Research Article, Endocrinol Diabetes Res Vol: 5 Issue: 1
Serum Ischemia Modified Albumin as a Marker of Complications in Patients withType 2 DiabetesMellitus
Mahmoud Saleh OF1*, Nehal Hamdy El Said2, Youssef Bahgat HM3, El Ghaffar Mohamed NA4, Hussein Said El Fishawy5 and Maha Assem6
1Nephrology and Internal Medicine, Specialist Ministry of Health and Population, Cairo, Egypt
2Department of InternalMedicine, CairoUniversity, Cairo, Egypt
3Department of Internal medicine, Cairo University, Cairo, Egypt
4Department of Clinical and Chemical Pathology, National Research Center, Cairo, Egypt
5Department of Internal Medicine and Nephrology, Cairo University, Cairo, Egypt
6Department of Internal Medicine, Cairo University, Cairo, Egypt
*Corresponding Author : Mahmoud Saleh OF
Nephrology and Internal Medicine, Specialist Ministry of Health and Population, Cairo, Egypt
Tel: +201282137818;
E-mail: omarfarouksaleh@hotmail.com
Received: June 18, 2018 Accepted: December 03, 2018 Published: January 22, 2019
Citation: Mahmoud Saleh OF, Said NHE, Youssef Bahgat HM, El Ghaffar Mohamed NA, Fishawy HSE, et al. (2019) Serum Ischemia Modified Albumin as a Marker of Complications in Patients withType 2 Diabetes Mellitus. Endocrinol Diabetes Res 5:1. doi: 10.4172/2470-7570.1000138
Abstract
Background: The term diabetes mellitus describes several diseases of abnormal carbohydrate metabolism that are characterized by hyperglycemia. It is associated with a relative or absolute impairment in insulin secretion along with varying degrees of peripheral resistance to the action of insulin, Type 2 diabetes mellitus is by far the most common type of diabetes in adults (>90 percent), Diabetes is associated with complications such as cardiovascular diseases, retinopathy and neuropathy which can lead to chronic morbidities and mortality, Increased level of oxidative stress markers like malondialdehyde (MDA) has been detected in diabetes associated with complications. Ischemia modified albumin (IMA) is an altered type of serum albumin that forms under conditions of oxidative stress. Increased IMA has been described as a marker of ischemia reperfusion injury and dysfunction of endothelial L-arginine/nitric oxide pathway (affecting NO levels).
Aim of the work: To evaluate serum IMA among diabetic nephropathy, type 2 diabetes mellitus complications in comparison to healthy controls. Results: The serum IMA were higher among group B than group A and group C with P value<0.005 which is statistically significant, there is positive predictive value of serum IMA to detect diabetic nephropathy and other diabetic complications.
Conclusion: There is positive predictive value of serum IMA to detect diabetic complications.
Keywords: Albumin; Diabetes mellitus
Abbrevations
ACS: Acute Coronary Syndrome; AOPP: Advanced Oxidation Protein Products; CRP: C-Reactive Protein; DM: Diabetes Mellitus; DN: Diabetic Nephropathy; DR: Diabetic Retinopathy
Introduction
In vivo studies showed that the molecule of albumin changes the ability of the first three amino acids N-Asp-Ala-His to bind free metal ions as cobalt, copper and nickel after modifications in hypoxia conditions [1], the ischemia-modified albumin (IMA) is a novel ischemia marker developed by quantifying the decrease in the metal binding capacity of albumin [2], Although the definitive and precise mechanism for IMA production In vivo is unknown as yet, it appears to be related to the generation of ROS that modifies metal binding domains of albumin. Both indirect and direct evidence supports this concept, Cobalt chloride is a well-established chemical inducer of hypoxia-like responses, such as erythropoiesis and angiogenesis In vivo, likely involving an increased DNA binding activity of hypoxiainducible factor-1α (HIF-1α) to its target genomic sequences, It has been speculated that cobalt might stabilize HIF-1α through generation of ROS by a nonenzymatic, mitochondrial mechanism. Under normoxic conditions, the main mediator HIF-1α is rapidly degraded by the proteasome [3].
Reference values
Reference values of IMA were determined from a population of 283 healthy individuals and ranged from 52 to 116 kilounits/L, with a 95th percentile at 85 kilounits/L (Cobas Mira) [4], after multivariate analysis, results of some studies IMA levels>85 U/ml suggested myocardial ischemia [5].
Relation to albumin
In particular study, a significant inverse association was observed between IMA and serum albumin, accordingly, it has been suggested that IMA serum concentrations in patients with extremely low or high serum albumin levels (<20 or >55 g/L) may be unreliable and lacking in clinically informative value [6].
Normal values
While the optimum cut-off for IMA for ruling out ACS is 85 kilounits/L, the manufacturer has suggested a higher value of 100 kilounits/L for risk stratification. IMA is normally distributed in a group of apparently healthy volunteers and is not correlated with smoking, age, race, gender, or Framingham risk score [7].
Importance of ischemia modified albumin
In clinical and experimental studies conducted so far, it has been demonstrated that IMA elevates depending on oxidative stress after acute ischemia and returns to normal levels in hours after reperfusions [8], In the body, IMA formation occurs not only acutely but also due to the chronic oxidative damage [9].
It has been proposed by various authors that IMA increases wherever the albumin passes through ischemic tissues, Hence, though approved as a marker in cardiac ischemia, now it is being studied in context of other diseases where there is a prevalence of increased oxidative stress and ischemia, like diabetes mellitus, liver diseases, obese post-menopausal women, complicated pregnancies and cancers [10].
Role of ischemia modified albumin in diagnosis of diabetes mellitus and diabetic complications
Hyperglycaemia in Type 2 Diabetes mellitus has been implicated in the pathogenesis and progression of the disease process with microvascular complications as per various researches [11]. Hyperglycaemia and inflammation reduces the capacity of albumin to bind cobalt, resulting in higher IMA levels [12], in published studies, formation of IMA appears to be involved in the initial stage of development of atheromatous plaques, and may be associated with early assessment of overall patient risks [13], Correlations between the modified albumin and HbA1c and homocysteine were described [14], IMA may not be specific applicable to be a marker for the detection of endothelial ischemic damage in diabetic patients who are already at a high risk for cardiovascular disease, Significance of IMA to those metabolic parameters might reflect a chronic production of ROS, but on the other hand, there is a concern that the increased IMA was a response to other factors such as glycation of albumin by chronic hyperglycemia [15], Plasma IMA levels are reported to correlate with parameters of oxidative stress like Advanced Oxidation Protein Products [AOPP] and thiol groups [16], Higher levels of IMA and CRP were detected in patients with T2 DM and T2 DKA [17]. There had been various studies performed on diabetic complications and serum IMA levels and close relationship has been determined [18], Hyperglycaemia-induced ischemia, inflammation, and oxidative stress might increase the levels of IMA in the serum and also in the kidney, resulting in podocyte malfunction, their excess accumulation along the extracellular matrix in the glomerulus and tubulointerstitium leads to vascular endothelial damage in diabetes [19,20].
Patients, Materials and Methods
Population of study and disease condition
Patients with type 2 DM with and without nephropathy.
Inclusion criteria: Patients with type 2 DM.
Exclusion criteria: Patients with type I DM, gestational DM, patients with other diseases of the kidney not related to DN, Patients with coronary heart disease.
Methodology in details
A total of 62 patients with type 2 diabetes mellitus who attended the outpatient clinic of the Department of Internal Medicine, Kasr Al Aini Hospital, Cairo University, and 29 healthy age-matched control individuals were included in the study. The study was performed from December 2015 to December 2016. Participants were subdivided into three groups: Group A: 33 type 2 DM patients without nephropathy, Group B: 29 type 2 DM patients with nephropathy and Group C: 29 healthy subjects as control.
Ethical aspects
Research protocols were approved by the medical ethics committee of Kasr al Aini Medical School, Cairo University. All participants provided a written informed consent after the research protocols were explained carefully to them. Informed consent was obtained from all the study participants and their approval was taken by signature.
