Research Article, Biomater Med Appl Vol: 8 Issue: 1
Recent Advances of Chitosan and Natural Polymer-based Hydrogels and their Potential Applications in Wound Healing
Amritendu Chakraborty1,2, Sangram Keshari Samal2,3, Srikanta Sahu1,4* and Susanta Kumar Biswal1
1Department of Chemistry, School of Applied Science, Centurion University of Technology and Management, Odisha, India
2Deaprtment of Chemistry, Materials Research Center, Indian Institute of Sciences, Bengaluru, Karnataka, India
3Deaprtment of Biomaterials and Regenerative Medicine, Laboratory of Biomaterials and Regenerative Medicine for Advanced Therapies, ICMR-Regional Medical Research Center, Bhubaneswar, Odisha, India
4Department of Chemistry, Government Women’s College, Sambalpur, Odisha, India
*Corresponding Author:Srikanta Sahu
Department of Chemistry, School of Applied Science, Centurion University of Technology and Management, Odisha, India
E-mail: srikantachem.iitd@gmail.com
Received date: 24 September, 2023, Manuscript No. BMA-23-114658; Editor assigned date: 26 September, 2023, PreQC No BMA-23-114658 (PQ); Reviewed date: 11 October, 2023, QC No. BMA-23-114658; Revised date: 18 January, 2024, Manuscript No. BMA-23-114658 (R); Published date: 25 January, 2024, DOI: 10.4172/2577-0268.1000537
Citation: Chakraborty A, Samal SK, Sahu S, Biswal SK (2024) Recent Advances of Chitosan and Natural Polymer-based Hydrogels and their Potential Applications in Wound Healing. Biomater Med Appl 8:1.
Abstract
Wound healing is a complex process which severely impairing the quality of life in patients. Most significantly, non-healing wounds are always alarming for patients. These non-healing wounds have increased the mortality rate in patients, specifically in the case of type 2 diabetes and non-compressible hemorrhages. Therefore, last few years have witnessed enormous research focus on wound healing, using naturally occurring chitosan and other natural polymers-based hydrogel as one of emerging materials for wound dressing. These special hydrogels have numerous numbers of promising biological properties including: biodegradable, biocompatible, non-toxic, antimicrobial, and hemostatic etc. Owing to their highly fascinating biological properties, these hydrogels are extensively utilized in the recent years for wound healing applications in biomedical domain. In this review, we have more precisely highlighted the recent advances of wound healing applications, using specifically chitosan and other natural polymer-based hydrogels. We have also underlined the role of chitosan-based hydrogel in the different phases of wound healing pathways, such as: Coagulation, inflammation, proliferation, and remodeling. In the subsequent part, it covers the potential therapeutic applications of various chitosan and natural polymer-based hydrogels in wound healing applications in great details. Finally, we describe the emerging and future scope of chitosan-based hydrogel in wound healing.
Keywords: Wound healing; Biomaterials; Hydrogels; Chitosan; Chitosan and natural polymers-based hydrogels
Introduction
Wound can be described as any injury that breaks the skin or other tissues which can range from minor cuts and bruises to punctured skin.
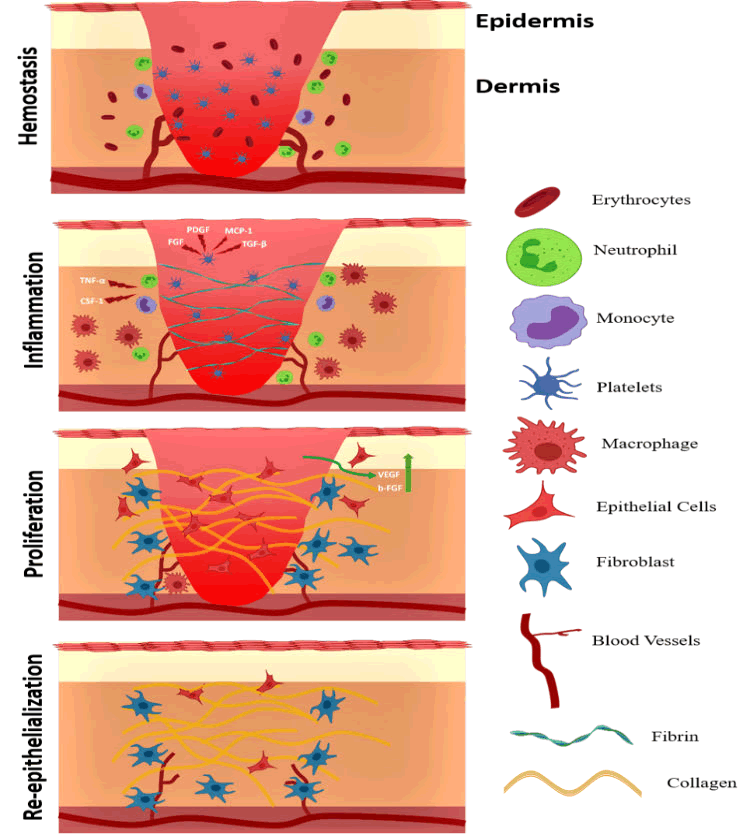
Normal acute wounds that generally progress through the normal phases of wound healing ultimately resulting in the wound closure (Figure 1). Sometimes, the acute wounds are stuck at a certain stage of wound healing and as a result do not lead to wound closure. These wounds are defined as chronic wounds. Chronic wounds not only delay the healing process but come with added complications such as consistent pain and additional burden on healthcare [1].
Figure 1: Mechanism of wound healing process (Involved in the main stages).
To susceptible patients, chronic wounds are incapacitating and are sometimes associated with high morbidity and mortality [2]. As of 2022, more than 6 million people are affected by acute and chronic wounds in the USA alone with a cost of $25 billion (USD) [3]. Moreover, from the onset of tissue injury to the final closure of the wound, the wound tissue is susceptible to wide range of infectious agents like bacteria, fungus, viruses etc. (Table 1).
| Group | Species |
|---|---|
| Gram negative | Psuedomonas aeruginosa, Klebsiella pneumoniae, Serratia marcescens, Enterobacter spp, Proteus spp, Bacteoidss spp, Actinetobacter baumanni, Enterobacteriaceae, Escherichia coli |
| Gram positive | Staphylococcus aureus, Corynebacterium diphtheria, Coagulase-negative staphylocci, Enterococcus, Micrococcus, Corynebacterium, Sreptococcuspyogens. |
| Fungi | Candida spp, non-albicans Candida, Fusarium spp, Alternaria spp, Rhizopus spp, Mucor spp, Aspergillus, Blastomycosis, Mucorcircinelloides |
| Viruses | Herpessimplex, Varicella-zoster |
| Drug-resistant strains | Staphylococcus aureus (Methicillin-resistant), Enterococci (Vancomycin-resistant), Beta-lactamases (extended-spectrum), Psuedomonas aeruginosa |
Table 1: List of pathogenic bacteria causing acute wound infection.
Microorganisms like Staphylococcus aureus (S. aureus), Pseudomonas aeruginosa (P. aeruginosa), and β-hemolytic streptococci are some of the common pathogens that have been known to cause significant damage to an already existing wound. These microorganisms deteriorate the wound tissue by the production of bacterial enzymes that destroy the tissue and during this process; they release various endo and exotoxins [4,5]. An added complication is the development of bacterial biofilms in chronic wounds [6]. Biofilms are a cluster of microbial cells that are primarily composed of Extracellular Polymeric Substances (EPS). These are made up of exopolysaccharides, proteins, bacterial DNA, and enzymes etc. It provides a protective shielding against antibiotic medications as well as host immune cells [7]. Poly-microbial biofilms play a major role in preventing the eradication of chronic wounds.
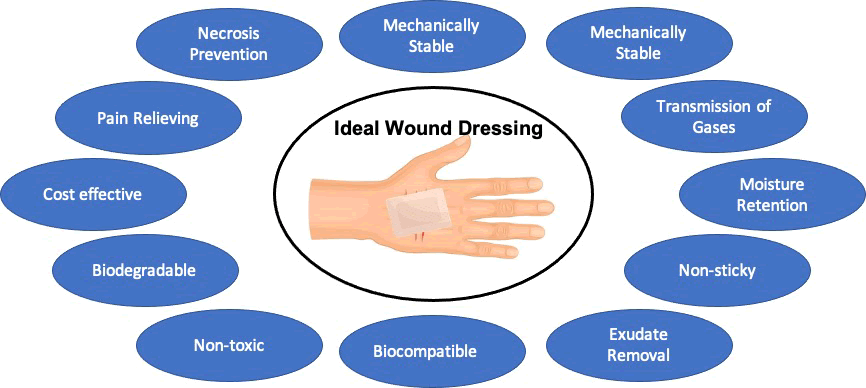
Hence, a wide range of wound care products has been introduced to mitigate these infections. Before the innovative research of Winter et al., wound dressings were almost considered redundant. It was believed that wounds would heal faster, if they were kept open and moisture free. And hence, wound dressings were assumed to have minimal effect in the healing process. Winter et al. showed for the very first time that when the wounded pig epithelial layer was covered with an inert layer of protection that prevented loss of moisture, the epithelization of the wound was twice as fast compared to the wounds exposed to air [8]. Since then, the research on wound healing and dressings was furthered, suggesting that an ideal wound dressing should have excellent mechanical strength, higher biocompatibility which would retain the moisture in the wound microenvironment, would allow gaseous exchange, and prevent bacterial infection thereby protecting against secondary infections to accelerate and improve the quality of the wound healing process [9–11].
