The Infrared (IR) spectrum peaks of nebivolol hydrochloride at the following wavenumbers 1210.83 cm-1 corresponds to C-O stretching, indicating the alcohol functional group; 1489.24 cm-1 likely represents the C=C stretching in the aromatic ring; 1071.94 cm-1 signifies C-N stretching, characteristic of the amine group; 866.58 cm-1 may be related to C-H bending in the aromatic or aliphatic structure; and 812.19 cm-1 represents C-Cl stretching, confirming the presence of the hydrochloride group, assisting in structural characterization and identification of nebivolol hydrochloride (Figure 3).
Figure 3: FTIR Spectrum of nebivolol hydrochloride.
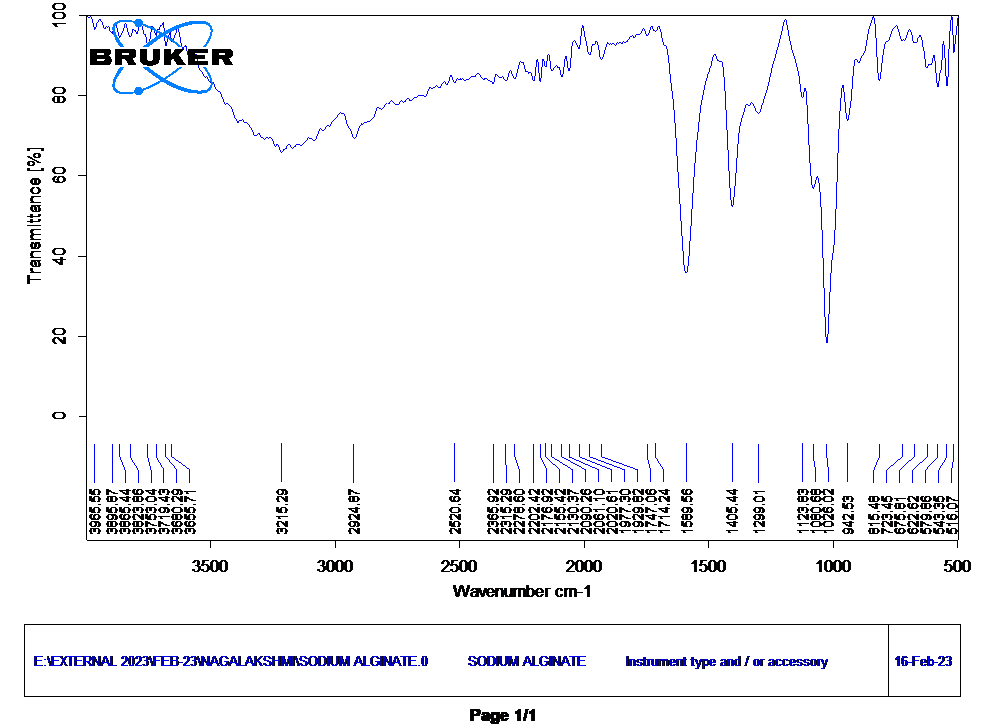
The Infrared (IR) spectrum of sodium alginate peaks at the following wavenumbers The wavenumbers 1589.56 cm-1 and 1405.44cm-1 correspond to the C=O and asymmetric stretching vibrations of carboxylate groups (-COO-) in sodium alginate, while 1026.02 cm-1 signifies C-O-C stretching in ether linkages, 942.53 cm-1 indicates C-O stretching in pyranose ring structures, and 815.48 cm-1 relates to C-H bending vibrations in the hydrocarbon portions of alginate, collectively revealing the presence of these characteristic functional groups and structural elements in the infrared spectrum of sodium alginate (Figure 4).
Figure 4: FTIR Spectrum of sodium alginate.