For each subject the following were done
-History taking, detailed physical examination, Measurement of weight, height and calculation of BMI (Wt(kg)/height2(m2), ECG to exclude coronary ischemia, Fundus examination, Abdominal ultrasound, Laboratory investigations in the form of Fasting and postprandial blood glucose, Lipid profile in the form of serum cholesterol, serum triglycerides, serum LDL, serum HDL, Serum creatinine. Urinary albumin/creatinine ratio. Urinary albumin were measured using immunoturbidmetric method and urinary creatinine was measured using Jaffe reaction) and Urinary albumin/creatinine ratio was calculated [21]. Serum ischemia modified albumin which was measured by colorimetric method [22]. All routine investigations were determined on auto-analyzer using colorimetric methods. The determination of fasting blood glucose, post prandial blood glucose, serum creatinine and lipid profile were carried out on Dimension RxL Max analyzer (Siemens Healthcare GmbH Henkestr. 127, 91052 Erlangen, Germany) by colorimetric techniques. HbA1c was determined using cation exchange resin. Albumin concentrations were measured in urine using a Minineph microalbumin kit based on nephlometry method on Mininephelometer (AD200) (The Binding Site, Birmingham, UK) [23]. Urinary creatinine was determined on Dimension RxL Max analyzer by colorimetric technique and the ratio of urine albumin to creatinine was used to define microalbuminuria.
IMA determination
Sample collection: Two ml of venous blood samples were collected from each subject participating in the study and was left to clot then the serum was separated by centrifugation at 3000 xg for 10 minutes and IMA assay was performed immediately.
Principle and assay procedure: IMA concentration was determined by addition of a known amount of cobalt (2) to a serum sample and measurement of the unbound cobalt (2) by the intensity of colored complex formed after reacting with dithiothreitol (DTT) by colorimeter. An inverse relationship thus exists between the level of albumin bound cobalt and the intensity of the color formed. The preparations for the Co (2) albumin binding protocol involved the addition of 200 μL of patient serum to 50 μl of a solution of 1 g/1 cobalt chloride, followed by vigorous mixing and 10-min incubation. Dithiothreitol (50 μl of a 1.5 g/1 solution) was then added and mixed. After 2-min incubation, 1.0 ml of a 9.0 g/1 solution of NaCl were added. The absorbance of the assay mixture was read at 470 nm, the blank was prepared similarly with the exclusion of DTT. The values are expressed in U/ml, standard curve was prepared in the range 6.0- 60.0 μg CoCl2/ml. One IMA unit was defined as μg of free Co (2) in the reaction mixture per ml of serum sample [24].
Statistical analysis
Data were collected, tabulated then statistically analyzed using the Statistical Package for Social Sciences (SPSS) computer software version 20.0 (IBM, Armonk, NY, USA). Numerical variables were presented as mean and standard deviation (± SD), while categorical variables were presented as number and percentage. Chi-square test (χ²) was used for comparison between groups as regard qualitative demographic data and score variables. Independent t-test and Oneway ANOVA with LSD post-hoc tests were used for comparison and multiple comparisons between groups as regard quantitative variables. A difference with a P value ≤0.05 was considered statistically significant.
Results
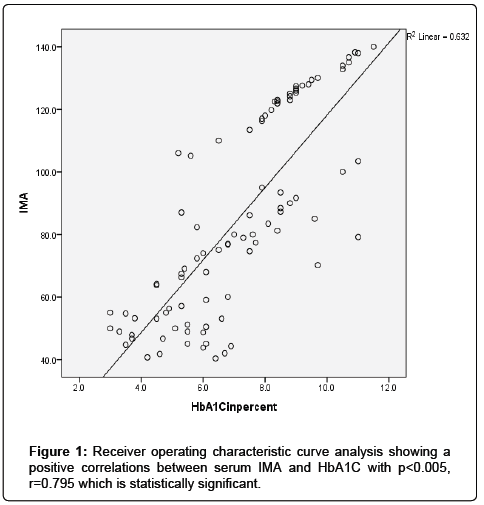
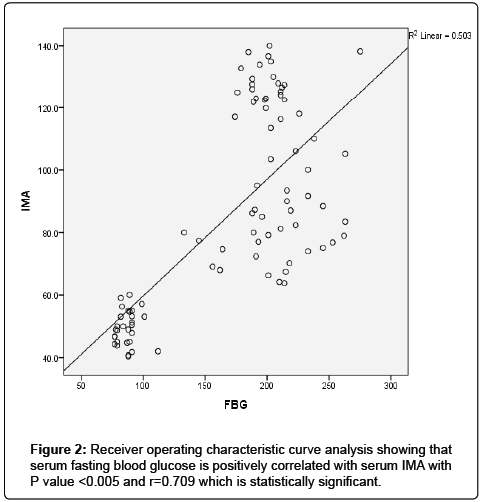
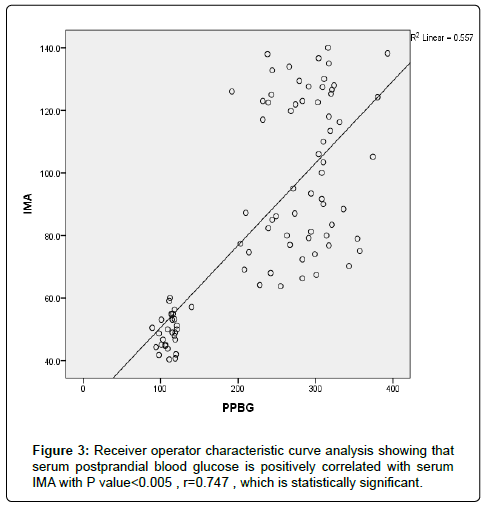
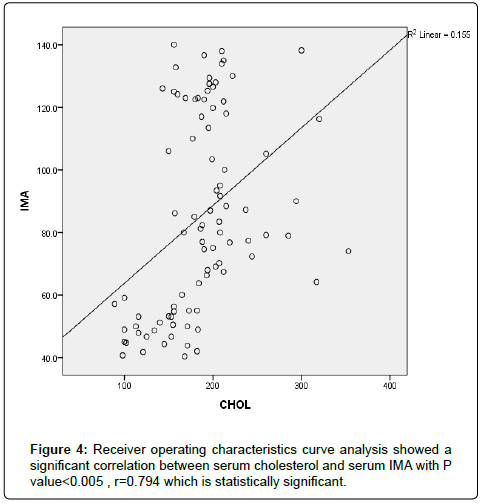
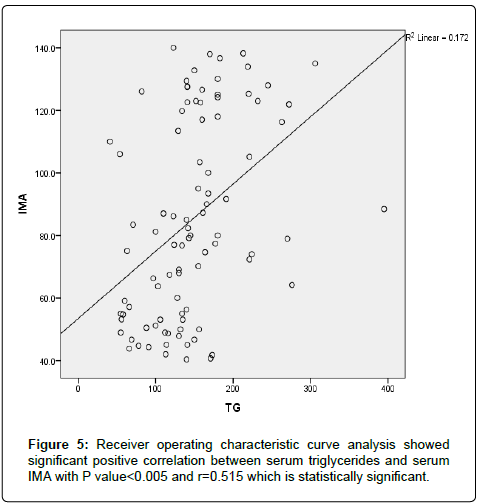
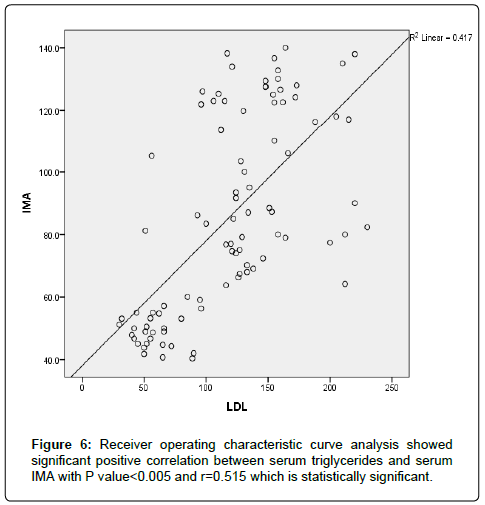
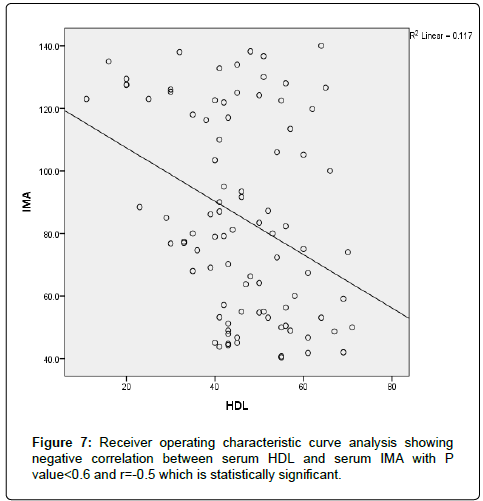
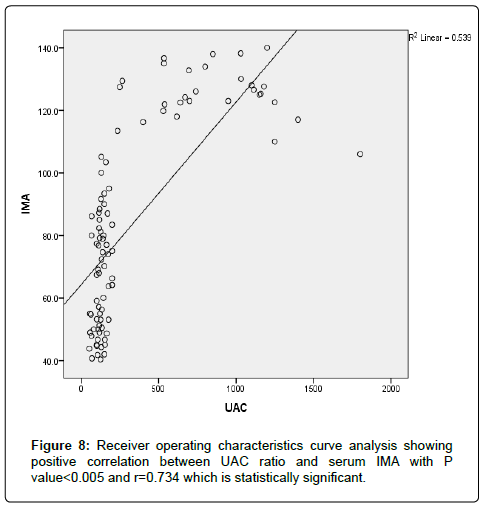
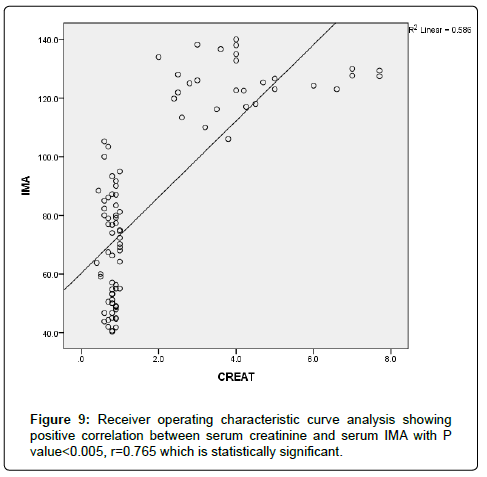
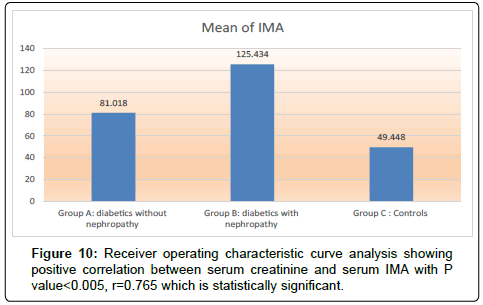
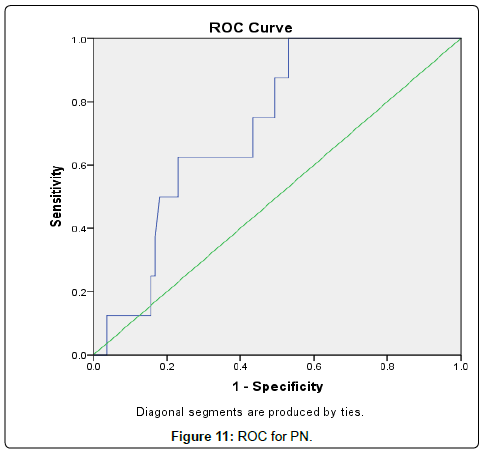
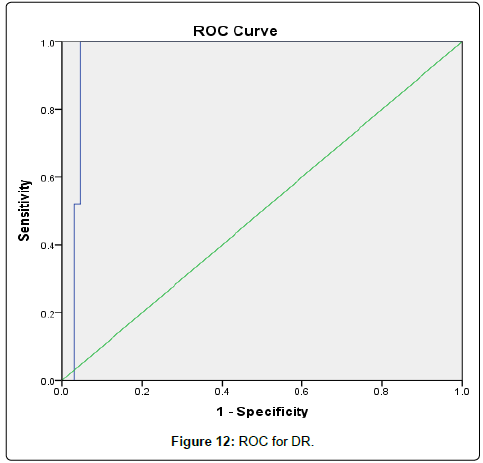
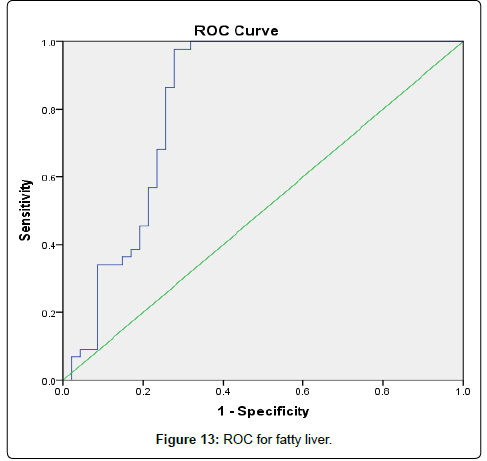
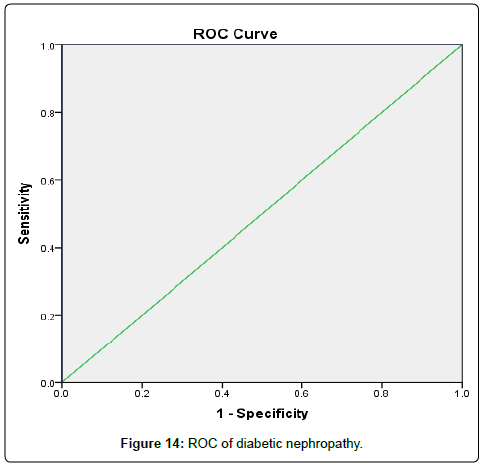