In its early stages, wound dressings were generally made of woven or non-woven gauzes or from a blend of composites which mostly included polyester, rayon, and cotton. One of the major drawbacks of these dressings was that they absorbed the wound exudates and rendered the wound environment dry which reduced the proficiency of wound healing. Moreover, the wound exudates would accumulate at the wound surface that facilitated contamination of the wound. Finally, the dried exudates would also stick to the skin which not only made removing the dressing painful but also damaged the newly formed epithelial layer. Gauze dressings have also been found to reduce the temperature of the wound environment during the evaporation of the wound exudates. This feature has been found to introduce multiple problems including hypoxia, compromised phagocytic activity that impairs wound healing [12].
In an ideal scenario, wound dressings should provide with a wet wound environment which ensures renewed skin formation with inflammation and eschar formation [13]. Moreover, an ideal wound dressing should have the following characteristics:
- Retain/control moisture content in the wound environment.
- Allow for gaseous exchange.
- Elimination of wound exudates.
- Provide barrier against microorganisms and secondary infection.
- Provide physical protection.
- Prevent/reduce surface necrosis.
- Easily replaceable.
- Cost effective.
- Biocompatible, and biodegradable, nontoxic.
- Ability to alleviate pain (Figure 2) [14].
Figure 2: Features of Ideal wound dressing.
Taking the above factors into consideration, hydrogels have emerged to be an ideal candidate for wound dressings in recent years [10]. Hydrogels are three-dimensional network structure composed of cross-linked hydrophilic polymers [15]. This 3D network structure of hydrogel allows absorbing large quantities of water, which can be multiple times the hydrogel’s weight. The presence of the hydrophilic structures in the polymeric backbone enhances the water uptake ability in hydrogels [16]. However, this network structure makes the hydrogel more insoluble and allows retaining more water into it which is many folds with respect to the equivalent weight of water. Hydrogels have multiple advantages when it comes to wound dressings. Hydrogels can retain moisture in the wound environment that enhances the resolution of the wound and facilitates effective debridement [17]. Interestingly, hydrogels have multiple advantages such as: Reduce the stimulation of surrounding tissues, decrease the chances of negative immune response, act as supporting material to the wound tissues [18,19]. Hydrogels can also act as drug delivery vehicles owing to their highwater content [20]. There are several polymeric hydrogels such as: Poly Acrylate Acid (PPA), Poly-Ethylene Glycol (PEG), and Poly-Vinyl Alcohol (PVA) used extensively as drug delivery materials with significant increase of drug efficiency [18]. Hydrogels can be composed of both natural and synthetic polymers and their combination. The main advantage of natural polymers is their resemblance to the Extracellular Matrix (ECM). It is owing to similar composition and mechanical properties of the hydrogel with respect to ECM. Therefore, the hydrogels are served as suitable supporting material for tissue regeneration in the living system and also act as potential drug delivers carrier.
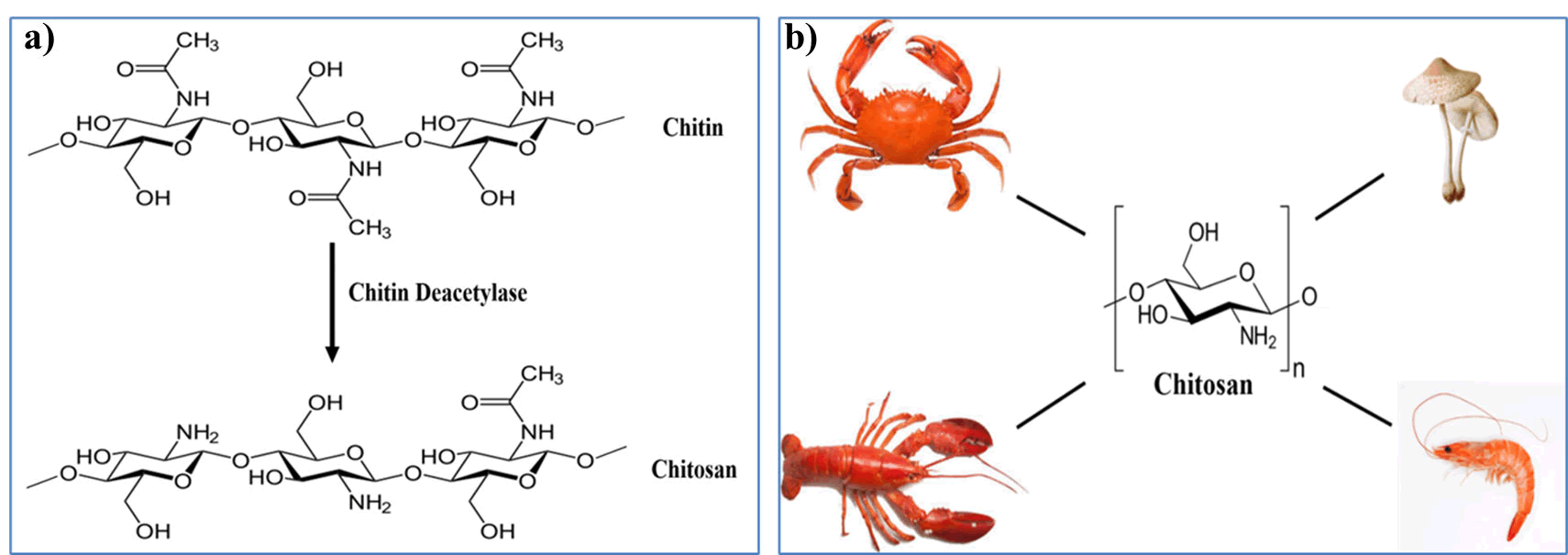
Among the naturally available polysaccharides, chitosan has been used extensively in the fields of wound dressings, medicine, tissue engineering and cosmetics amongst others. Chitosan, a derivative of chitin, has been used extensively in the preparation of hydrogels in recent years. Chitin is a biopolymer most abundantly exists in outer shell of shrimps and crabs. It is also found in many algae and bacterial cell walls as well. Chitin is known to be the second most abundant biopolymer after cellulose. Chitin is found to insoluble in water and therefore, it is converted to chitosan using chemical reaction to increase the solubility in water (Figure 3).
Figure 3: a) Deacetylation of Chitin to Chitosan, b) Common sources of chitosan.
The structural skeleton of chitosan and chitin is same. But only difference is, the chitin is having additional acetyl group at the C-2 position [10]. Chitosan has numerous amicable biological properties including; non-toxic, biodegradable, biocompatible, non-antigenic, antimicrobial etc. Owing to its bio-friendly properties, chitosan has been extensively used in drug delivery and tissue engineering. Chitosan is a polycationic polymer due to the presence of primary amines that bind to protons. It is the positive charge of the amine (NH2) functional group that interacts with the negative charge of the phospholipids and proteins in the bacterial plasma membrane. As a result, the polycationic nature confers an inherent antibacterial property to chitosan. This distinct antimicrobial effect has been found to cause the disruption of the cell membrane in gram-negative bacteria. Consequently, it can easily penetrate the microbial plasma membrane, leading to release of cytosolic components present in the bacteria. Moreover, chitosan has been found to have the ability to open epithelial tight junctions in human epithelial cells by inducing translocation of tight junction proteins. Chitosan has also been found to be highly biocompatible. It has been found that chitosan can be easily degraded in the human body by the presence of lysozyme and by enzymes released by the bacterial flora in the gut. Chitosan is muco-adhesive in nature due to the presence of positively charged amino group in it. Owing to their intrinsic properties of chitosan, it brings the ionic interactions between the positively charged amino groups of chitosan with that of the negatively charged environment of the mucus layer. Chitosan is available in varying molecular weights with varying degrees of deacetylation. Multiple formulations of chitosan-based hydrogel with different functional groups having varying degrees of acetylation can accelerate and aid in the various stages of the wound healing process. Commercially, a wide variety of chitosan based wound dressings are available in the form of hydrogels, films, and sponges.
One of the major drawbacks of such hydrogel is that it is a natural polysaccharide and there is a high possibility of eliciting the host immune response which may lead to adverse immunological response. Moreover, chitosan alone has shown poor bio-stability owing to be a polysaccharide, which lacks of protein structure. Therefore, it has an inherent ability to form a matrix by itself. Hence, to overcome these problems, it naturally demands to cross linked with other polymers to enhance the properties. Furthermore, chitosan-based hydrogels have been known to possess weak mechanical properties with poor dissolution rate and hence need to be combined with other polymers.
There is several review papers reported in the literature, highlighting the potential applications of hydrogel in wound healing. In the recent years, numerous researches have been done specifically on developing new hydrogels-based materials using the combination of chitosan and other natural polymers. Moreover, these special hydrogels are widely used in wound healing applications in the recent year owing to their better wound healing ability than chitosan itself. However, there is several review articles reported on chitosan-based hydrogels, highlighting the potential application in wound healing till date. But, most of these review papers have very limited information on these special types of hydrogels that are formulated by the combination of chitosan and other natural polymers. To the best of our knowledge, in this review article we have extensively highlighted on the recent progress of wound healing applications specifically using the hydrogel, formulated by the combination of chitosan and other natural polymers, in great details. In this review, we aim to highlight the recent advances of chitosan and natural polymer-based hydrogel that are used exclusively for wound healing application in the recent years. Moreover, we have also covered the mechanism of wound healing pathways, such as: coagulation, inflammation, proliferation, and remodeling. It also covers the major potential disadvantage of chitosan-based hydrogel only and describes the need of hydrogel derived from the combination of chitosan and natural polymers. In the subsequent part, it covers the potential therapeutic applications of various chitosan and natural polymer-based hydrogels in wound healing applications in great details. Finally, we describe the emerging and future scope of chitosan-based hydrogel in wound healing.
