Research Article, Int J Ment Health Psychiatry Vol: 9 Issue: 2
Retrospective Chart Review Comparing MeRT and Standard rTMS Depression Protocols
Maxwell W. Hand1* and Mark Liker2
1California Neurological Institute, Brain Stim Center, Valencia, USA
2Department of Neurosurgery, University of Southern California, Los Angeles, USA
*Corresponding Author: Maxwell W. Hand
Department of Neurosurgery,
California Neurological Institute, Brain Stim Center, Valencia, USA
E-mail: maxwell_hand@yahoo.com
Received date: 25 March, 2023, Manuscript No. IJMHP-23-92789;
Editor assigned date: 27 March, 2023, PreQC No. IJMHP-23-92789 (PQ);
Reviewed date: 10 April, 2023, QC No. IJMHP-23-92789;
Revised date: 25 May, 2023, Manuscript No. IJMHP-23-92789 (R);
Published date: 01 June, 2023, DOI: 10.4172/2471-4372.1000221
Citation: Hand MW, Liker M (2023) Retrospective Chart Review Comparing MeRT and Standard rTMS Depression Protocols. Int J Ment Health Psychiatry 9:2.
Abstract
Transcranial Magnetic Stimulation (rTMS) has been widely explored as an effective therapeutic tool for treating a wide range of persistent difficult to treat neurological and physiological disorders. Currently, TMS studies are focused on intervening in cases of severe mental health disorders (Depression, OCD, PTSD, etc…). While there are an increasing number of studies which look at different applications of TMS, there has been little research on how different approaches compare across each modality.
This paper aimed to compare the effectiveness of two unique approaches in treating MDD with rTMS. Intermittent Theta- Burst Stimulation (iTBS) and Magnetic e-Resonance Therapy (MeRT).
Major Depressive Disorder patients who came in for treatment were given PHQ-9 assessments every week for the duration of their treatment session. The pre and post PHQ-9 scores were analyzed from both stimulation groups and compared. Nexstim NBT 2 TMS chair and coil used for all patients.
Both methods resulted with individually significant decrease in patient PHQ-9 scores, but there was no inter-method differences observed. Both protocols resulted in about 60% of patients experiencing significant reductions in PHQ-9 scores.
Retrospective comparison of these methods showed no significant difference in effectiveness in treating MDD. Both iTBS and MeRT TMS approaches reveal insight into the pathology of depression as a leading health concern. Further research should include larger numbers of patients as well as increasing the homogeneity of the population. No significant difference may point to the conclusion that we do not completely understand the complexity of MDD and should further investigate about the structural vs. functional aspect of this disorder.
Keywords: rTMS, Neuromodulation, Major depressive disorder, Depression, Retrospective chart review
Abbreviations
TMS: Transcranial Magnetic stimulation; rTMS: repetitive Transcranial Magnetic Stimulation; spTMS: singlepulse Transcranial Magnetic Stimulation; iTBS: intermittent- Theta Burst Stimulation; MDD: Major Depressive Disorder; trMDD: treatment resistant Major Depressive Disorder; MeRT: Magnetic e-Resonance Therapy; DLPFC: Dorsolateral Prefrontal Cortex; ACC: Anterior Cingulate Cortex; EEG: Electroencephalogram; QEEG: Quantitative Electroencephalogram; DTI: Diffusion Tensor Imaging; MRI: Magnetic Resonance Imagine; BOLD: Blood Oxygen Level Dependent; fMRI: Functional Magnetic Resonance Imaging; PET: Positron Emission Tomography; GAD: General Anxiety Disorder; PHQ-9: Personal Health Questionnaire 9; LTP: Long Term Potentiation; rMT: Resting Motor Threshold; APB: Abductor Pollicis Brevis ; MEP: Motor-Evoked Potential; EMG: Electromyograph.
Introduction
Michael Faraday was one of the first scientists to discover the concept of electromagnetic induction in the early 1800’s [1]. Technology began to develop around this concept in the form of Electro-Convulsive Therapy (ECT), Transcranial Electric Stimulation (TES), and eventually Transcranial Magnetic Stimulation (TMS). The first TMS device was invented in 1985 by a scientist named Anthony Barker, successfully showing that Faraday’s principle could be applied as a tool to alter the electrical signaling in the human brain.
Over the past two decades, Transcranial Magnetic Stimulation (TMS) has grown in popularity as a therapeutic tool for persistent and difficult to treat neurological and psychiatric disorders. Several types of TMS systems, coils, chairs, and equipment have been created since the first successful application of altering human brain function using TMS.