The results were divided into three groups group A which consists of patients diagnosed with diabetes mellitus which included 33 patients aged from 50 to 55 years old, with 18 males and 15 females, 16 of them treated with insulin and 17 of them treated by oral hypoglycemic drugs, no one of them had diabetic peripheral neuropathy in general examinations, no one of them showed diabetic retinopathy by fundus examinations, group B included 29 patients of the same age consists of 15 males, 14 females, 20 patients treated with insulin, 9 patients treated with oral hypoglycemic drugs, with 25 patients diagnosed with diabetic peripheral neuropathy with general examinations, 25 patients were diagnosed with diabetic retinopathy by fundus examinations, 20 patients were diagnosed with fatty liver by abdominal ultrasound, and group C included 29 healthy control subjects. On comparison of group B with group A, the number of patients diagnosed with diabetic neuropathy by general examination, and those with diabetic retinopathy by fundus examination, and the number of patients diagnosed by fatty liver by abdominal ultrasound in group B were higher than those in group A with P values<0.05 which were statistically significant, the values of systolic, diastolic blood pressure, HbA1C, UAC ratio, serum creatinine, and serum IMA were higher in group B than those in group A with P values<0.001 which is statistically significant, while on comparison between group A and group C no significant difference was found between group A and control subjects regarding presence of neuropathy, retinopathy or fatty liver, the value of BMI, systolic, diastolic blood pressure, serum glucose (fasting and postprandial ), HbA1c, lipid profile except HDL, UAC ratio, and serum IMA were higher in patients with group A than those in healthy controls (group C) with p value<0.001 which is statistically significant, serum HDL-c is statistically significantly higher in control subjects than patients with group A, while on comparison between B and C, the frequency of diabetic neuropathy, diabetic retinopathy by fundus examinations, fatty liver by abdominal ultrasound were higher in group B than those in healthy control (group C ) with P values<0.001 which were statistically significant, the values of BMI, systolic, diastolic blood pressure, fasting, postprandial blood glucose, HbA1C, Lipid profiles except HDL, Serum creatinine, UAC ratio, and serum IMA were higher in group B than those in healthy control (group C ) with P value<0.001 which is statistically significant, While HDL-c is significantly lower in group B, on correlating serum IMA and various parameters in the study there is statistically significant positive correlation between serum IMA and duration of diabetes mellitus, systolic and diastolic blood pressure, FBG, PPBG, serum cholesterol, LDL c, TG, UACR, serum creatinine, and statistically significant negative correlation between serum IMA and HDL, while on correlating serum IMA to HbA1c receiver operating characteristic curve analysis in (Figure 1) showing a positive correlations between serum IMA and HbA1C with p<0.005, r=0.795 which is statistically significant, while on correlating serum IMA to serum fasting blood glucose receiver operating characteristic curve analysis (Figure 2) showing that serum fasting blood glucose is positively correlated with serum IMA with P value<0.005 and r=0.709 which is statistically significant, while on correlating serum IMA to serum postprandial blood glucose receiver operator characteristic curve analysis in (Figure 3) showing that serum postprandial blood glucose is positively correlated with serum IMA with P value<0.005, r=0.747, which is statistically significant, while on correlating serum IMA to lipid profile as cholesterol receiver operating characteristics curve analysis in (Figure 4) showed a significant correlation between serum cholesterol and serum IMA with P value<0.005, r=0.794 which is statistically significant while on correlating serum IMA to serum triglycerides receiver operating characteristic curve analysis in (Figure 5) showed significant positive correlation between serum triglycerides and serum IMA with P value<0.005 and r=0.515 which is statistically significant while on correlating serum IMA to serum LDL in (Figure 6) receiver operating characteristic curve analysis showed significant correlation between serum LDL and serum IMA with P value<0.005, r=0.646 which is statistically significant, while on correlating serum IMA to serum HDL receiver operating characteristic curve analysis in (Figure 7) showing negative correlation between serum HDL and serum IMA with P value<0.6 and r=-0.5 which is statistically significant, while on correlating serum IMA to urine albumin creatinine ratio receiver operating characteristic curve analysis in (Figure 8) showing positive correlation between UAC ratio and serum IMA with P value<0.005 and r=0.734 which is statistically significant, and when correlating serum IMA to serum creatinine receiver operating characteristic curve analysis in (Figure 9) showing positive correlation between serum creatinine and serum IMA with P value<0.005, r=0.765 