Literature Review
Role of chitosan based hydrogels in wound healing process
There are several scientific reports in the literatures clearly reveals that Chitosan and its hydrogel impart a positive impact on wound healing processby influencing its effect at different stages (Figure 2). The process of wound healing is meticulously orchestrated by multiple cells like ECM, white blood cells and various cells of the immune system, etc. Chitosan-based hydrogels plays significant role in wound healing by increasing exudate absorption capacity of wounds. It also enhances adherent and anti-bacterial properties of wounds. In addition to that it helps in stimulating angiogenesis and re-epithelialization of skin tissue. Furthermore, it supports collagen deposition and sustains delivery of drugs. The main role of chitosan in the wound healing process is elucidated in the section below.
Coagulation or hemostasis: Immediately after the injury, the wound healing process is initiated by the coagulation or hemostasis phase which prevents excessive blood loss from the site of injury and provides a framework for the immunological cells that are required for the wound healing process. In the initial coagulation phase, platelets play an important role where they release specific cytokines like SDF-1. Chitosan plays multiple roles in the hemostatic process. Firstly, chitosan is a hemostat which rapidly coagulates blood. The positively charged amine groups in chitosan interacts with the negatively charged sialylated glycoproteins present in the membrane of the red blood cells to arrest the bleeding and seal the site of injury. Secondly, chitosan is also anti-pyrogenic which aids in the reduction of pain at the wound site. Most importantly, chitosan has been known to in platelets leads to activation of thrombosis and glycoproteins like GPIIb/IIIa and release of inducing the activation of platelets by increasing the levels of calcium in platelets.
Inflammation: The inflammatory phase of inflammation starts shortly after coagulation. In this phase, damaged cells, bacteria, and other pathogens are removed from the wound microenvironment. For this, many components of the immune system play an important role. Components like cytokines, chemokines, immune cells, growth factors, etc. Cytokines like interleukin-1 are responsible for the recruitment of neutrophils, fibroblast via chemotaxis. Chitosan-based hydrogels can accelerate the wound healing process by regulating the activity of the inflammatory mediators in the wound environment that encourages healing.
Studies have shown that chitosan can regulate the secretion of inflammatory mediators and chemokines such as interleukin 8, prostaglandin E, and interleukin 1b. In other studies, researchers have shown that chitosan and chitosan-based hydrogels can recruit inflammatory cells such as poly-morphonuclear leukocytes that not only phagocytize but also secrete inflammatory mediators such as Interleukin (IL)-1, IL-8, IL-12, Macrophage Inflammatory Protein (MIP)-1a, and MIP-1b. The binding of N-Acetyl Glucosamine (GlcNAc) present in chitosan to its specific receptors in macrophages is thought to be important for macrophage activation and accelerate cytokine production from macrophages conducive to wound healing.
Proliferation: In this phase, the wound environment undergoes some major changes where the new cells and tissues are formed. For this, the wound site contracts, and tissues proliferate for reepithelialization. During the proliferation stage, angiogenesis and granulation tissue formation take place along with the synthesis of new extracellular matrix. Ueno et al. have shown that chitosan promotes type III collagen synthesis thereby promoting the formation of granulation tissue. Moreover, chitosan functionalized with RGD peptide sequences have been found to bind α5β1, αvβ5 and αvβ3 integrins, which facilitate signals crosstalk between the Extracellular Matrix (ECM) and cytoskeleton and aid in proliferation.
Remodeling: The final stage of wound healing results in the development of a new epithelium layer and maturation of the scar tissue. In this phase, the type I collagen laid down in the earlier phase of wound healing is gradually replaced by more stable type III collagen. Wang, F, et al., reported that chitosan accelerates the secretion of type III collagen and also accentuates granulation in the wound site. Contrary to normal wounds chronic wounds present a different set of complications. Chronic wounds like diabetic foot ulcers and pressure sores have an excess of inflammatory cells in the wound environment which might lead to tissue necrosis and can delay the wound healing process and ultimately threaten life. Moreover, another concern is bacterial infections in these wounds. Due to the nature of certain chronic wounds like diabetic foot ulcers which are open, the bacterial infection is persistent, for which the immune cells have to consistently phagocytize bacterial cells which increases the concentration of free radicals and tissue destroying enzymes like proteases in the wound and ultimately accentuates physiological tissues damage and delays the wound healing. Chitosan and chitosanbased hydrogels have an advantage in these situations. Primarily, chitosan being non-immunogenic as well as highly anti-bacterial provides a favorable environment for treating chronic wounds as it prevents excessive inflammatory reaction in the wound site and also prevents bacterial infection that is the second reason that prevents wound healing (Figure 4).
Figure 4: Schematic representation of mode of action of chitosanbased hydrogels in wound healing process.
Drawbacks of chitosan-based hydrogels
One of the major drawbacks of such hydrogel is that it is a natural polysaccharide and there is a high possibility of eliciting the host immune response which may at times provoke adverse immunological response if the chitosan used for the preparation of the wound dressing is not carefully selected. Moreover, being a polysaccharide, chitosan lacks a protein structure that leads to inefficient bio-stability and an inherent ability to form a matrix by itself for which it needs to be cross-linked with other chemicals that may be hazardous. Furthermore, chitosan-based hydrogels have been known to possess weak mechanical properties with poor dissolution rate and hence need to be combined with other polymers.
Applications of chitosan as wound dressings
As discussed above, chitosan has an essential role in wound repairing and therefore, it has been widely used in dressing of wounds. Chitosan-based hydrogels can be further improved to increase the efficacy in various aspects of wound healing (Figure 5).
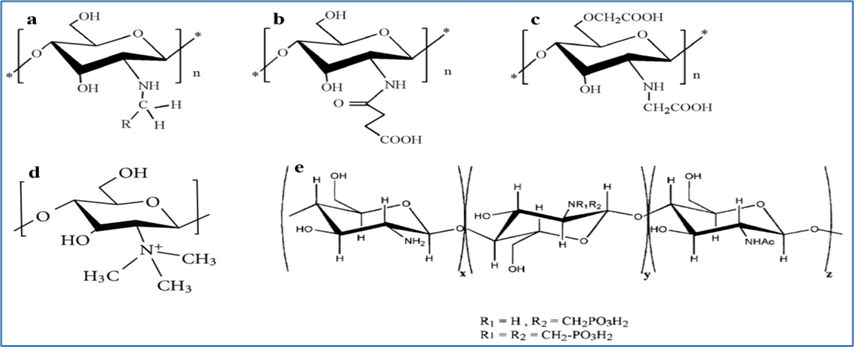
Figure 5: Modified forms of Chitosan commonly used in biomedical applications. a) Alkylated Chitosan, b) Succinylated Chitosan, c) Carboxymethylated Chitosan, d) N,N,N-Tri-methylated Chitosan, e) N-Methylene phosphonic Chitosan.
This can be done by the various modifications on the polymeric surface or by using chitosan of different degrees of deacetylation or by crosslinking chitosan with other polymers and crosslinking agents. Furthermore, these modifications can lead to enhance the properties of the hydrogel by enhancing wound exudate absorption capacity and antibacterial activity. These modified hydrogels also help in increasing the re-epithelialization activity, and others. In the following sections, the various modifications of chitosan-based hydrogels in wound dressing’s application will be discussed.
Chitosan-based hydrogels for wound dressings: These hydrogels have added advantages as wound repairing material as they can easily provide a moist environment for wound healing that essentially seals the wound site from secondary infection, can provide innate antibacterial effect, and can increase the healing process faster and thereby prevent scab formation.
Chitosan hydrogels in combination with other polymers: Chitosan can also be mixed with other polymers in the preparation of the hydrogel to exert the beneficial effect of both the polymers in wound healing. The other polymer can be either natural or synthetic.
The natural polymer can be defined as a polymer that has been obtained from an animal, plant, or microbial source and one that is protein or polysaccharide in nature. Natural polymers have the inherent advantage of being able to simulate the local wound environment and the ECM and are easily biodegradable. On the other hand, natural polymers have some drawbacks like having poor stability, having batch-to-batch variations as they are isolated from the animal or plant source, and the high cost of complexity of extraction from the source. The following section will shed light on the chitosanbased hydrogels in combination with other polymers.
Combination of Chitosan with other natural polymers-based hydrogels for wound healing applications: Alginate: Alginate is a widely available anionic biological polymer mostly obtained from brown algae (Phaeophyceae), Ascophyllum nodosum, Laminaria Hyperborea, Laminaria japonica, Macrocystis pyrifera, and other species. It is mainly composed of repeating building blocks of (1,4)- linked β-D-mannuronate (M) and α-L-guluronate (G) residues. Alginate has been found to be highly biocompatible in nature and therefore, it has been extensively used in diverse biomedical applications such as: Tissue engineering and drug delivery. One of the major drawbacks of alginate is low muco-adhesiveness and as a result of which alginates have been mixed with chitosan to increase its mucoadhesive property in wound repair applications.