Medical devices have been approved by multiple governing bodies around the world to apply rTMS in a variety of ways. As of 2022, the disorders that have been approved by the U.S. food and drug administration are depression (2008), obsessive compulsive disorder (2018), and migraines/headaches (2013). Different medical devices have been approved for each of these illnesses due to the complexity and differences in each pathology.
Research has been conducted on several successful applications of TMS, but there is an overall tendency towards quantity over quality. This study hopes to add to the growing number of papers looking at the specific parameters needed in rTMS to improve the current therapeutic applications. The need for optimization of current approaches is more relevant than ever.
Additionally, there is a gap in current literature revealing the differences between clinical results and experimental results. There is a great deal of complexity behind neurological and physiological disorders, so there is only so much that can be concluded from tightly regulated experimental groups. One of the advantages of this study is that it puts into perspective the difficulties faced when treating real patients in a clinical setting.
TMS uses a changing magnetic field to induce an electrical charge across an insulate surface bone. This electrical charge has been shown to alter the excitability of neurons in the human cortex when the coil is placed against a patient’s head [2]. This study, as well as other forms of TMS therapy, uses a series of repeated stimulations to induce deeper and longer lasting effects-called rTMS or repetitive TMS [3-5]. rTMS has been shown to mimic the effects of neural plasticity as well as Long Term Potentiation (LTP) [6]. One more complexity that rTMS faces over single pulse TMS is that there are more variables to account for during stimulation.
There are multiple parameters to vary, of which intensity, location, and frequency (Hz) is some of the most important. Depending on the frequency, rTMS can have either an excitatory or inhibitory effect on the targeted neurons. Typically, it is thought that 1 Hz and below will result in an inhibitory effect, while 5 Hz and above will result in an excitatory effect [7]. Deciding which frequency to administer depends on the desired therapeutic effect as well as the neurophysiological state of the treated patient.
Major Depressive Disorder (MDD) was the leading cause of disability in 2017 in people between the ages of 15 and 44 [8]. While there is a wide range of therapeutic options for the purposes of treating MDD, there are a high percentage of people who are considered treatment resistance. Up to 30% of people diagnosed with MDD are classified as treatment resistant, meaning that they respond poorly or not at all to standard methods of therapy (antidepressants, psychotherapy, etc…). Many in this treatment resistant population are looking for alternative therapies. Additionally, there has been a movement away from reliance on medication. rTMS has been studied at length and is an FDA approved option for patients who are classified as treatment resistant.
The pathology of depression has been thoroughly studied as a disorder caused by underactive neuron activity in certain brain regions. The Dorsolateral Pre-Frontal Cortex (DLPFC) and the Anterior Cingulate Cortex (ACC) are two brain regions of interest in MDD studies [9-11]. The DLPFC and the ACC are connected through a series of pathways in the brain, as such; this overall pathway is referred to as the depression network. Brain imaging studies have shown that hypoactivity in the left-DLPFC has a strong correlation with patients who suffer from depression [12].
Standard rTMS protocols use this depression network to treat major depression, targeting the DLPFC with varying parameters to excite the underactive neurons. Most rTMS coils can only reach the outer cortex, due to a diminishing electrical induction effect weakening by the square of distance traveled. The focal point of a rTMS coil is typically within 5 mm from the actual coil, meaning that standard rTMS is not able to stimulate subcortical brain regions like the ACC.
While there is already significant research for the application of rTMS on the DLPFC, more recent research that takes an alternative approach. This newer approach uses lobe specialization as well as electrical signals from a patient’s brain. This approach targets emotional and higher functioning areas located in the frontal lobe to treat MDD.
This approach is named, Magnetic e-Resonance Therapy (MeRT), and utilizes quantitative analysis of a patient’s Electro-Encephalogram (EEG) to personalize the treatment. Quantitative EEG or qEEG is a diagnostic tool used to read the amplitude and frequency of brain waves. MeRT focuses on Alpha waves, which are most common in states of rest and relaxation and oscillate in the frequency range 8-12 Hz. People who have suffered brain injuries, emotional or physical trauma, and different forms of neurological disorders may have irregular brain waves that are produced and received across different brain regions or dysrhythmia. Dysrhythmia in alpha waves is believed to be associated with higher incidents of MDD and other neurological disorders [13].