which is statistically significant, The values of serum IMA among different groups showed that value of serum IMA among patients with diabetic nephropathy (group B) were higher than that their values in diabetic patients without nephropathy (group A ) that were higher than their values in healthy controls (group C) with p value<0.001 which is statistically significant, so there is strong positive correlation between serum IMA and diabetic nephropathy also (Figure 10) showing the mean value of serum IMA in group B (diabetic nephropathy ) were higher than their values in group A and group C, the value of serum IMA among those with peripheral diabetic neuropathy (108.899 U/ml) were higher than their value among those with no peripheral diabetic neuropathy (82.899 U/ml ) with P value 0.024 which is statistically significant, so there is positive correlation between serum IMA and diabetic peripheral neuropathy, the ability of serum IMA to predict diabetic peripheral neuropathy with AUC 0.722, 95%C.I 0.583-0.861 with P value 0.039 which is statistically significant, and with cut-off 89.2 with sensitivity 62.5 and specificity 62.7% (Figure 11), the value of serum IMA among those with diabetic retinopathy patients were higher than their values in patients without diabetic retinopathy with P value<0.001 so there is significant correlation between serum IMA and diabetic retinopathy, The mean values of serum IMA among patients with fatty liver disease by abdominal ultrasound were higher than their values among patients without fatty liver disease by abdominal ultrasounds with (Figure 12) P value<0.001 which is statistically significant, so there is strong positive correlation between serum IMA and fatty liver as a complication of DM, the ability of serum IMA to predict fatty liver with AUC 0.820, 95%C.I 0.726-0.914 with P value<0.001 with sensitivity 72.7 % and specificity 74.5%., significant difference of serum IMA in group B than that of group A and group C with P value<0.001 which is statistically significant, a moderate positive correlation exists between IMA and creatinine in the whole sample R=0.765 and P<0.001, and the ability of serum (Figures 13 and 14) IMA in detecting diabetic nephropathy has 100% specificity, and 100% sensitivity (Tables 1-19).
| Item | Group A N=33 |
Group B N=29 |
Group C N=29 |
|||
|---|---|---|---|---|---|---|
| Frequency | % | Frequency | % | Frequency | % | |
| Sex | ||||||
| Male | 18 | 54.5% | 15 | 51.7% | 15 | 51.7% |
| Female | 15 | 45.45% | 14 | 48.27% | 14 | 48.27% |
| Treatment | ||||||
| Insulin | 16 | 48.48% | 20 | 68.9% | ----- | --- |
| Oral hypoglycemic drugs | 17 | 51.51% | 9 | 31.03% | ----- | ---- |
| PN | ||||||
| Negative | 33 | 100% | 4 | 13.7% | 29 | 100% |
| Positive | 25 | 86.2% | ||||
| DR | ||||||
| Negative | 33 | 100% | 4 | 13.7% | 29 | 100% |
| Positive | 25 | 86.2% | ||||
| U/S | ||||||
| Normal | 33 | 100% | 9 | 31.03% | 29 | 100% |
| F.I. | 20 | 68.9% | ||||
DR: Diabetic Nephropathy; F.L: Fatty Liver; N: Number of Patients; PR: Peripheral Neuropathy; U/S: Abdominal Ultrasound.
Table 1: Comparison of the studied groups.
| Item | Group A N=33 |
Group B N=29 |
Group C N=29 |
|---|---|---|---|
| Mean ± SD | Mean ± SD | Mean ± SD | |
| Age in years | 51.9 ± 6.545 | 50.5 ± 5.56 | 51.5 ± 4.5 |
| Duration in years | 8.66 ± 4.80 | 11.18 ± 6.526 | ----- |
| BMI | 27.39 ± 3.992 | 27.93 ± 4.317 | 24.48 ± 2.148 |
| BP systolic | 130.61 ± 10.589 | 147.59 ± 8.724 | 118.97 ± 3.099 |
| BP Diastolic | 86.97 ± 5.855 | 91.38 ± 4.411 | 78.62 ± 5.158 |
| HbA1C in percent | 7.388 ± 1.798 | 8.990 ± 1.3857 | 4.028 ± 1.2265 |
| FBG | 203.66 ± 20.274 | 208.36 ± 32.951 | 86.90 ± 7.993 |
| PPBG | 283.73 ± 45.229 | 291.72 ± 44.524 | 111.55 ± 10.284 |
| CHOL | 195.86 ± 28.150 | 218.267 ± 43.046 | 138.52 ± 30.264 |
| TG | 161.27 ± 64.456 | 170.66 ± 60.309 | 108.03 ± 36.954 |
| LDL | 138 ± 40.464 | 152.34 ± 34.52 | 60.38 ± 18.204 |
| HDL | 44.97 ± 10.987 | 40.93 ± 14.919 | 52.48 ± 9.661 |
| UAC | 13.5 ± 5.5 | 848 ± 379.043 | 11.6 ± 3.4 |
| IMA | 81.018 ± 10.9490 | 125.434 ± 8.4077 | 49.448 ± 5.5295 |
| CREAT | 0.808 ± 0.1733 | 4.295 ± 1.6258 | 0.793 ± 0.1223 |
BMI: Body Mass Index; BP: Blood Pressure; Chol: Serum Cholesterol; CREAT: Serum Creatinine; FBG: Fasting Blood Glucose; Hba1c: Glycated Hemoglobin; HDL: Serum High Density Lipoprotein; IMA: Serum Ischemia Modified Albumin; LDL: Serum Low Density Lipoprotein; PPBG: Postprandial Blood Glucose; SD: Standard Deviation; TG: Serum Triglycerides;
UAC: Urinary Albumin Creatinine Ratio
Table 2: Demographic, clinical and laboratory data of the studied groups.