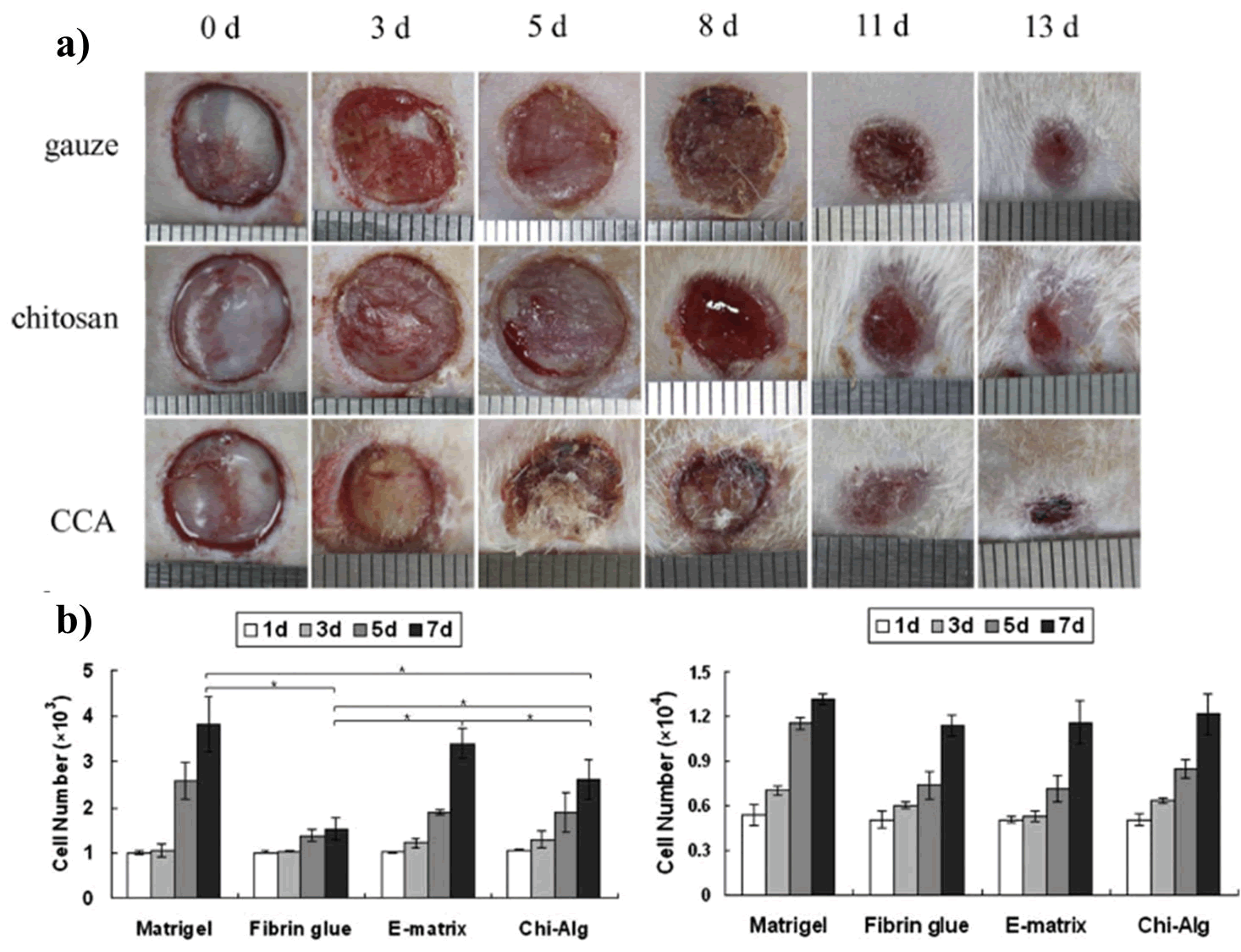
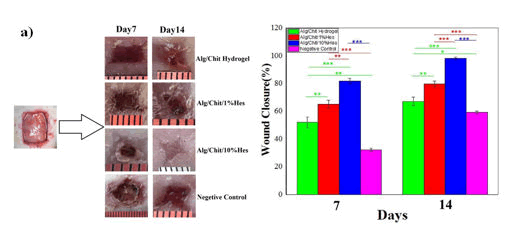
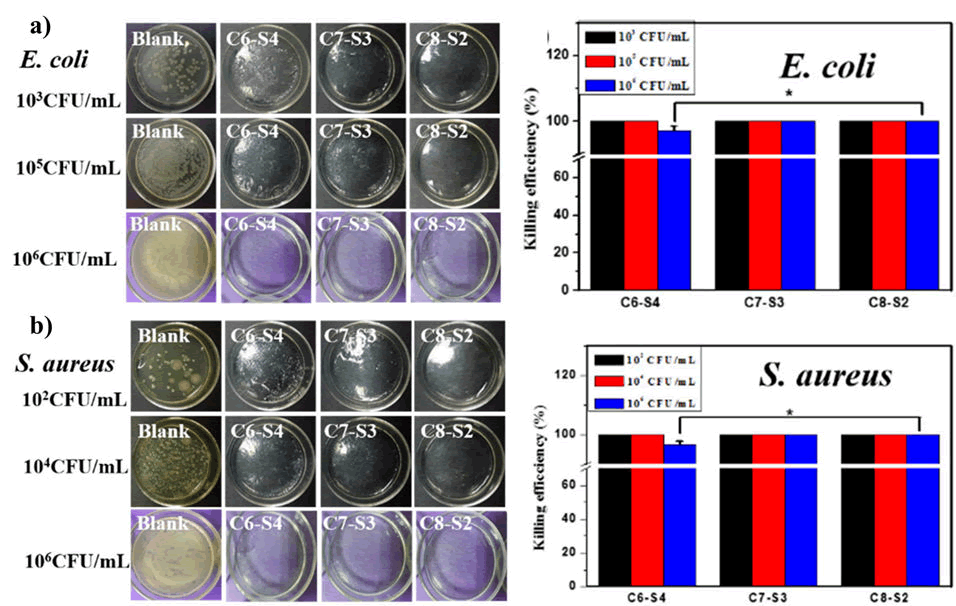
Xie et al., prepared a composite dressing material for wound healing using the combination of Chitosan-Collagen-Alginate (CCA). The material was prepared using paint-coat and freeze-drying techniques. The authors have tested the wound healing potency of CCA composite into a rat wound model. The wound in the rat model was observed to be recovered much faster rate upon treatment with CCA than either chitosan or gauze alone (Figure 6). Moreover, they also reported that the composite dressings had a positive effect on fibroblast and other growth factors growth and facilitated reepithelialization. Chitosan and Alginate (CS-Alg) were integrated to synthesize a hydrogel (CS-Alg) containing 10% hesperidin, a flavonoid present in fruits and vegetables. The hydrogel had a progressive effect on cellular proliferation. The authors have reported a better wound closure and higher antibacterial activity than gauze alone (Figure 7). Another study showcased the ability of chitosan alginate hydrogel that assist in proliferating Olfactory Ensheathing Cells (OECs) and Neural Stem Cells (NSCs) with ease. In this study, Wang et al. compared the ability of the hydrogel along with other commercially available gels (Matrigel®, Fibrin glue, and E-matrix) in cell proliferation study. The data showed that both OECs and NSCs cells were proliferated effortlessly and equally well after treating with newly fabricated hydrogel as they did in the commercially viable alternatives. This provided a promising outcome on the use of the hydrogel complex on the growth and proliferation of neural tissue and might open a new avenue in drug delivery application of the biological polymer-based hydrogels in disorders related to the nervous system. Wei et al synthesized a chitosan alginate hydrogel loaded with growth factors (VE-cadherin and FGF) was synthesized by that exhibited unexceptionable cytocompatibility to L929 and EC cell lines along with near 100% antibacterial activity towards both gram positive and negative bacteria (Figure 8).
Figure 6: (a) Study of CCA material in wound healing, (b) Cellular proliferation of OECs and NSCs cells on CS-Alg hydrogel.
Figure 7: Study of chitosan-alginate hydrogel in wound healing, a) CS-Alg hydrogel containing 10% hesperidin shows maximum wound closure.
Figure 8: a) Antibacterial activity of chitosan-alginate hydrogel against E. coli, b) antibacterial activity of chitosan-alginate hydrogel against S. aureus.
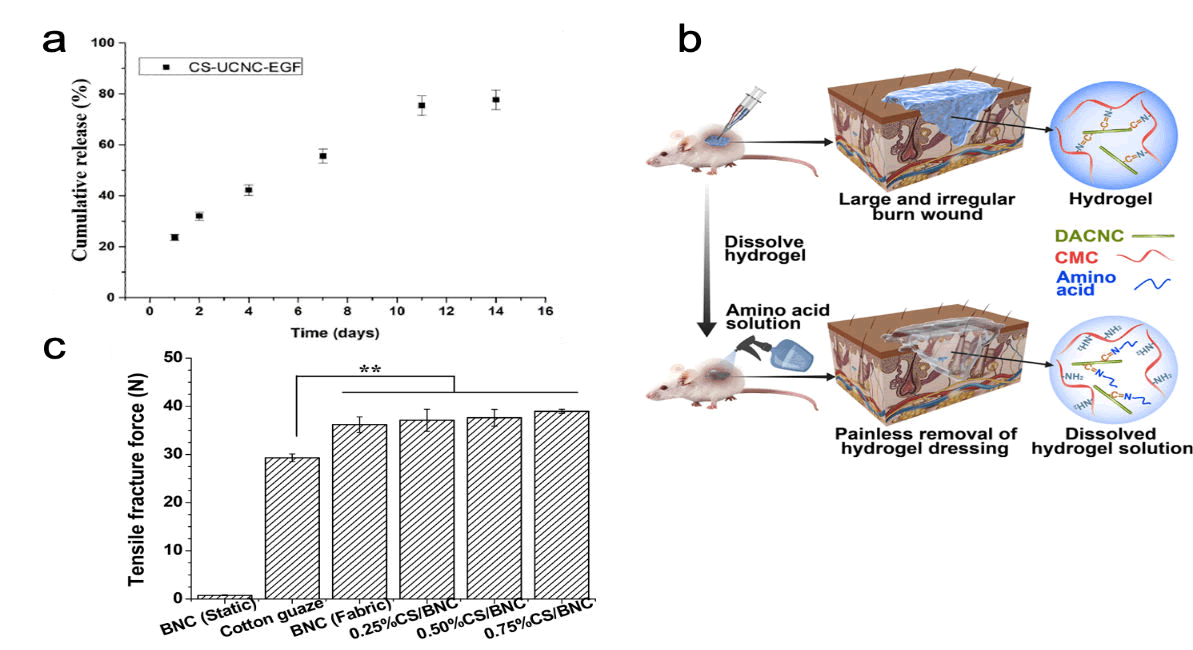
Cellulose: The most abundant naturally occurring biopolymer on earth is cellulose. It is a linear polysaccharide composed of (1, 4)- linked β-D-glucopyranose subunits. Cellulose does not incite an inflammatory response in a foreign body and hence is exceptionally biocompatible. The major sources of cellulose are plants, seaweed, and bacterial species like Komagateibacter, Acetobacter, and Gluconacetobacter. Cellulose derived from wood (nanofibrillar cellulose) is the most cost-efficient material that could be used in biomedical applications and has its distinct advantages like having the ability to be molded into films, hydrogels and also having high elastic modulus. Cellulose from bacterial sources (bacterial cellulose) can absorb high amounts of water and maintain a moist environment. The broad-scale applications of Bacterial Cellulose (BC) are restricted by the fact that being from a microbial source, the biodegradation of BC remains a hurdle due to bacterial fermentation conditions which greatly limits ways to control the porosity of the material using additive materials. Moreover, the applications are also restricted due to inefficient production and under desired strength of the material when swollen. Despite all the hurdles, Zhang et al., fabricated a Chitosan/ Bacterial Nanocellulose (CS/BNC) composite hydrogel as an antibacterial wound dressing material. The composite hydrogel was found to greatly boost the tensile strength of the material as compared to cellulose alone. Moreover, in the lyophilized sponge state, the hydrogel displayed good broad-spectrum antibacterial activity against both gram positive and negative bacteria. Furthermore, the addition of chitosan also improved the water in taking and holding properties of the hydrogel. Chen et al. synthesized a dissolvable self-healing hydrogel using Carboxymethyl Chitosan (CMC) with that of modified form of Dialdehyde Cellulose Nanocrystal (DACNC). The self-healing is anticipated due the presence of various active junctions in the hydrogel which could readily break and reform. The removal of the hydrogel from the wound was rendered effortless as the hydrogel was easily dissolved when exposed to amino acid solution enabling painless removal. The cell viability study of the hydrogel was observed to be as high as 98%, which makes the hydrogel practically non-toxic and safe for biomedical applications. The in vivo wound healing study demonstrated that the hydrogel was effective in treating deep burn wounds after 2 weeks of application of the hydrogel without forming scars (Figure 9). Recently Mariia et al., synthesized a chitosan-ulvan hydrogel in combination with Cellulose Nanocrystals (CNC). The study clearly exhibited substantial improvement in the mechanical stress as well as swelling behavior of hydrogel that is obtained after the incorporation of CNC into chitosan. It has shown 100% wound contraction in 15 days. It is expected owing to the continuous release of Epidermal Growth Factor (EGF) at the wound site in presence of hydrogel.