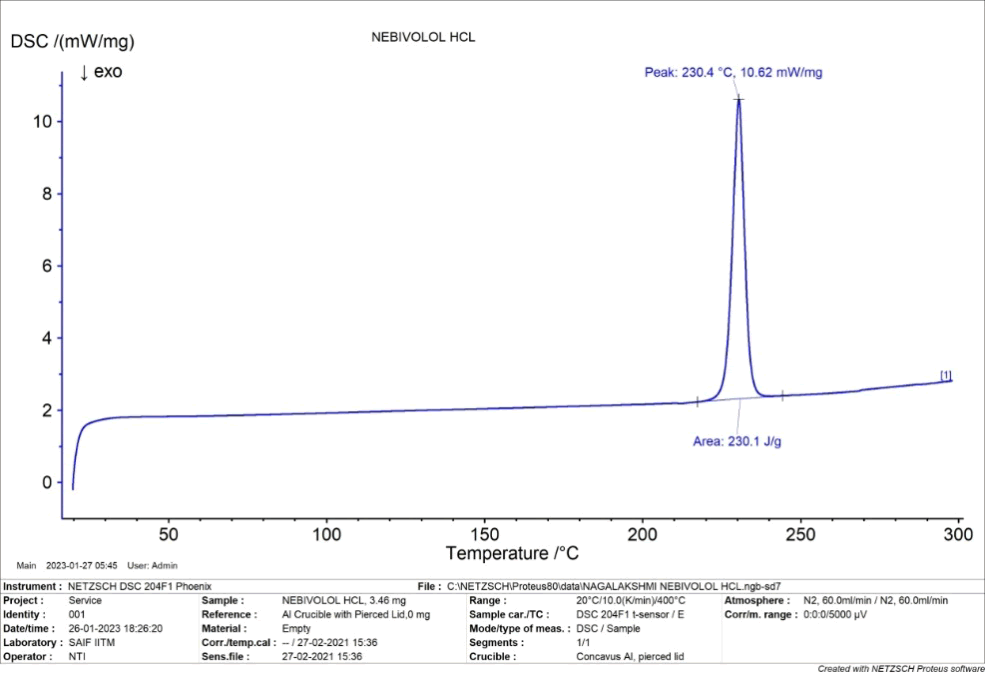
DSC thermogram of nebivololhydrochloride, showed a sharp exothermic peak corresponding to the melting point at 230.4°C (Figure 5).
Figure 5: DSC of nebivolol hydrochloride.
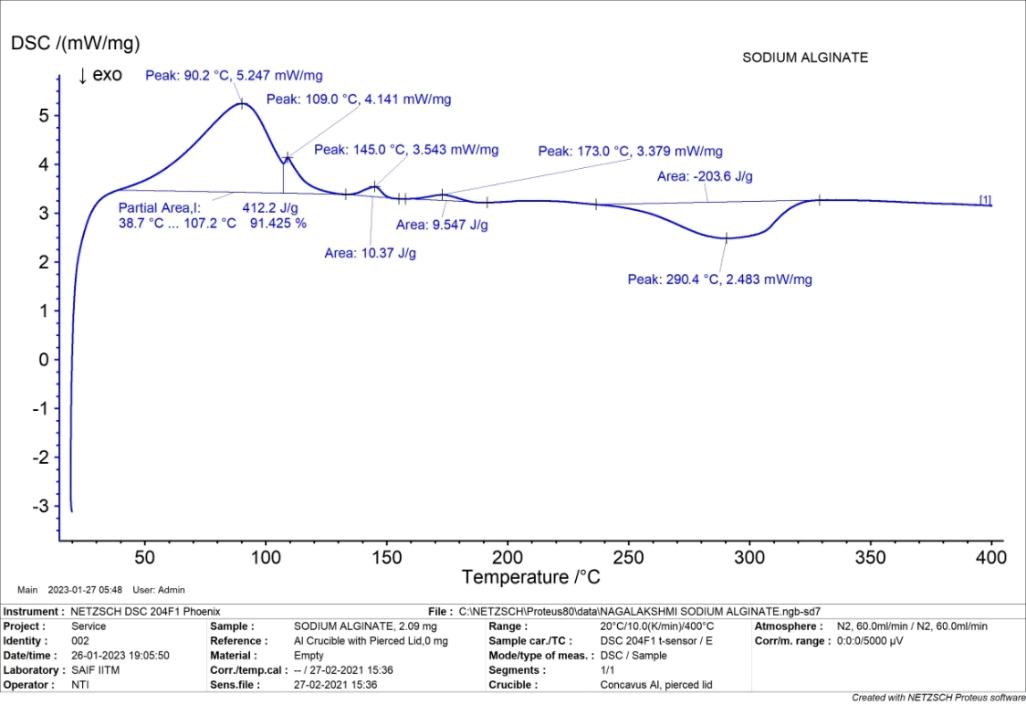
The DSC thermogram of sodium alginate showed both endothermic peak corresponding to the melting point at 290.4°C and exothermic peak of corresponding to the melting point at 90.2°C (Figure 6).