| Item | Group A N=33 |
Group B N=29 |
P Value |
||
|---|---|---|---|---|---|
| Frequency | % | Frequency | % | ||
| Sex | 0.78 | ||||
| Male | 18 | 54.54% | 15 | 51.7% | |
| Female | 15 | 45.45% | 14 | 48.27% | |
| Treatment | <0.001* | ||||
| Insulin | 16 | 48.48% | 20 | 68.9% | |
| Oral hypoglycemic drugs | 17 | 58.6% | 9 | 31.03% | |
| PN | <0.001* | ||||
| Negative | 33 | 100% | 4 | 13.7% | |
| Positive | 25 | 86.2% | |||
| DR | <0.001* | ||||
| Negative | 33 | 100% | 4 | 13.7% | |
| Positive | 25 | 86.2% | |||
| U/S | <0.001 | ||||
| Normal | 33 | 100% | 9 | 31.03% | |
| F.I. | 20 | 68.9% | |||
The number of patients diagnosed with diabetic neuropathy by general examination , and those with diabetic retinopathy by fundus examination ,and the number of patients diagnosed by fatty liver by abdominal ultrasound in group B were higher than those in group A with P values <0.05 which were statistically significant
Table 3: Comparison between groups A and B regarding the enlisted variables.
| Item | Group A N=33 |
Group B N=29 |
P value |
|---|---|---|---|
| Mean ± SD | Mean ± SD | ||
| Age in years | 51.97 ± 6.545 | 50.5 ± 5.56 | 0.78 |
| Duration in years | 8.66 ± 4.80 | 11.18 ± 6.526 | 0.092 |
| BMI | 27.39 ± 3.992 | 27.93 ± 4.317 | 0.613 |
| BP systolic | 130.61 ± 10.589 | 147.59 ± 8.724 | 0.0001* |
| BP Diastolic | 86.97 ± 5.855 | 91.38 ± 4.411 | 0.002* |
| HbA1Cinpercent | 7.388 ± 1.7980 | 8.990 ± 1.3857 | 0.0001* |
| FBG | 203.66 ± 20.247 | 208.36 ± 32.951 | 0.508 |
| PPBG | 283.73 ± 45.229 | 291.72 ± 44.524 | 0.487 |
| CHOL | 195.86 ± 28.150 | 218.267 ± 43.046 | 0.032* |
| TG | 161.27 ± 64.546 | 170.66 ± 60.309 | 0.558 |
| LDL | 138 ± 40.464 | 152.34 ± 34.4452 | 0.141 |
| HDL | 44.97 ± 10.987 | 40.93 ± 14.919 | 0.226 |
| UAC | 13.5 ± 5.5 | 848.83 ± 379.043 | <0.001* |
| IMA | 81.018 ± 10.9490 | 125.434 ± 8.4077 | <0.001* |
| CREAT | 0.808 ± 0.1733 | 4.295 ± 1.6258 | <0.001* |
The values of systolic , diastolic blood pressure , HbA1C , UAC ratio , serum creatinine , and serum IMA were higher in group B than those in group A with P values <0.001 which is statistically significant.
Table 4: Comparison between groups A and B regarding the enlisted variables.
| Item | Group A N=33 |
Group C N=29 |
P value |
||
|---|---|---|---|---|---|
| Frequency | % | Frequency | % | ||
| Sex | 0.9 | ||||
| Male | 18 | 54.54% | 15 | 51.7% | |
| Female | 15 | 51.7% | 14 | 48.27% | |
| Treatment | a | ||||
| Insulin | 16 | 48.48% | ------ | ---- | |
| Oral hypoglycemic drugs | 17 | 51.51% | ------ | ----- | |
| PN | 0.99 | ||||
| Negative | 33 | 100% | 29 | 100% | |
| Positive | |||||
| DR | 0.99 | ||||
| Negative | 33 | 100% | 29 | 100% | |
| Positive | |||||
| U/S | 0.99 | ||||
| Normal | 33 | 100% | 29 | 100% | |
| F.I. | |||||
a: undetermined..
no significant difference was found between group A and control subjects regarding presence of neuropathy , retinopathy or fatty liver
Table 5: Comparison between groups A and C regarding the enlisted variables.
| Item | Group A N=33 |
Group C N=29 |
P value |
|---|---|---|---|
| Mean ± SD | Mean ± SD | ||
| Age in years | 51.97 ± 6.545 | 50.5 ± 5.56 | 0.78 |
| Duration in years | 8.66 ± 4.80 | 11.18 ± 6.526 | 0.092 |
| BMI | 27.39 ± 3.992 | 27.93 ± 4.317 | 0.613 |
| BP systolic | 130.61 ± 10.589 | 147.59 ± 8.724 | 0.0001* |
| BP diastolic | 86.97 ± 5.855 | 91.38 ± 4.411 | 0.002* |
| HbA1Cinpercent | 7.388 ± 1.7980 | 8.990 ± 1.3857 | 0.0001* |
| FBG | 203.66 ± 20.247 | 208.36 ± 32.951 | 0.508 |
| PPBG | 283.73 ± 45.229 | 291.72 ± 44.524 | 0.487 |
| CHOL | 195.86 ± 28.150 | 218.267 ± 43.046 | 0.032* |
| TG | 161.27 ± 64.546 | 170.66 ± 60.309 | 0.558 |
| LDL | 138 ± 40.464 | 152.34 ± 34.4452 | 0.141 |
| HDL | 44.97 ± 10.987 | 40.93 ± 14.919 | 0.226 |
| UAC | 13.5 ± 5.5 | 848.83 ± 379.043 | <0.001* |
| IMA | 81.018 ± 10.9490 | 125.434 ± 8.4077 | <0.001* |
| CREAT | 0.808 ± 0.1733 | 4.295 ± 1.6258 | <0.001* |
The value of BMI , systolic , diastolic blood pressure , serum glucose ( fasting and postprandial ) , HbA1c , lipid profile except HDL , UAC ratio , and serum IMA were higher in patients with group A than those in healthy controls (group C) with p value <0.001 which is statistically significant, serum HDL-c is statistically significantly higher in control subjects than patients with group A
Table 6: Comparison between groups A and C regarding the enlisted variables.
| Item | Group B N=29 |
Group C N=29 |
P value |
||
|---|---|---|---|---|---|
| Frequency | % | Frequency | % | ||
| Sex | 0.99 | ||||
| Male | 15 | 51.7% | 15 | 51.7% | |
| Female | 14 | 48.2% | 14 | 48.27% | |
| Treatment | undetermined | ||||
| Insulin | 20 | 68.9% | ----- | ||
| Oral hypoglycemic drugs | 9 | 31.03% | ----- | ||
| PN | 0.0001* | ||||
| Negative | 4 | 13.7% | 29 | 100% | |
| Positive | 25 | 86.2% | |||
| DR | 0.0001* | ||||
| Negative | 4 | 13.7% | 29 | 100 | |
| Positive | 25 | 86.2% | |||
| U/S | 0.0001* | ||||
| Normal | 9 | 31.03% | 29 | 100 | |
| F.I. | 20 | 68.96% | 0 | ||
| The frequency of diabetic neuropathy , diabetic retinopathy by fundus examinations , fatty liver by abdominal ultrasound were higher in group B than those in healthy control (group C ) with P values <0.001 which were statistically significant. | |||||
Table 7: Comparison between groups B and C regarding the enlisted variables.