Figure 9: Significance of chitosan-cellulose hydrogel in wound healing; a) Impact of cellulose nano-crystals into wound site in releasing EGF, b) CMC and DACNC hydrogel showcasing painless removal of hydrogel dressing, c) Improved tensile strength of chitosancellulose hydrogel than cellulose or cotton gauze.
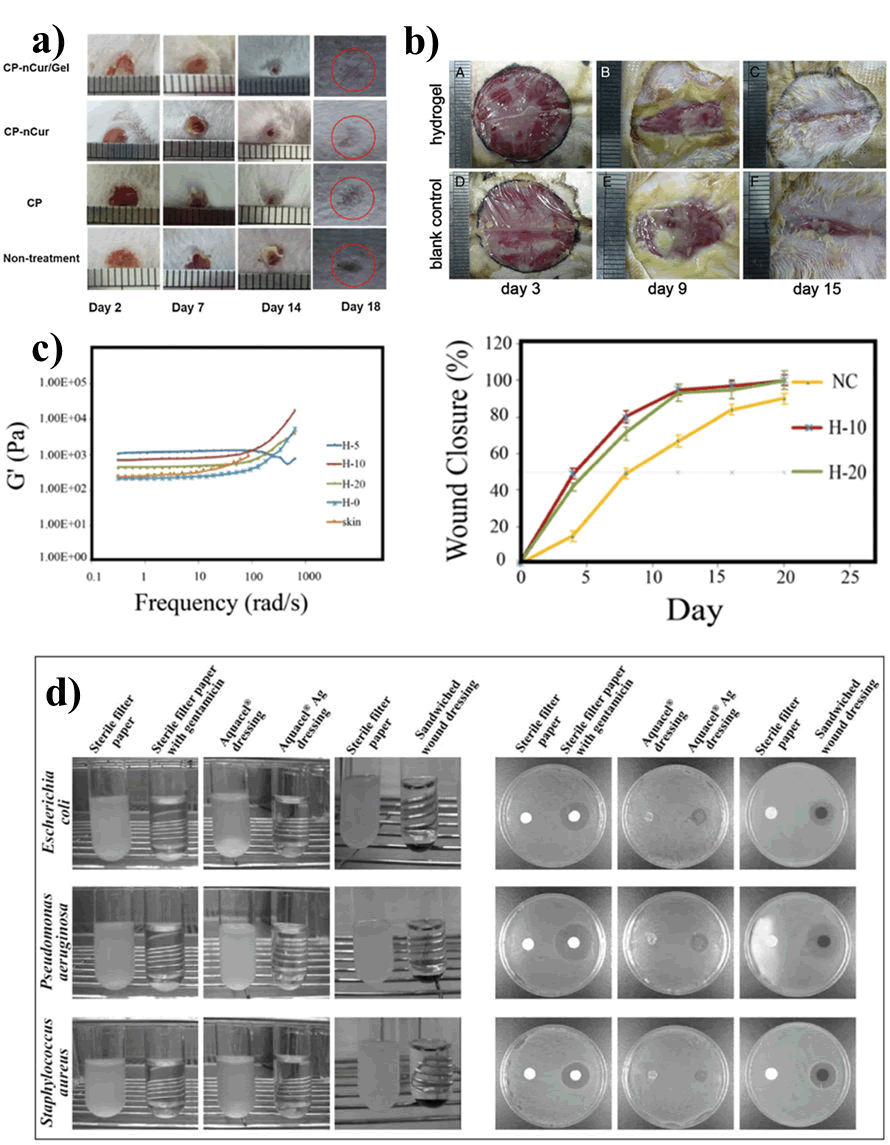
Gelatin: Gelatin, derived from denaturing collagen, is acquired by breaking down the triple helical structure of collagen. Gelatin is typically acquired from bovine and porcine skin. Their skin contains an attractive polymer which has been extensively used in different biomedical application due to its biocompatibility nature. Moreover, these are low cost and can be availed easily. Gelatin has been used for many years as a prosthetic sealant and as a drug delivery carrier because of its excellent biodegradability and non-immunogenicity nature. The major limitations of gelatin are that it is not stable at temperatures at or above 37°C and also it undergoes quick enzymatic degradation. As a result, gelatin is often combined with other natural or synthetic polymers for the use of various biomedical applications. Different studies have been carried out, using chitosan in combination with gelatin for wound healing applications. For example, Pham and co-workers have developed an injectable hybrid hydrogel (Chitosan- Pluronic P123) containing gelatin and curcumin as an effective dressing agent for potential wound healing application. The presence of gelatin and curcumin in the hydrogel, promoted the mobilization of fibroblasts. As a consequence, it leads to faster wound healing at the edge of the wound (Figure 10). They also demonstrated that wound remedial process was faster. It is mainly as due to the presence of hydrogel, which acting as an ECM and also, it assists to keep the wound environment moist and thereby, increasing the epithelial cell migration. Yang et al. developed a hydrogel by the combination of gelatin (aqueous solution) and Carboxymethyl chitosan (CM-chitosan) which encouraged cell attachment and promoted the growth of fibroblasts. Thereby acquiring the ability to accelerating the wound healing process (Figure 11). In another study, Shamloo and co-workers fabricated a physically cross-linked 3D hydrogel using the combination of Chitosan, Gelatin, and PVA (CG-PVA). It was developed by freezing and thawing method. This hydrogel was observed to be highly porous in nature. The authors showed that the microstructure of the hydrogel was found to be quite similar with the ECM structure of the skin. Moreover, the hydrogels incorporated with honey were able to replicate the viscoelastic properties of the native skin tissue. It also displayed 100% wound closure which was observed to be better than gauze alone. This is also biocompatible in nature. Lin et al. developed a nanoparticle for repairing wound using gelatin, chitosan, and epigallocatechin gallate. This nano particle was fused in the outer surface of poly (c-glutamic acid)/gelatin hydrogel. This hydrogel was sandwiched between the nanoparticle, coated in the outer surface, and activated carbon fibers with gentamicin which is incorporated inside the hydrogel to facilitate the wound regeneration. The presence of nano-particle enhances the re-epithelialization process and whereas the presence of activated carbon fibers with gentamicin encourages the antimicrobial activity.
Figure 10: Study of Chitosan and gelatin-based hydrogel in wound healing; a) Study of CP-nCur/Gel hydrogel for wound healing application, b) Study of CM-chitosan and gelatin-based hydrogels for treatment of wound, c) Viscoelastic properties and wound closure behavior CG-PVA hydrogel, d) Antimicrobial study of gallate nanoparticle sandwiched hydrogel material.
Hyaluronic Acid (HA): One of the few natural polysaccharides that are a major component of the extracellular matrix is hyaluronic acid (also known as hyaluronic). It is an anionic non-sulfated glycosaminoglycan, which is intertwined within the extracellular space. HA can be isolated from an animal source or can be synthesized using a bacterial source (Streptococcus sp.). HA aids in wound healing with its ability to interact with cell surface receptors such as CD44, which initiates cellular signaling that regulates cell proliferation and differentiation and cellular migration of multi-potent stem cells and can induce angiogenesis. HA has an inherent ability to grip large amounts of water, which provides strength to the ECM. This capability to absorb water leads to the formation of a hydrogel.