Taken together with the different target location and the qEEG, one of the advantages of MeRT is its ability in personalizing each treatment. Levy and Crawford explain how “MeRT Stimulation is an individualized TMS treatment protocol, which utilizes individual’s intrinsic alpha EEG frequency and its closest frequency relationship with the higher harmonic of heartbeat to determine the magnetic stimulus rate [14]. Stimulus location was set at the most apparent abnormal EEG site revealed by quantitative EEG mapping.
Standard rTMS protocols assume that the average human brain oscillates around 10 Hz, which is true, but very limiting and varies with age. A therapeutic tool which targets the average, may be able to treat a large percentage of the population, but will fall short in treating patients which have outlying brain frequencies.
For personal as well as community benefit, this study looks to further understand the differences between the two different rTMS MDD protocols. This is a pilot study whose results will be relevant to future rTMS and depression studies.
Materials and Methods
Clinical data from twelve patients diagnosed with treatment resistant Major Depression Disorder (trMDD) was retrospectively analyzed. These patients underwent treatment during a twelve-month period (June 2020 to June 2021). The mean age of participants was 48.7 with a range of 19-77 years (83% female). The age range of male subjects was 54-68 years and the age range of female subjects 19-77 years. Patients were enrolled based on (trMDD) psychiatric evaluation and had not previously undergone any form of TMS treatment.
Patients were given one of two protocols based on psychiatric evaluation and medical professional advisement: Standard, which includes either 3-minute intermittent Theta-Burst Stimulation (iTBS) or 19-minute 10 Hz left DLPFC protocols, or Magnetic e-Resonance Therapy (MeRT).
One female (77 years of age) patient received both protocols 3 minute iTBS then MeRT, with a 3 month intertreatment period. The 3- minute iTBS and 19-minute 10 Hz standard protocols were used interchangeably, as studies have shown that these two protocols show no significant difference in therapeutic outcome [15]. Any patients in the standard category ended with the standard protocol, initially started with the 3-minute iTBS protocol but were moved to the longer 19 minute 10 Hz protocol if the stimulation intensity reached over 40. This change of protocol was used to limit the discomfort of the patient, as the 19-minute protocol is administered at a lower frequency over a longer period.
Patients in both protocol groups received an MRI before initial mapping and treatment. Initial motor threshold mapping involved defining each patient’s resting motor threshold (rMT). Resting motor threshold was measured by performing an Electromyograph (EMG) of each patient’s Abductor Pollicis Brevis (APB) muscle, located in their right hand, and simultaneously stimulating the contralateral primary motor representation of the ABP muscle. Single-pulse Transcranial Magnetic Stimulation (spTMS) was used to elicit a Motor-Evoked Potential (MEP) in the right APB muscle and locate a precise target for motor threshold determination. Stimulation intensity was increased at a gradual rate until an appropriate MEP was shown on the electromyograph (between 15-25 ms of latency and 100-1000 mV amplitude) and could be reproduced multiple times at that intensity. When an appropriate brain region was found for the APB representation, the coil was held over the exact target (within 5 mm) and varying stimulation intensities were administered. This process was automated through the Nexstim TMS machine which varied the stimulation intensities until one was found that elicited a significant MEP of the APB muscle 50% of the time. This rMT was used to determine the maximum intensity that was safe to deliver over the frontal cortex.
Images from the patient’s MRI were used to create a 3-dimentional cortical representation which was used to target specific brain regions and increase accuracy and precision throughout the entire treatment. Initial mapping differed slightly between the protocol types: Standard mapping involved located the DLPFC using the method and the MeRT mapping involved locating an EEG node, either FpZ or Fz. FpZ and Fz were found by measuring the distance between the nasion and inion of each patient and using the 10-20 method [16]. The nexstim Navigated Brain Therapy System (NBT) 2 chair with a figure of 8 cooled coils was used for all treatments in this study.
The TMS treatment involved 36 sessions of varying time, depending on the protocol. The iTBS is a 3-minute protocol: 50 Hz, 120% intensity goal, 3 pulses per burst, 10 bursts per train, 160 ms inter-burst interval, 20 trains, 8000 ms inter-train interval. The 19- minute protocol is longer with a lower frequency: 10 Hz, 120% intensity goal, 40 pulses per burst, 75 bursts per train and 11000 ms inter-burst time, 1 train. Both standard protocols were administered over the DLPFC, while the MeRT protocol was administered over either the FpZ or Fz electroencephalogram node. The parameters for the MeRT protocol were different for each patient, but overall included a duration time between 29-30 minutes, frequency range from 9-12 Hz, and varying numbers of pulses, trains, and bursts. The MeRT protocol parameters were determined using quantitative analysis of a 20 channel Zeto EEG headset (Santa Clara, CA). EEG results were evaluated by Wave Synchrony (Newport Beach, CA) software and analyzed based on their proprietary computer algorithm. The parameters that were produced correlated with the hypothetically optimal target and range based on the electrophysiological status of each patient.