| Item | Group B N=29 |
Group C N=29 |
P value |
|---|---|---|---|
| Mean ± SD | Mean ± SD | ||
| Age in years | 50.5 ± 5.56 | 51.5 ± 4.5 | 0.69 |
| Duration in years | 11.18 ± 6.526 | 0a | 0.0001* |
| BMI | 27.93 ± 4.317 | 24.48 ± 2.148 | 0.0001* |
| BP systolic | 147.59 ± 8.742 | 118.97 ± 3.099 | 0.0001* |
| BP Diastolic | 91.38 ± 4.411 | 78.62 ± 5.1585 | 0.0001* |
| HbA1Cinpercent | 8.990 ± 1.3857 | 4.028 ± 1.2265 | 0.0001* |
| FBG | 208.36 ± 32.951 | 86.90 ± 7.993 | 0.0001* |
| PPBG | 291.72 ± 44.524 | 111.55 ± 10.284 | 0.0001* |
| CHOL | 218.267 ± 43.046 | 138.52 ± 30.264 | 0.0001* |
| TG | 170.66 ± 60.309 | 108.03 ± 36.954 | 0.0001* |
| LDL | 152.34 ± 34.452 | 60.38 ± 18.204 | 0.0001* |
| HDL | 40.93 ± 14.919 | 52.48 ± 9.661 | 0.001* |
| UAC | 848.83 ± 379.043 | 11.6 ± 3.4 | 0.0001* |
| IMA | 125.434 ± 8.4077 | 49.448 ± 5.5295 | 0.0001* |
| CREAT | 4.295 ± 1.6258 | 0.793 ± 0.1223 | 0.0001* |
The values of BMI , systolic , diastolic blood pressure , fasting , postprandial blood glucose , HbA1C , Lipid profiles except HDL , Serum creatinine , UAC ratio , and serum IMA were higher in group B than those in healthy control (Group C ) with P value <0.001 which is statistically significant, While HDL-c is significantly lower in group B.
Table 8: Comparison between groups B and C regarding the enlisted variables.
| Correlation between Serum ischemia modified albumin and different variables as a whole (Serum IMA) | |||
|---|---|---|---|
| P value | R | ||
| Variable | |||
| Age in years | 0.101 | 0.173 | |
| Duration of DM | 0.056* | 0.244 | |
| BMI | 0.003* | 0.706 | |
| B.P systolic | 0.0001* | 0.776 | |
| B.P diastolic | 0.0001* | 0.631 | |
| HbA1c | 0.0001* | 0.795 | |
| F.B.G | 0.0001* | 0.709 | |
| P.P.B.G | 0.0001* | 0.747 | |
| Cholesterol | 0.0001* | 0.794 | |
| TG | 0.0001* | 0.515 | |
| LDL | 0.0001* | 0.646 | |
| HDL | 0.6 | -0.5 | |
| UAC ratio | 0.0001* | 0.734 | |
| Serum creatinine | 0.0001* | 0.765 | |
P value <0.005 is significant statistically, R explanation
1-Positive or negative according to the sign
2-<0.5 weak correlation
3-Between 0.5-0.7 moderate correlation
4->0.7 strong correlation
5-As shown in this table there is statistically significant positive correlation between serum IMA and duration of diabetes mellitus , systolic and diastolic blood pressure , FBG, PPBG, serum cholesterol , LDL –c , TG , UACR, serum creatinine , and statistically significant negative correlation between serum IMA and HDL.
Table 9: Correlation between serum IMA and different variables.
| IMA | |||||
|---|---|---|---|---|---|
| N | Mean | Std. Deviation | P value | ||
| Group A: diabetics without nephropathy (63.8-105.2 U/ml) | 33 | 81.018 | 10.9490 | <0.001* | |
| Group B: diabetics with nephropathy (106-140 U/ml) | 29 | 125.434 | 8.4077 | ||
| Group C: Controls (40-60 U/ml) | 29 | 49.448 | 5.5295 | ||
| Total | 91 | 85.112 | 31.8435 | ||
The values of serum IMA among different groups showed that value of serum IMA among patients with diabetic nephropathy (group B) were higher than that their values in diabetic patients without nephropathy (group A ) that were higher than their values in healthy controls ( group C) with p value <0.001 which is statistically significant, so there is strong positive correlation between serum IMA and diabetic nephropathy
Table 10: IMA among different study groups.
| Group Statistics | |||||
|---|---|---|---|---|---|
| PN | N | Mean | Std. Deviation | ||
| IMA | Negative | 66 | 82.899 | 31.6980 | 0.024* |
| Positive | 25 | 108.075 | 24.6273 | ||
The value of serum IMA among those with peripheral diabetic neuropathy (108.899 U/ml) were higher than their value among those with no peripheral diabetic neuropathy (82.899 U/ ml ) with P value 0.024 which is statistically significant, so there is positive correlation between serum IMA and diabetic peripheral neuropathy.
Table 11: IMA among different nerve status either diabetic peripheral neuropathy or normal nerves.
| AUC* | 95% CI | P value | Cut-Off | Sensitivity | Specificity | |
|---|---|---|---|---|---|---|
| IMA | 0.722 | 0.583– 0.861 | 0.039* | 89.2 | 62.5% | 62.7% |
The ability of serum IMA to predict diabetic peripheral neuropathy with AUC 0.722, 95%C.I 0.583-0.861 with P value 0.039 which is statistically significant , and with cut-off 89.2 with sensitivity 62.5 and specificity 62.7.
Table 12: Ability of IMA to predict PN.
| Group Statistics | |||||
|---|---|---|---|---|---|
| DR | N | Mean | Std. Deviation | P value | |
| IMA | Negative | 66 | 69.952 | 23.1448 | <0.001* |
| Positive | 25 | 125.136 | 7.1417 | ||
The value of serum IMA among those with diabetic retinopathy patients were higher than their values in patients without diabetic retinopathy with P value <0.001 so there is significant correlation between serum IMA and diabetic retinopathy.
Table 13: IMA among different status of the retina either diabetic retinopathy or normal retina.
| AUC* | 95% CI | P value | Cut-Off | Sensitivity | Specificity | |
|---|---|---|---|---|---|---|
| IMA | 0.962 | 0.919– 1.000 | <0.001* | 111.7 | 96.0% | 95.5% |
The ability of serum IMA to predict diabetic retinopathy with AUC 0.962 , 95%C.I 0.919-1.00, with P value <0.001 , and cut-off 111.7 with sensitivity 96% and specificity 95.5.
Table 14: Ability of IMA to predict DR.
| Group statistics | |||||
|---|---|---|---|---|---|
| US | N | Mean | Std. Deviation | P value | |
| IMA | Normal | 71 | 69.357 | 31.0556 | <0.001* |
| F.l. | 20 | 101.941 | 23.0112 | ||
The mean values of serum IMA among patients with fatty liver disease by abdominal ultrasound were higher than their values among patients without fatty liver disease by abdominal ultrasounds with P value<0.001 which is statistically significant , so there is strong positive correlation between serum IMA and fatty liver as a complication of DM.
Table 15: IMA among different abdominal ultrasound either in fatty liver or normal liver.
| AUC* | 95% CI | P value | Cut-Off | Sensitivity | Specificity | |
|---|---|---|---|---|---|---|
| IMA | 0.820 | 0.726– 0.914 | <0.001* | 80.6 | 72.7% | 74.5% |
The ability of serum IMA to predict fatty liver with AUC 0.820, 95%C.I 0.726-0.914 with P value <0.001 with sensitivity 72.7% and specificity 74.5%.
Table 16: Ability of IMA to predict fatty liver.
| Group statistics | |||||
|---|---|---|---|---|---|
| Group | N | Mean | Std. Deviation | P value | |
| IMA | Group A and C: diabetics and controls without nephropathy | 62 | 66.252 | 18.1416 | <0.001* |
| Group B: diabetics with nephropathy | 29 | 125.434 | 8.4077 | ||
| CREAT | Group A and C: diabetics and controls without nephropathy | 62 | 0.801 | .1505 | <0.001* |
| Group B: diabetics with nephropathy | 29 | 4.295 | 1.6258 | ||
Significant difference of serum IMA in group B than that of group A and group C with P value <0.001 which is statistically significant.