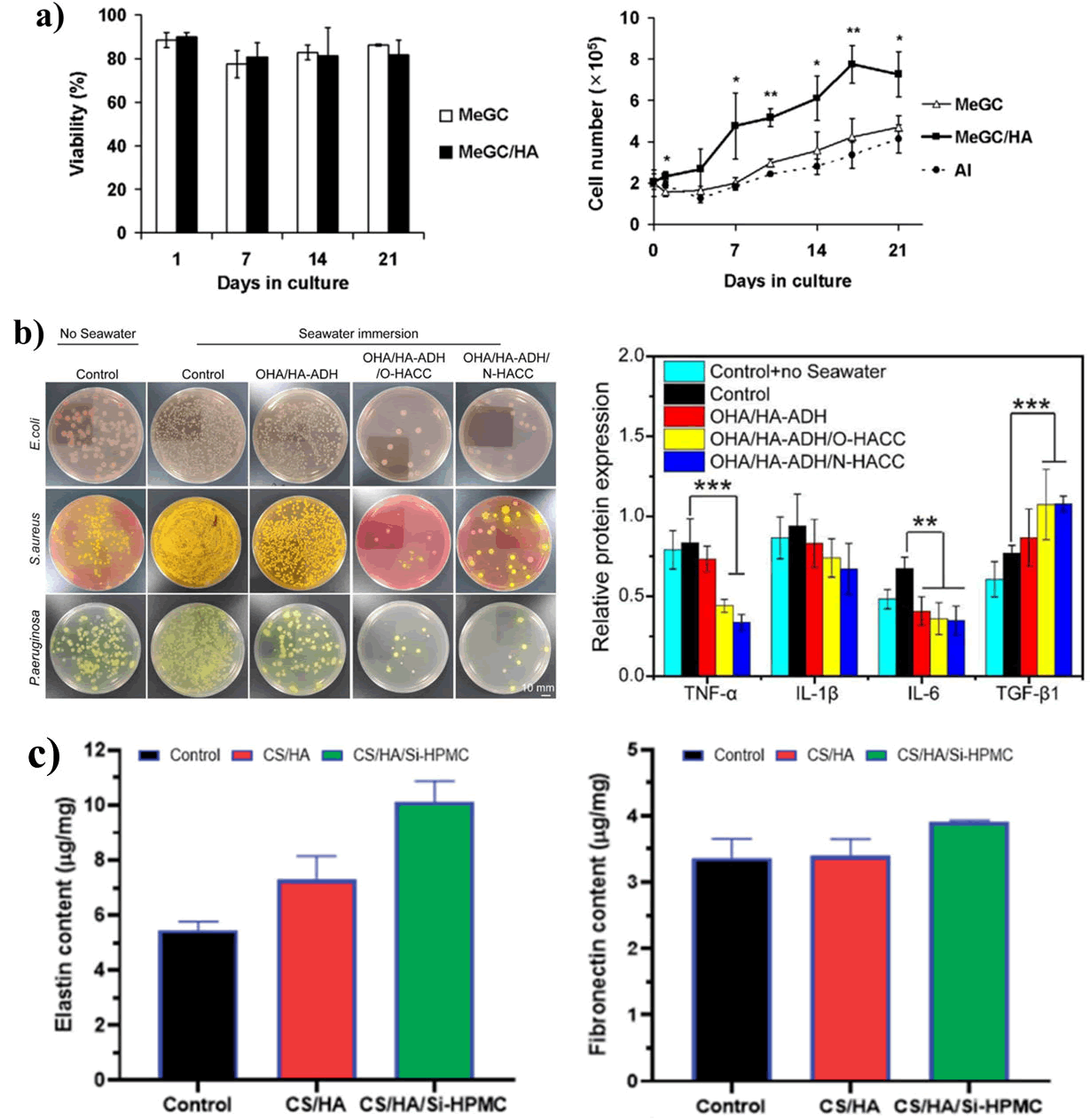
Despite the advantages of the biopolymer, its application is limited by its mechanical weakness and as a result, it has been combined with other polymers to broaden its application [10]. Several unique hydrogels have been fabricated by taking the advantage of polyanionic nature of HA with that of poly-cationic nature of chitosan for wound healing applications. Park et al. fabricated an injectable hydrogel for cartilage repairing. It was prepared by using Methacrylate Glycol Chitosan (MeGC) with HA through a photo-crosslinking reaction with riboflavin. The hydrogel was successfully able to encapsulate and provide a platform for the viability of cartilage cells showing high cytocompatibility (Figure 11). However, it was also observed that the presence of HA in the hydrogel enhanced the proliferation and differentiation properties of the hydrogel. Chitosan- HA hydrogels have been shown to have very good biodegradability with excellent wound healing capacities and antibacterial activity. A similar hydrogel was fabricated using HA, Poly-Vinyl Phosphonic Acid (PVPA), and chitosan (HA-PVPA-CS hydrogel) by Phuc et al., that displayed promising properties in wound healing along with a degradation time of two weeks that provided sufficient time for the regeneration of the ECM framework in the wound environment. An exciting approach to treat seawater immersed wounds was developed by fabricating a hydrogel containing HA with Quaternized Chitosan (QCS). The hydrogel displayed not only antibacterial properties but also enhanced the release of anti-inflammatory cytokines in the wound site. It is observed to be highly biocompatible. The presence of oxidized hyaluronic acid significantly promoted wound closure by promoting re-epithelialization and the presence of quaternized chitosan enhanced the antibacterial properties of the hydrogel, both of which contributed Figure 10: Study of Chitosan and gelatin-based hydrogel in wound healing; a) Study of CP-nCur/Gel hydrogel for wound healing application, b) Study of CM-chitosan and gelatin-based hydrogels for treatment of wound, c) Viscoelastic properties and wound closure behavior CG-PVA hydrogel, d) Antimicrobial study of gallate nanoparticle sandwiched hydrogel material to the healing of sea-water immersed wounds. Recently, Hu et al., fabricated an injectable hydrogel, resulting from the combination CS, HA and Silanized-Hydroxypropyl Methylcellulose (Si-HPMC) for repairing of cartilage tissue. It was observed that the hydrogel assisted in the proliferation of chondrocytes and cartilage ECM. In addition to that, it also improved the tissue organization of the wound environment. Furthermore, the data clearly revealed that the injectable hydrogel significantly enhanced the content of fibronectin and elastin after implantation compared to CS/HA and control, resulting in the improvement of material-cell interaction.
Figure 11: Study of Chitosan/HA-based hydrogels; a) Cell viability study of incorporated chondrocytes in MeGC and MeGC/HA; b) Antibacterial activity and protein expression levels of pro inflammatory cytokines of HA and QCS; c) Comparative study of cell-materials interaction between CS/HA hydrogel, Si-HPMC hydrogel and control.
Silk Fibroin (SF): It is a natural protein-polymer obtained mostly from silk worms. It has been used in numerous biomedical applications. It is attributed to have various attractive features. Among these, two paramount qualities of SF that attract the scientific communities are; its ability to promote tissue formation, and its excellent biodegradability nature. SF has exceptional thermal stability and flexibility as compared to other polymers such as gelatin and collagen. Moreover, since SF contains the RGD (Arg-Gly-Asp) tripeptide sequence, it can help in the cellular adhesion, and proliferation of various types of cells that further strengthens its versatility. However, one of the major disadvantages of SF is its susceptibility of its α-sheets that provide mechanical strength to enzymatic degradation of various proteases (MMPs, Proteinase K etc.). Hence, it becomes quite evident to combine SF with other polymers for use in biomedical applications. The material derived from SF in combination with chitosan has been explored in wound healing applications.
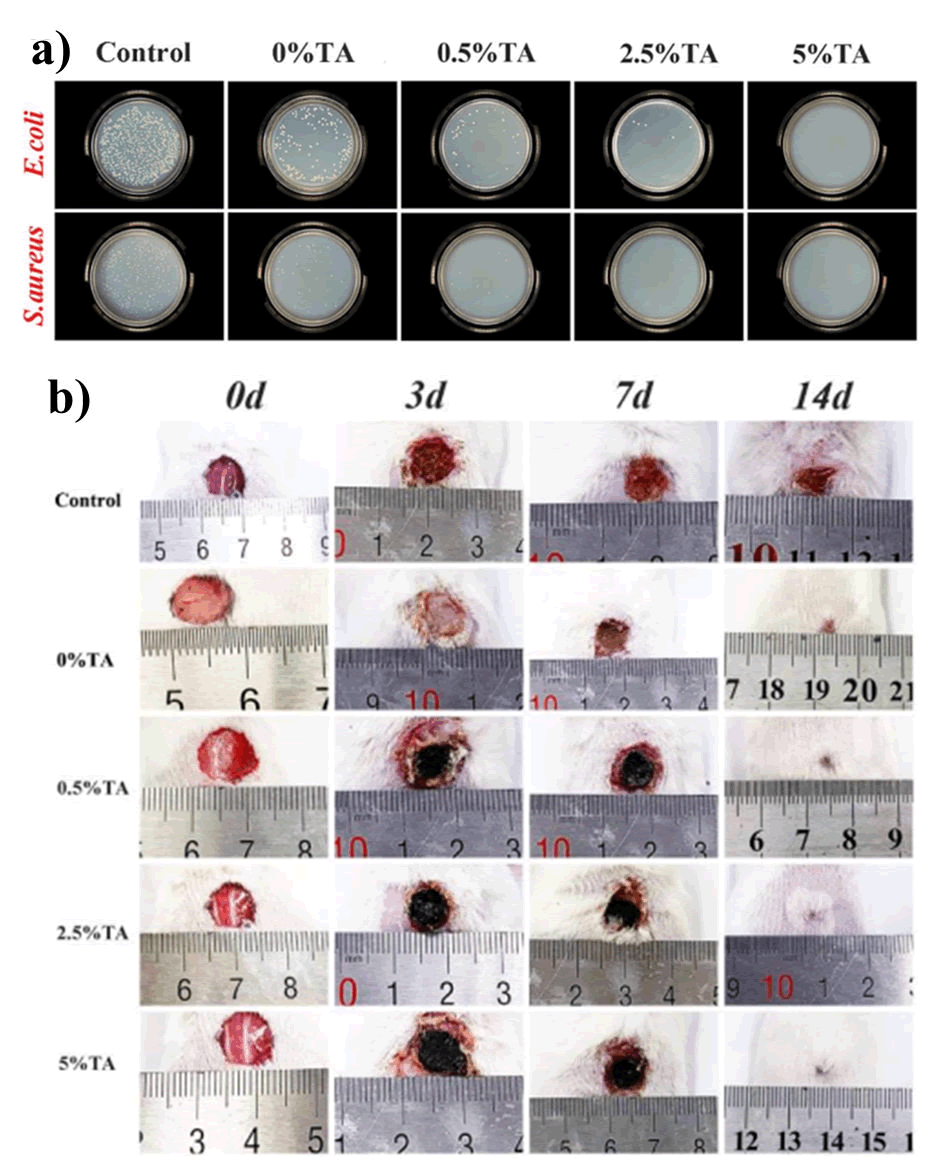
He et al., fabricated a novel Tannic acid (TA)-reinforced Methacrylated Chitosan (CSMA)/Methacrylated Silk Fibroin (SFMA) hydrogels. The hydrogels significantly promoted wound healing in a full-thickness skin defect model (Figure 12). Moreover, the presence of Tannic acid significantly improved antibacterial activity as well as fibroblast cytocompatibility.