Figure 6: DSC of sodium alginate
Particle size
In ionotropic gelation method mean particle size of micro-beads for batch C1 to C5 ranges from 427.2 µm to 697.7 µm. It was observed that as the concentration of sodium alginate increased and size of micro-beads also increased. Increasing concentration of polymer causes increasing viscosity of solution which in turn increases the droplet size during extrusion of the polymer dispersion to the harvesting medium which results formation of larger size beads (Tables 3 and 4).
| S. no. | Division of stage micrometer (y) | Division of eye piece micrometer (x) | Calibration factor (y/x × 10) |
| 1 | 7 | 5 | 14 |
| 2 | 10 | 7 | 14.28 |
| 3 | 13 | 9 | 14.44 |
| Average weight=14.24 | |||
Table 3: Calibration of the eye piece micrometer.
| S. no. | Particle size distribution | Size in microns |
| 1 | 30 | 427.2 |
| 2 | 39 | 555.3 |
| 3 | 40 | 569.6 |
| 4 | 28 | 398.7 |
| 5 | 42 | 598 |
| 6 | 40 | 569.6 |
| 7 | 45 | 640.8 |
| 8 | 37 | 526.8 |
| 9 | 32 | 455.6 |
| 10 | 38 | 541.1 |
| 11 | 42 | 598 |
| 12 | 33 | 469.9 |
| 13 | 45 | 640.8 |
| 14 | 44 | 626.5 |
| 15 | 23 | 327.5 |
| 16 | 39 | 555.3 |
| 17 | 40 | 569.6 |
| 18 | 34 | 484.1 |
| 19 | 44 | 626.5 |
| 20 | 49 | 697.7 |
Table 4: Measurment of practicle size.
In ionotropic gelation method batches (C1 to C5) %swelling in pH 7.4 ranges from 100 to 620 respectively. At acidic pH, alginate is protonated into insoluble form of the alginic acid this displays low swelling and in intestinal pH, at pH 7.4 carboxyl groups of alginate ionize, which weakens the electrostatic interactions, thus making the bead structure loose resulting in increased swelling (Table 5).
| Time | Initial weight | Final weight avg | S.I |
| 0 | 50 mg | 50 mg | 100 |
| 30 | 50 mg | 242 mg | 484 |
| 60 | 50 mg | 265 mg | 530 |
| 120 | 50 mg | 280 mg | 560 |
| 180 | 50 mg | 310 mg | 620 |
Table 5: Swelling index.
In ionotropic gelation method, % drug encapsulation efficiency for batches (C1-C5) ranges from 27.6% to 45.8%. The higher encapsulation efficiency was observed as the concentration of alginate increased. This is due to the greater availability of active calcium binding sites in the polymeric chains and consequently the greater degree of cross linking (Table 6 and Figure 7).
| Formulation code | Encapsulation efficiency (%) |
| C1 | 27.6 |
| C2 | 32.2 |
| C3 | 45.8 |
| C4 | 38.6 |
| C5 | 33.2 |
Table 6: Encapsulation efficiency.
Figure 7: Encapsulation efficiency.
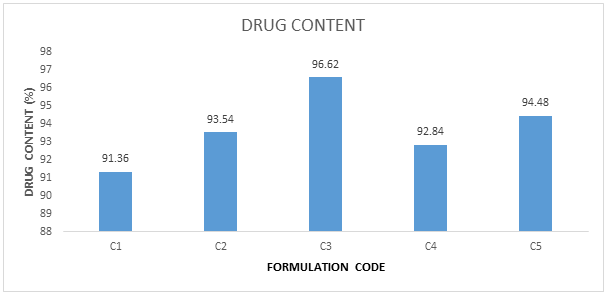
In ionotropic gelation method, drug content for batches (C1-C5) ranges from 91.36 to 96.62%. The higher drug content was observed as the C3 (Table 7 and Figure 8).
|
Formulation code |
Drug content (%) |
|---|---|
|
C1 |
91.36 |
|
C2 |
93.54 |
|
C3 |
96.62 |
|
C4 |
92.84 |
|
C5 |
94.48 |
Table 7: Drug content.