Table 17: Serum IMA among different states of renal functions.
| Correlations | ||||||||
|---|---|---|---|---|---|---|---|---|
| CREAT | ||||||||
| IMA | Pearson Correlation | .765** | ||||||
| Sig. (2-tailed) | .000* | |||||||
| N | 91 | |||||||
| A moderate positive correlation exists between IMA and creatinine in the whole sample R=0.765 P <0.001 |
||||||||
| N | Mean | Std. Deviation | ||||||
| P value | ||||||||
| IMA | Group A: diabetics without nephropathy (63.8-105.2 U/ml) | 33 | 81.018 | 10.9490 | <0.001* | |||
| Group B: diabetics with nephropathy (106-140 U/ml) | 29 | 125.434 | 8.4077 | |||||
| Group C: Controls (40-60 U/ml) | 29 | 49.448 | 5.5295 | |||||
| Total | 91 | 85.112 | 31.8435 | |||||
| CREAT | Group A: diabetics without nephropathy (63.8-105.2 U/ml) | 33 | .808 | .1733 | <0.001* | |||
| Group B: diabetics with nephropathy (106-140 U/ml) | 29 | 4.295 | 1.6258 | |||||
| Group C: Controls (40-60 U/ml) | 29 | .793 | .1223 | |||||
| Total | 91 | 1.914 | 1.8757 | |||||
A moderate positive correlation exits between serum IMA and creatinine in the whole sample with P value <0.001 and R=0.765 which is statistically significant , The serum IMA is higher in group B than that in group A and C which is statistically significant.
Table 18: Correlation between serum IMA and serum creatinine in the whole sample.
| AUC* | 95% CI | P value | Cut-Off | Sensitivity | Specificity | |
|---|---|---|---|---|---|---|
| IMA | 1.000 | 1.000– 1.000 | <0.001* | 105.6 | 100% | 100% |
Excellent ability of IMA to predict nephropathy (p value ˂0.001) (AUC ˃ 0.7)
Table 19: Ability of IMA to predict Nephropathy.
Discussion
Our case control study included 91 patients, 33 patients with diabetes mellitus type 2, 29 patients with diabetic nephropathy complicating type 2 diabetes mellitus, and 29 healthy controls, The aim of the study was to evaluate IMA in type 2 diabetic patients with various complications, Significant difference was observed between mean duration of disease in group B than that in group A with p value<0.092 which is statistically significant, while HbA1c as a marker of glycemic control was statistically significantly higher in group B patients with diabetic nephropathy than those of group A without nephropathy, this could reflect the association between poor glycemic control and duration of DM and development of diabetic complications as nephropathy this was supported and agreed by Kaefer et al. [12,25-27].
On correlation between serum IMA and duration of diabetes mellitus among populations of study, it showed positive correlation between serum IMA, and duration of diabetes mellitus with p value<0.056, r=0.244 which is statistically significant which is agreed and supported by Sowjanya et al. [25]. The correlations between serum IMA and different parameters of glucose control as HbA1c, FBG, PPBG showed positive correlation between them with p value<0.05 which is statistically significant these results were supported and agreed by Chawla et al. [26]. Serum level of IMA was positively correlated with HbA1c, a marker of glycaemic control and an indicator of development of complications, This finding is supported by Sowjanya who reported that the positive association of raised plasma IMA with HbA1c may be an effect of increased oxidative stress and free radical generation leading to widespread inflammation of vascular endothelium et al. [25-27], the resultant tissue hypoxia might have contributed to the increased modification of albumin attributing towards raised IMA level, The correlations between serum IMA and lipid profiles shows positive correlation between elevated serum cholesterol, serum triglycerides, and serum LDL, with serum IMA in the whole population of study and negative correlation between serum IMA and HDL these results were supported by Refaat et al. [28], There is significant difference between BMI in group B than that in group C, and there is significant difference between BMI in group A than that in group C with P value<0.001 which is statistically significant this was supported and agreed by Arslan et al. [29], In our study IMA and BMI were positively correlated (p-value<0.003) this was in agreement with Joha et al. [30], who found a positive correlation between serum ischemia modified albumin and BMI in obese patients above 30 kg/m.2 and with Arslan et al. [29] study which positively correlated serum ischemia modified albumin with obesity and metabolic syndrome, on the contrary Yigitbasi et al. [31], found no significant correlation between ischemia modified albumin and BMI. Significant difference in UAC was observed between group B and group A, also between group A and group C with P values<0.001 this was supported and agreed by Ahmed et al. [32-34], also significant difference was observed between serum creatinine in group B than that in group A and group C this was supported and agreed by Ahmed et al. [32], Moreover there is significant positive correlation between serum IMA and serum creatinine in the three groups which is significant statistically, this were supported and agreed by Ahmed et al. [32], the correlations of serum IMA and various parameters in the whole population of study showed a positive correlations between serum IMA and UAC ratio (p value<0.05, r=0.734 ) which is statistically significant, also it showed positive correlation between serum IMA, and serum creatinine (p<0.05, r=0.765) which is statistically significant these results were supported and agreed by Ahmed et al. [32], The ability of serum ischemia modified albumin to detect diabetic nephropathy with 95% confidence interval equals 1 with (p value<0.001) with (AUC>0.7) with 100% specificity and 100% sensitivity, this was supported and agreed by Ahmed et al. [32], The positive correlation between IMA and the UAC ratio implies that IMA increased progressively with the degree of albuminuria. This was supported by Krzystek-Korpacka et al. [16-32]. Results relating serum IMA to the presence of diabetic peripheral neuropathy by neurological examination and others who haven’t diabetic peripheral neuropathy, showed that also the ability of serum IMA to predict diabetic peripheral neuropathy had sensitivity and specificity of 62.5% and 62.7%, furthermore, serum IMA among patients with diabetic retinopathy was significantly higher than those with normal retina (125.13 vs. 69.95)(P<0.001), Also, the ability of serum IMA to predict diabetic retinopathy with AUC 0.962, 95% C.I 0.919-1.00, cutoff 111.7 had 96% sensitivity and 95.5% specificity, these results were supported by Turk et al. [35,36]. Also relating serum IMA to abnormal finding on abdominal ultrasound as fatty liver as a complication of diabetes mellitus showed positive correlation between serum IMA and fatty liver by p value<0.001 which is statistically significant, Moreover the ability of serum IMA to predict fatty liver with AUC 0.820, 95% C.I 0.726 - 0.914, had sensitivity of 72.7 % and specificity of 74.5%, these were agreed and supported by Amirtharaj et al. [37].
Conclusion
There is positive predictive value of serum IMA to detect diabetic microvascular complications as diabetic nephropathy, neuropathy and retinopathy.
Ethical Aspects
Research protocols were approved by the medical ethics committee of Kasr al Aini Medical School, Cairo University. All participants provided a written informed consent after the research protocols were explained carefully to them. Informed consent was obtained from all the study participants and their approval was taken by signature.
Conflicts
No conflicts of interest.
Acknowledgment
I am very grateful to Nehal hamdy Elsaid, Heba Morad Youssef Bahgat, Nagwa Abd ElGhaffar Mohamed, Hussein Said ElFishaway, Maha Assem for their continuous help, guidance and support.
References
- Christenson RH, Duh SH, Sanhai WR, Wu AH, Holtman V, et al. (2001) Characteristics of an albumin cobalt binding test for assessment of acute coronary syndrome patients: A multicenter study. Clin Chem 47: 464-470.
- Ertekin B, Kocak S, Defne Dundar Z, Girisgin S, Cander B, et al. (2013) Diagnostic value of ischemia-modified albumin in acute coronary syndrome and acute ischemic stroke. Pak J Med Sci 29: 1003-1007.