All participants underwent a psychiatric evaluation prior to treatment. Case notes on patient’s well-being were taken daily and involved before, during, and after session notes about patient wellbeing and any experienced or noticed side effects. The personal health questionnaire 9 (PHQ-9), which is used to assess the current level of depression, was obtained from each patient after initial mapping and once weekly after that for the duration of the treatment.
A decrease of 50% from initial PHQ-9 depression severity score to final PHQ-9 depression severity score was used as the measure of response rate for rTMS therapeutic effect on MDD.
Results
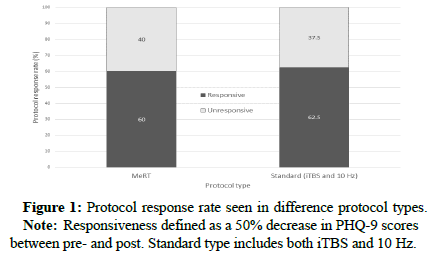
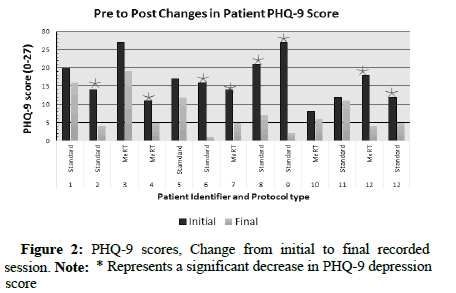
Both protocol groups resulted in a clinically significant decrease in PHQ-9 scores between the initial scoring and final scoring. Initial review of this data showed a similar response rate between both protocol groups, around 60% (Figures 1 and 2).
The difference between the two protocol groups was not significant, so it can only be reported as preliminary and retrospective.
One patient received both protocols, with a three-month intertreatment period, and experienced a significant reduction of PHQ-9 scores from both MeRT and iTBS. The significance of these results comes from repeatedly testing each protocol under different conditions and different patients to show that they have consistent results in decreasing depressive symptoms. These results are not novel in the world of TMS therapy, but they are very important to further solidify the evidence of rTMS therapeutic effects on MDD.
Discussion
This study is retrospective and preliminary. It highlights very interesting trends in the world of rTMS therapy. Both protocol types show a clinical response as a therapeutic tool for treating MDD. While both protocols in treating MDD with rTMS show significant results, they employ different approaches. This information shows that depression does not result from a single focus of abnormal activity in the brain. Instead, mental health may be seen as an error or difference in a region of brain circuitry. With increasing rates of mental illness [9-10] there is a huge need for improved methods of treating psychological disorders like MDD and General Anxiety Disorder (GAD). Mental illness is a fluid concept as no two people’s symptoms or affliction are alike, so the therapies which are used to these disorders need to personalize to the disease pattern.
Fundamentally, the neurochemistry of depression varies widely between different people [17]. It has been customary for the past two centuries to use medication as the first line of therapy for people with psychological or emotional disorders, but there is always concern about the interaction between different medication and their attending side effects on those who take them.
Side effects were not discussed in this paper due to limited reporting. The most common side effects are minor headaches and fatigue which can be explained by the effect of the electromagnetic pulses on the brain and local musculature. Both protocols show similar side effects in previous literature, so the question about whether different side effects play any part in the effectiveness of treatment may be a productive line of questioning. The difference in intensity between the two protocols is another interesting direction for future studies.
Further research is needed to explore the difference between the two protocols, incorporating more stringent study parameters as well as larger populations. Larger studies can give a clearer description of how each of these protocols affects a patient and how they differ in effectiveness. Magnetic e-resonance therapy is a very new concept in the field of neuromodulation and more than likely will continue to change and may become more effective over time. The standard rTMS approaches are limited in their adaptation personalize treatment.
Conclusion
Potential future studies should incorporate neuroimaging and cortical mapping to add an additional level of comparison. Functional MRI, BOLD, PET, and Diffusion Tensor Imaging (DTI) may all be viable methods of quantifying the therapeutic effects of rTMS on MDD.