Figure 12: Therapeutic effect of chitosan-silk fibroin-based hydrogels; a) Antibacterial studies of CMSA/SFMA-TA hydrogels at varying percentage of TA; b) Progressive monitoring (0-14 days) of wound healing effect of CMSA/SFMA-TA hydrogels at varying percentage of TA.
Discussion
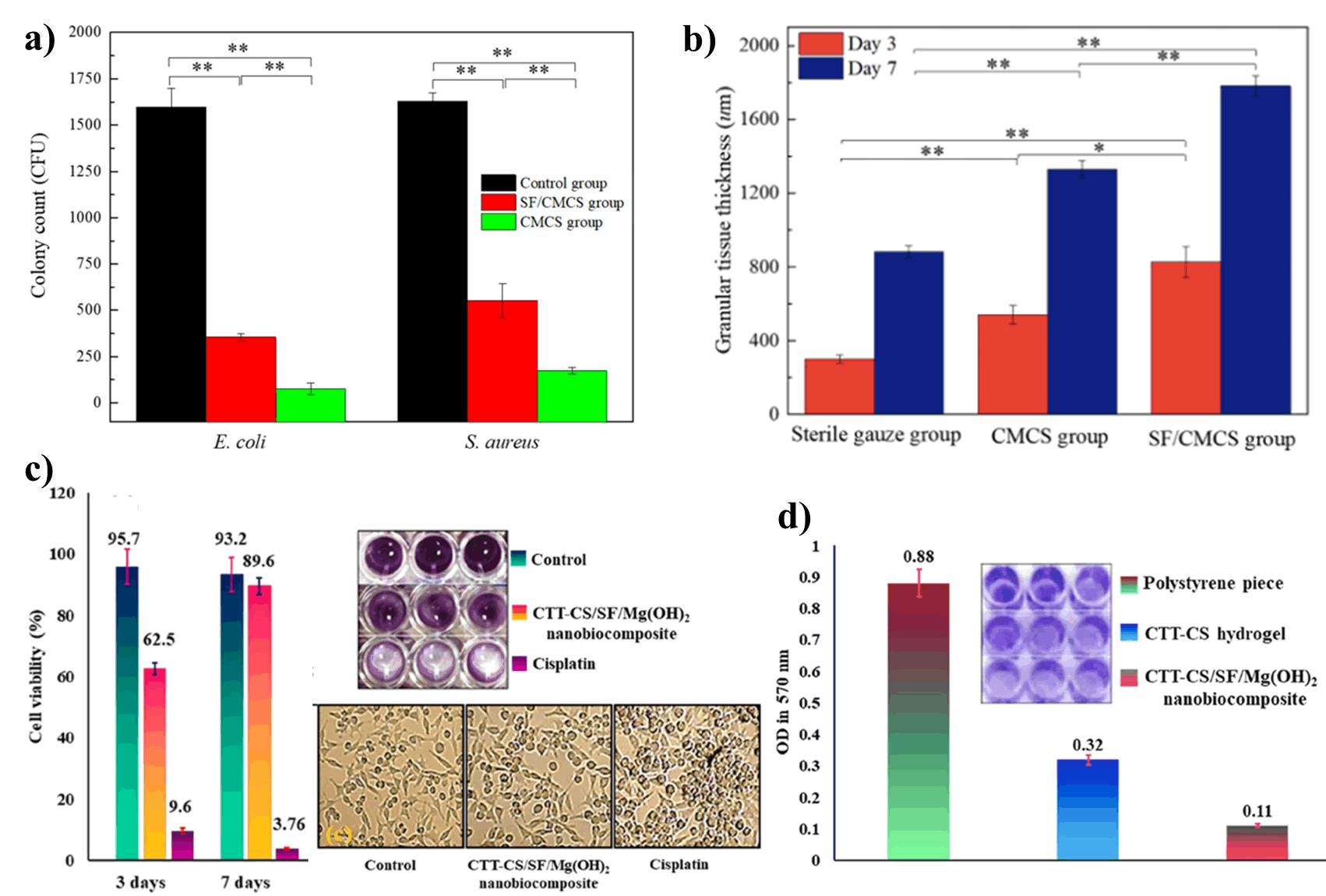
In another study, Chen and coworkers developed a hydrogel (SF/ CMCS) by cross-linking SF with Carboxymethyl Chitosan (CMCS) through hydrogen bonding, then co-deposited on the graphite electrode under low voltage. They observed that the SF/CMCS hydrogels displayed antibacterial activity against Escherichia coli and Staphylococcus aureus as well as promoted wound re-epithelization and granulation tissue formation (Figure 13). A nano-biocomposite of Mg(OH)2 nanoparticle was synthesized using the combination of Cross-linked Terephthaloyl Thiourea-Chitosan hydrogel (CTT-CS hydrogel) with SF biopolymer by Keihan and co-workers. It strongly inhibits the bio-film formation on the surface of P. aeruginosa. The scaffold also demonstrated increased cell viability which expressed its excellent biocompatibility.
Figure 13: Effect of chitosan and silk fibroin-based hydrogel; a) Antibacterial activity of SF/CMCS, and CMCS hydrogels; b) Comparative study of granulation tissue formation of SF/CMCS, CMCS and sterile gauze group; c) Cell viability study of CTT-CS/SF/ Mg(OH)2 and inverted microscopic images; d) Anti-biofilm study of CTT-CS/SF/Mg(OH)2 nano-biocomposite.