- Lippi G, Montagnana M, Guidi GC (2006) Albumin cobalt binding and ischemia modified albumin generation: an endogenous response to ischemia? Int J Cardiol 108: 410-411.
- Aslan D ,and Apple FS. (2004) Ischemia modified albumin: Clinical and analytical update. Lab Med 35: 1-5.
- Roy D, Quiles J, Aldama G, Sinha M, Avanzas P, et al. (2004) Ischemia Modified Albumin for the assessment of patients presenting to the emergency department with acute chest pain but normal or non-diagnostic 12-lead electrocardiograms and negative cardiac troponin T. Int J Cardiol 97: 297-301
- Gaze DC, Crompton L, Collinson P (2006) Ischemia-modified albumin concentrations should be interpreted with caution in patients with low serum albumin concentrations. Med Princ Pract15: 322-324.
- Apple FS, Wu AH, Mair J, Ravkilde J, Panteghini M, et al. (2005) future biomarkers for detection of ischemia risk Stratification in acute coronary syndrome. Clin chem 51: 810-824.
- Turedi S, Gunduz A, Mentese A, Karahan SC, Yilmaz SE, et al. (2007) Value of ischemia-modified albumin in the diagnosis of pulmonary embolism. Am J Emerg Med 25: 770-773.
- Valle Gottlieb MG , Manica da Cruz IB, Duarte MMF (2010) Associations among metabolic syndrome , ischemia , inflammatory , oxidative lipid biomarkers. J Clin Endoc Metab 95: 586-591.
- Ellidag HY, Eren E, Aydin O (2013) Ischemia modified albumin levels and increased oxidative stress in patients with multiple myeloma. Asian Pac J Cancer Prev 14: 2759-2763.
- Stitt AW, Jenkins AJ, Cooper ME (2002) Advanced glycation end products and diabetic complications. Exp Opini Invest Drug 11: 1205-1223
- Kaefer M, Piva SJ, De Carvalho JA, Da Silva DB, Becker AM, et al. (2010) Association between ischemia modified albumin, inflammation and hyperglycaemia in type 2 diabetes mellitus. Clin Biochem 43: 450-454.
- Kazanis K , Dalamaga M, Nounopoulos C (2009) Ischemia modified albumin, high-sensitivity c-reactive protein and natriuretic peptide in patients with coronary atherosclerosis. Clinica Chimica Acta. International J Clin Chem 408: 65-69.
- Ma SG, Xu W, Wei CL, Wu XJ, Hong B, et al. (2011) Role of ischemia-modified albumin and total homocysteine in estimating symptomatic lacunar infarction in type 2 diabetic patients. Clin Biochem 44: 1299-1303.
- Ukinc K, Eminagaoglu S, Ersoz HO, Erem C, Karahan C, et al. (2009) A novel indicator of widespread endothelial damage and ischemia in diabetic patients : ischemia-modified albumin. Endocrine 36: 525-535.
- Krzysek KM, Neubauer K, Berdowska I, Boehm D, Zielinski B, et al. (2008) Enhanced formation of advanced oxidation products in IBD. Inflamm Bowel Dis 14: 749-802.
- Shang-gang MA, Yao Jin, Wen Hu, Feng Bai, Wen Xu, et al. (2012) Evaluation of ischemia modified albumin and C-reactive protein in type 2 diabetes with and without ketosis . Bio In 7: 19-26.
- D’Souza JM, D’ Souza RP, Vijin VF, Shetty A, Arunachalam C, et al. (2016) High predictive ability of glycated hemoglobin on comparison with oxidative stress markers in assessment of chronic vascular complications in type 2 diabetes mellitus. Scand J Clin Lab Invest 76: 51-57.
- Piwowar A, Kapik-Kordecka M, Warwas M (2010) Comparison of usefulness of plasma levels of oxidatively modified forms of albumin in estimating kidney dysfunctions in diabetic patients. Clin Invest Med 33: 109-116.
- Bucay AC, Viswanathan G (2012). Urinary markers of glomerular injury in diabetic nephropathy. Int J Nephrol 2012: 146987.
- Comper WD, Osicka TM, Jerums G (2003) High prevalence of immuno-unreactive intact albumin in the urine of diabetic patients. Am J Kidney Dis. 41: 336–342.
- Kumar A, Sivakanesan R, Singh S (2008) Oxidative stress, endogenous antioxidant and ischemia modified albumin in normolipidemic acute myocardial infarction patients. J Health Sci 54:482-487.
- Showell PJ, Matters DJ, Long JM Smith HC, Bradwell AR (2002) Evaluation of latex-enhanced nephelometric reagents for measuring free immunoglobulin light-chains on a modified MININEPHTM. Clin Chem 48: 22.
- Choudhury JR, Karmakar A, Guha B, Das B, Rout JK (2015) Ischaemia modified albumin and malondialdehyde level in subjects suffering from hypothyroidism. Int J Biochem Res Rev 7: 160-165.
- Sowjanya UVPU, Sridevi C, Rajkumari DMM, Kasibabu A (2015) Study of ischemia Modified albumin in type 2 diabetes as a marker of severity. J Dent Med Sci 14: 14-17.
- Chawla R, Loomba R, Guru D, Loomba V (2016) Ischemia modified albumin (IMA)-A marker of glycaemic control and vascular complications in type 2 diabetes mellitus. J Clin Diagn Res 10: 13-16.
- Patil P, Rao AV, Shetty (2017) Association of ischemia modified albumin with glycemic status in type II diabetes mellitus. In J Sci Res: 15374-15378.
- Refaat S, Ghaffar NA, Khalil A (2014) The Relationship between Ischemia Modified albumin and lipids in type 2 Egyptian diabetic patients. Advance Bio Res 18-22.
- Arslan B, Çuhadar S, Yiğitbaşı T, Uzun R, Oruk G, et al. (2015) Relationship between ischemia modified albumin and metabolic syndrome. Siriraj Med J 67.
- Joha MS, Krita J, Ibraheem A (2016) Influence of obesity on ischemia modified albumin and fructosamine levels. Int J Clin Biol Sci 2 .
- Yigitbasi T, Baskin Y, Akgol E, Kocal GC, Ellidokuz H, et al. (2017) Association of ischemia-modified albumin with oxidative stress status and insulin resistance in obese patients. Revista Română de Med Lab 25.
- Ahmed A, Manjrekar P, Yadav C, Agarwal A, Srikantiah RM, et al. (2016) Evaluation of ischemia-modified albumin, malonaldialdehyde and advanced oxidation end-product as a marker of vascular injury in diabetic nephropathy. Biomark Insights 11: 63-68.
- Gajjar M, Sirajwala HB (2016) Evaluation of ischemia modified albumin in hyperlipidemia patients. J Bio Biochem 2: 32-35.
- Ghosh K, Muddeshwer MG, Ghosh K (2017) Ischaemia modified albumin test to detect early diabetic complications. Asian J Med Sci 34: 467-470.
- Turk A, Nuhoglu I, Mentese SC, Karahan SC, Erdol H, et al. (2011) The relationship between diabetic retinopathy and serum levels of ischemia-modified albumin and malondialdehyde. Retina 31: 602-608.
- Reddy VS, Sethi S, Agrawal P, Gupta N, Garg R (2016) Ischemia modified albumin (IMA) and albumin adjusted-IMA (AAIMA) as biomarkers for diabetic retinopathy Biomarkers for diabetic retinopathy. Nepal J Ophthalmol 7: 117-123.
- Amirtharaj GJ , Natarajan SK , Mukhopadhya A, Zachariah UG, Hegde SK, et al. (2008) Fatty acids influence binding of cobalt to serum albumin in patients with fatty liver. Biochimica et Biophysica Acta 1782: 349.
 Spanish
Spanish  Chinese
Chinese  Russian
Russian  German
German  French
French  Japanese
Japanese  Portuguese
Portuguese  Hindi
Hindi