Overall, this study confirmed the effectiveness of noninvasive neuromodulation in treating MDD. Additional studies are required to understand how different parameters, locations, and patients change the effectiveness of these therapies.
Acknowledgment
This research received funding from Waveneuro inc. and Nexstim inc. Funding was accepted from both companies as to not incentivize any biases when reporting the data. Funding included educational grants from both companies of equal amounts for the purposes of paying Institutional Review Board (IRB) costs.
References
- Faraday M (1832) Experimental researches in electricity. Phil Trans R Soc Lond 122: 125-162.
- Barker AT, Jalinous R, Freeston IL (1985) Non-invasive magnetic stimulation of human motor cortex. Lancet 325: 1106-1107.
- Rush AJ, Trivedi MH, Wisniewski SR, Nierenberg AA, Stewart JW, et al. (2006) Acute and longer-term outcomes in depressed outpatients requiring one or several treatment steps: A STAR*D report. Am J Psychiatry 163: 1905-1917.
- Siebner HR, Hartwigsen G, Kassuba T, Rothwell JC (2009) How does transcranial magnetic stimulation modify neuronal activity in the brain? Implications for studies of cognition. Cortex 45: 1035-1042.
- Sackeim HA, Aaronson ST, Carpenter LL, Hutton TM, Mina M, et al. (2020) Clinical outcomes in a large registry of patients with major depressive disorder treated with transcranial magnetic stimulation. J Affect Disord 277: 65-74.
- Peng Z, Zhou C, Xue S, Bai J, Yu S, et al. (2018) Mechanism of repetitive transcranial magnetic stimulation for depression. Shanghai arch psychiatry 30: 84-92.
- Banerjee J, Sorrell ME, Celnik PA, Pelled G (2017) Immediate effects of repetitive magnetic stimulation on single cortical pyramidal neurons. PLoS One 12: e0170528.
- Experimental researches in electricity
- Mayberg HS, Liotti M, Brannan SK, Ginnis MS, Mahurin RK, et al. (1999) Reciprocal limbic-cortical function and negative mood: Converging PET findings in depression and normal sadness. Am I psychiatry 156: 675-682.
- Rogers MA, Kasai K, Koji M, Fukuda R, Iwanami A, et al. (2004) Executive and prefrontal dysfunction in unipolar depression: A review of neuropsychological and imaging evidence. Neurosci Res 50: 1-11.
- Fox MD, Buckner RL, White MP, Greicius MD, Leone PA, et al. (2012) Efficacy of transcranial magnetic stimulation targets for depression is related to intrinsic functional connectivity with the subgenual cingulate. Biol psychiatry 72: 595-603.
- Grimm S, Beck J, Schuepbach D, Hell D, Boesiger P, et al. (2008) Imbalance between left and right dorsolateral prefrontal cortex in major depression is linked to negative emotional judgment: An fMRI study in severe major depressive disorder. Biol psychiatry 63: 369-376.
- Leuchter AF, Cook IA, Jin Y, Phillips B (2013) The relationship between brain oscillatory activity and therapeutic effectiveness of transcranial magnetic stimulation in the treatment of major depressive disorder. Front hum neurosci 7: 37.
- Levy D, Crawford J (2016) The potential of magnetic resonant therapy in children with autism spectrum disorder. Austin J Autism & Relat Disabil 2: 1029.
- Blumberger DM, Rodriguez VF, Thorpe KE, Feffer K, Noda Y, et al. (2018) Effectiveness of theta burst versus high-frequency repetitive transcranial magnetic stimulation in patients with depression (THREE-D): A randomised non-inferiority trial. Lancet 391: 1683-1692.
- Mylius V, Ayache SS, Ahdab R, Farhat WH, Zouari HG, et al. (2013) Definition of DLPFC and M1 according to anatomical landmarks for navigated brain stimulation: Inter-rater reliability, accuracy, and influence of gender and age. NeuroImage 78: 224-232.
- Yuan J, Yu H, Yu M, Liang X, Huang C, et al. (2022) Altered spontaneous brain activity in major depressive disorder: An activation likelihood estimation meta-analysis. J affect disord 314: 19-26.
 Spanish
Spanish  Chinese
Chinese  Russian
Russian  German
German  French
French  Japanese
Japanese  Portuguese
Portuguese  Hindi
Hindi