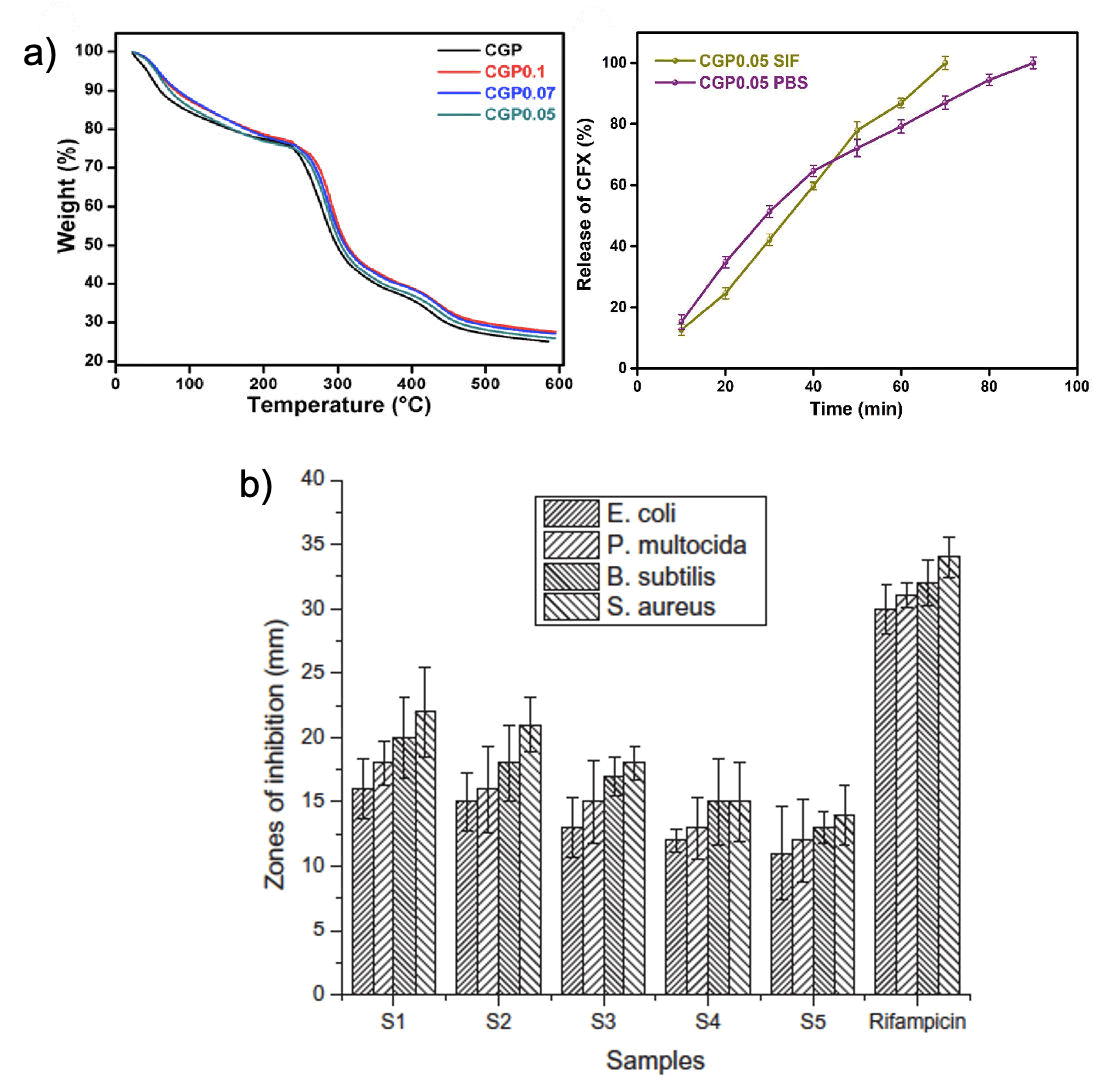
Guar gum: Guar Gum (GG) is a naturally occurring water-soluble polysaccharide obtained from the embryos of the leguminous plant Cyamopsis tetragonoloba. It contains a linear polymeric chain linking at the 1-4 position of repeated unit of β-D-mannopyranose and having a side chain linking at the 1-6 position of α-D-galactopyranose. The presence of large number of hydroxyl groups in its structure allows GG to undergo forming hydrogen bond in aqueous solution and renders much of its versatility. It is extensively used as a thickener, stabilizer, emulsifier, and binder etc. in diverse industries. Taking the numerous advantageous properties of GG including; biocompatibility, biodegradability, and non-toxicity nature etc, it has been extensively utilized in various wound healing applications. In addition to that, it has low antigenicity and shown strong interaction with mucus lining tissue indicating localized interaction. Due to its low mechanical properties, Guar gum hydrogels are found to be easily degradable in nature. Therefore, it has impacted the usability in wound healing application. However, it has been observed that the modified GG hydrogel in combination with other polymer enhances its usability. In the recent years, several GG-based hydrogels are fabricated and demonstrated their wound healing applications to an appreciating standard. Ghauri et al., have recently developed a pH-responsive hydrogel using chitosan/guar gum/polyvinyl pyrrolidone through a cross-linking reaction of sodium tripolyphosphate as a linker. The hydrogel was found to be thermally stable, with the ability to release drug molecule sustainably without tampering with the native structure of the drug. Moreover, the hydrogels were also shown to be bacteriostatic (Figure 14). In another study, Iqbal and co-workers have developed a series of polymeric blended membranes, using CS, GG, and Polyvinyl Alcohol (PVA) for sustainable delivery of antibacterial drugs. It was observed that the hydrogels were highly cyto-compatible as they do not lyse the red blood cells. In addition to that, hydrogels were shown notable antibacterial activity. Another series of CS/PVA/ GG hydrogels were prepared by Iqbal et al. that showed promising antibacterial activity against a wide range of bacterial species.
Figure 14: Study of chitosan and guar gum-based hydrogels; a) Thermal stability and drug release study of pH-sensitive biodegradable hydrogel (CS/GG/PVP) hydrogels, b) Antibacterial activity of (CS/ PVA/GG) hydrogels.
Conclusion
This review addresses the various properties of chitosan and the recent advances in chitosan-natural polymer-based hydrogels as potential wound healing materials. Chitosan, due to its intrinsic wound antibacterial ability and exceptional biocompatibility, and biodegradable nature has been used as wound healing material either alone or in combination with drugs and other natural or synthetic biomaterials. The major justification for the development of any wound healing material is to expedite the wound healing process and to alleviate the pain of the patient. As the science of wound dressings has evolved, the use of wound healing materials, as well as therapeutic agents has been fine-tuned to optimize the wound healing process. For this, chitosan has evolved as one of the better biomaterials owing to its above-mentioned properties. Taking the above advantages of Chitosan, in the recent year Chitosan and natural polymers-based hydrogels have been used as wound dressings for acute as well as chronic wounds as they can release therapeutic drugs, as well as growth factors to increase its efficacy. Moreover, the hydrogels can also be modified by combining with other biomaterials to be used as a delivery system for a wide variety of substances including but not restricting to growth factors, antibiotics, peptides, stem cells and release them in sustained methods. This article showcases the recent advances in the use of various chitosan and natural polymers-based hydrogels as wound healing materials. We think that chitosan and natural polymers-based hydrogels have a strong potential for clinical applications as wound healing materials and this article can provide helpful insight to researchers in biomedical and pharmaceutical fields to use these hydrogel as novel biomaterials as a wound healing process. In addition to that, this review article will open new avenue for researches to craft new type of hydrogels system for the treatment of wounds.
Conflicts of Interest
The authors declare that they have no conflict of interest.
Acknowledgements
The authors would like to gratefully acknowledge the support from DST (New Delhi), IISc (Bengaluru, Karnataka), ICMR-Regional Medical Research Center (BBSR, Odisha), and CUTM (BBSR, Odisha).
References
- Falcone M, de Angelis B, Pea F, Scalise A, Stefani S, et al., (2021) Challenges in the management of chronic wound infections. J Glob Antimicrob Resis 26: 140-147.
[Crossref] [Google Scholar] [PubMed]
- Mahmoudi M, Gould LJ (2020) Opportunities and challenges of the management of chronic wounds: a multidisciplinary viewpoint. Chronic Wound Care Management and Research. 27-36.
- Ding X, Tang Q, Xu Z, Xu Y, Zhang H, et al. (2022) Yin S. Challenges and innovations in treating chronic and acute wound infections: From basic science to clinical practice. Burns Trauma 10: t kac014.
[Crossref] [Google Scholar] [PubMed]
- Edwards R, Harding KG (2004) Bacteria and wound healing. Curr Opin Infect Dis 1: 91-96.
[Crossref] [Google Scholar] [PubMed]
- Jiang L, Loo SC (2020) Intelligent nanoparticle-based dressings for bacterial wound infections. ACS Appl Bio Mater 4: 3849-3862.
[Crossref] [Google Scholar] [PubMed]
- Di Lodovico S, Bacchetti T, D’Ercole S, Covone S, Petrini M, et al. (2022) Complex chronic wound biofilms are inhibited in vitro by the natural extract of Capparis spinose. Front Microbiol 13: 832919.
[Crossref] [Google Scholar] [PubMed]
- Kim MH (2016) Nanoparticle-based therapies for wound biofilm infection: Opportunities and challenges. IEEE Trans Nanobioscience 15: 294-304.
[Crossref] [Google Scholar] [PubMed]
- Winter GD (1962) Formation of the scab and the rate of epithelization of superficial wounds in the skin of the young domestic pig. Nature 193: 293-294.
[Crossref] [Google Scholar] [PubMed]
- Bhattarai N, Gunn J, Zhang M (2010) Chitosan-based hydrogels for controlled, localized drug delivery. Adv Drug Deliv Rev 62: 83-99.
[Crossref] [Google Scholar] [PubMed]
- Liu H, Wang C, Li C, Qin Y, Wang Z, et al. (2018) A functional chitosan-based hydrogel as a wound dressing and drug delivery system in the treatment of wound healing. RSC Adv 8: 7533-7549.
[Crossref] [Google Scholar] [PubMed]
- Xia G, Zhai D, Sun Y, Hou L, Guo X, et al. (2020) Preparation of a novel asymmetric wettable chitosan-based sponge and its role in promoting chronic wound healing. Carbohydr Polym 227: 115296.
[Crossref] [Google Scholar] [PubMed]
- Sood A, Granick MS, Tomaselli NL (2014) Wound dressings and comparative effectiveness data. Adv Wound Care 3: 511-529.
[Crossref] [Google Scholar] [PubMed]
- Fan Z, Liu B, Wang J, Zhang S, Lin Q, et al. (2014) A novel wound dressing based on Ag/graphene polymer hydrogel: Effectively kill bacteria and accelerate wound healing. Adv Funct Mater 24: 3933-3943.
- Rezvani Ghomi E, Khalili S, Nouri Khorasani S, Esmaeely Neisiany R, Ramakrishna S (2019) Wound dressings: Current advances and future directions. J Appl Polym Sci 136: 47738.
- Tavakoli S, Klar AS (2020) Advanced hydrogels as wound dressings. Biomolecules 10: 1169.
[Crossref] [Google Scholar] [PubMed]
- Ahmed EM (2015) Hydrogel: Preparation, characterization, and applications: A review. J Adv Res 6: 105-121.
[Crossref] [Google Scholar] [PubMed]
- Sahiner N, Sagbas S, Sahiner M, Silan C, Aktas N, et al. (2016) Biocompatible and biodegradable poly (Tannic Acid) hydrogel with antimicrobial and antioxidant properties. Int J Biol Macromol 82: 150-159.
[Crossref] [Google Scholar] [PubMed]
- Amsden B (2015) Novel biodegradable polymers for local growth factor delivery. Eur J Pharm Biopharm 97: 318-328.
[Crossref] [Google Scholar] [PubMed]
- Lee KY, Mooney DJ (2001) Hydrogels for tissue engineering. Chem Rev 01: 1869-1880.
[Crossref] [Google Scholar] [PubMed]
- Tessmar JK, Gopferich AM (2007) Matrices and scaffolds for protein delivery in tissue engineering. Adv Drug Deliv Rev. 59: 274-291.
[Crossref] [Google Scholar] [PubMed]
 Spanish
Spanish  Chinese
Chinese  Russian
Russian  German
German  French
French  Japanese
Japanese  Portuguese
Portuguese  Hindi
Hindi