Figure 8: Drug content
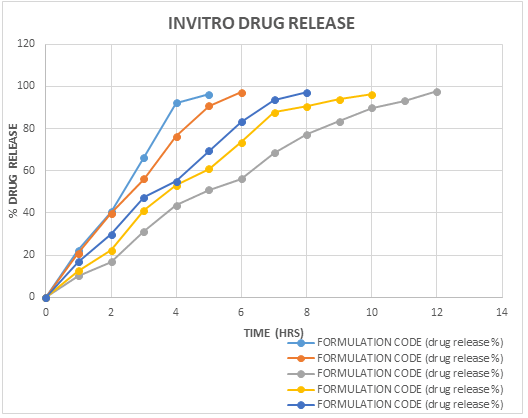
Drug release from batches C1, C2, C3, C4, C5 containing 2.0, 2.5, 3.0, 3.5, 4.0% sodium alginate respectively showed 96.17% in 5 h, 97.26% in 6 h, 93.31% in 7 h, 97.31% in 8 h, 96.18% in 10 h and 97.75 in 12 h respectively. It was observed from the swelling study that alginate beads had swollen in phosphate buffer pH 7.4 and hydrochloric acid buffer (pH 1.2). The release will depend on diffusion of nebivolol hydrochloride through the insoluble matrix of alginate polymer in pH 1.2 HCl buffer. On the other hand, rapid swelling and erosion of beads prepared form alginate were observed at pH 7.4 because at this pH exchange of Na+ ion and Ca2+ takes place and Ca-alginate is converted into Na-alginate which is more soluble. From ionotropic gelation method, batch C3 showed 97.75% encapsulation efficiency in 12 h, hence considered as optimized batch (Table 8 and Figure 9).
| Time in hrs | Formulation code (drug release %) | ||||
|---|---|---|---|---|---|
| C1 | C2 | C3 | C4 | C5 | |
| 0 | 0 | 0 | 0 | 0 | 0 |
| 1 | 22.43 | 20.98 | 10.16 | 12.76 | 16.98 |
| 2 | 40.48 | 39.8 | 16.97 | 22.27 | 29.98 |
| 3 | 66.41 | 56.17 | 31.45 | 41.32 | 47.36 |
| 4 | 92.32 | 76.34 | 43.58 | 53.12 | 55.12 |
| 5 | 96.17 | 90.76 | 50.93 | 60.89 | 69.45 |
| 6 | 97.26 | 56.17 | 73.54 | 83.24 | |
| 7 | 68.47 | 87.76 | 93.75 | ||
| 8 | 77.39 | 90.48 | 97.31 | ||
| 9 | 83.4 | 93.92 | |||
| 10 | 89.76 | 96.18 | |||
| 11 | 93.19 | ||||
| 12 | 97.75 | ||||
Table 8: In vitro drug release.
Figure 9: In vitro drug release.
Release kinetics In order to elucidate the mechanism of drug release, the data was fitted into various models such as zero order, first order, Higuchi and Koresmeyar Peppa’s. The data are shown in in vitro. The examination of the coefficient of correlation (r2) values indicated that the prepared beads followed koresmeyar Peppas kinetics with non-fickian diffusion mechanism of drug release.
| Code of formulation | Zero-order (r2) | First-order (r2) | Higuchi (r2) | Peppa’s | |
| n | (r2) | ||||
| C3 | 0.9817 | 0.8978 | 0.9516 | 0.9451 | 0.9871 |
Table 9: Release kinetics.
Scanning electron microscopy (SEM): The SEM of the drug loaded beads (C3) was found to be irregular in shape having smooth and dense surface with inward dent and shrinkage due to collapse of the wall during dehydration. The fibrous network was also observed on the surface of the beads as shown in Figure 10.
Figure 10: SEM photograph microbeads of ionotropic gelation method
From the results, it was optimized formulations C3 stored at three different storage conditions i.e. accelerated stability for 3 months, long term stability study for 3 months showed no significant change on product when compared to initial stage (Table 10).
| Days | Temperature | ||
|---|---|---|---|
| 100C | 25OC | 400c | |
| 0 | 99.8 | 99.2 | 99.1 |
| 30 days | 99.7 | 98.8 | 90.2 |
| 60 days | 99.6 | 98.2 | 97.2 |
| 90 days | 99.4 | 97.9 | 95.12 |
Table 10: Stability studies.
Summary
• The research project centered around the creation of microbeads through the ionotropic gelation method, integrating the active pharmaceutical component (API) Nebivolol Hydrochloride (NH) with the natural polymer Sodium Alginate (SA).
• The procedure involved a series of essential steps, commencing with the dissolution of sodium alginate in distilled water. This resultant polymer solution was subsequently dispersed into a solution of calcium chloride, which served as the crosslinking agent.
• The droplets of polymer solution underwent ionotropic gelation when they came into contact with the calcium chloride solution, culminating in the formation of spherical micro-beads. These micro beads were subsequently subjected to filtration, washing, and drying in a hot air oven, leading to the ultimate product.
• The resultant micro-beads underwent a comprehensive characterization utilizing diverse techniques. Fourier-Transform Infrared Spectroscopy (FTIR) was harnessed to scrutinize the chemical interactions between the polymers and the API, affirming the efficacious encapsulation of nebivolol hydrochloride.
• Differential Scanning Calorimetry (DSC) was employed to evaluate the microbeads' thermal behavior, thereby providing insights into their stability and compatibility.
• The micro-beads' morphological attributes and size distribution were evaluated through Scanning Electron Microscopy (SEM), which revealed their uniform spherical structure and the size was found to be irregular in shape having smooth and dense surface with inward dent and shrinkage due to collapse of the wall during dehydration.
• A microscope-based technique was used for particle size analysis, which further verified the consistent dimensions of the micro-beads. The capacity of the micro-beads to absorb and retain fluids was gauged through the assessment of their swelling index.
• Crucial performance parameters of the micro-beads were evaluated. Studies pertaining to encapsulation efficiency and drug content estimation were executed to quantify the extent of nebivolol hydrochloride encapsulation within the micro-beads.
• Furthermore, drug release studies were conducted to explore the sustained release of the API from the micro-beads over a specific duration, here the drug release for the optimized formulation was found to be C3 97.75% these investigations furnished insights into the potential of the micro-beads as a delivery mechanism for controlled and sustained drug release.
Conclusion
To conclude, the research endeavor successfully conceived, developed, and characterized micro-beads through the utilization of the ionotropic gelation method, wherein nebivolol hydrochloride functioned as the active ingredient and sodium alginate served as the polymer matrix.
The approach effectively facilitated the controlled implanting of the API within the micro-beads, thereby representing the method's ability for drug delivery applications.
The characterization methodologies, including FTIR, DSC, SEM, particle size analysis, swelling index determination, and drug release studies, collectively provided a comprehensive comprehension of the micro-beads' attributes.
The FTIR analysis substantiated the interaction between the polymers and the API, validating the encapsulation procedure. Insights into the micro-beads' stability under different temperatures were collected from the DSC data.
Demonstrating uniform size and spherical morphology, the micro beads were encapsulated by SEM and particle size analysis. The assessment of their swelling index underscored their potential for controlled fluid absorption. The encapsulation efficiency and drug content estimation analyses showcased the successful inclusion of nebivolol hydrochloride.
Furthermore, the in vitro drug release studies revealed a measured and prolonged release of the API from the micro-beads, underscoring their viability for sustained therapeutic effects.
Overall, the research contributes to the advancement of a promising drug delivery system through the ionotropic gelation method, offering a platform for calibrated and sustained drug release, with promising applications in the pharmaceutical and medical terms.
References
- Elsherif NI, Al-Mahallawi AM, Abdelkhalek AA, Shamma RN (2021) Investigation of the potential of nebivolol hydrochloride-loaded chitosomal systems for tissue regeneration: In vitro characterization and in vivo assessment. Pharmaceutics 13: 700.
[Crossref] [Google Scholar] [PubMed]
- Hanif M, Ullah khan HA, Afzal S, Mahmood A, Maheen S, et al. (2017) Sustained release biodegradable solid lipid microparticles: Formulation, evaluation and statistical optimization by response surface methodology. Acta Pharm 67: 441-461.
[Crossref] [Google Scholar] [PubMed]
- Panda PK, Verma A, Saraf S, Tiwari A, Jain SK (2021) Ionically Gelled Gellan Gum in Drug Delivery. Ionically Gelled Biopolysaccharide Based Systems in Drug Delivery 2021: 55-69.
- Parulkar AS (2016) Formulation, Development and Evaluation of Oral Multiparticulate Drug Delivery System of Arterolane. Master's thesis, Rajiv Gandhi University of Health Sciences; India.
- Yousif NZ, Salman ZD (2023) Microsponge as a Strategy for Effective Drug Delivery System. Al Mustansiriyah J Pharm Sci 23: 322-335.
- Kshirsagar NA (2000) Drug delivery systems. Indian J Pharmacol 32: 54-61.
- Bariya SH, Gohel MC, Mehta TA, Sharma OP (2012) Microneedles: An emerging transdermal drug delivery system. J Pharm Pharmacol 64: 11-29.
[Crossref] [Google Scholar] [PubMed]
- Ko JA, Park HJ, Hwang SJ, Park JB, Lee JS (2002) Preparation and characterization of chitosan microparticles intended for controlled drug delivery. Int J Pharm 249: 165-174.
[Crossref] [Google Scholar] [PubMed]
- Brannon-Peppas L (1995) Recent advances on the use of biodegradable microparticles and nanoparticles in controlled drug delivery. Int J Pharm 116: 1-9.
 Spanish
Spanish  Chinese
Chinese  Russian
Russian  German
German  French
French  Japanese
Japanese  Portuguese
Portuguese  Hindi
Hindi