Case Report, J Nephrol Ren Dis Vol: 6 Issue: 3
The Association between Serum Uric Acid Level and Hematological parameters in Healthy Chinese Adults
Zhibin Ye*, Xiaoli Zhang, Jing Xiao, Hui Guo, Fengqin Li, Chensheng Fu
Department of Nephrology, Huadong Hospital affiliated to Fudan University Shanghai, China
*Corresponding Author: Zhibin Ye Department of Nephrology, Huadong Hospital affiliated to Fudan University, Shanghai, China, E-mail: yezhibin3@126.com
Received: 11 December, 2019, Manuscript No. JNRD-19-5514; Editor assigned: 16 December, 2019, PreQC No JNRD-19-5514 (PQ); Reviewed: 30 December, 2019, QC No. JNRD-19-5514; Revised: 03 August, 2022, QI No. JNRD-19-5514, Manuscript No. JNRD-19-5514 (R); Published: 31 August, 2022, DOI: 10.4172/2576-3962.1000013.
Citation: Ye Z, Zhang X, Xiao J, Guo H, Li F, et al (2022) The Association between Serum Uric Acid Level and Hematological parameters in Healthy Chinese Adults. J Nephron Ren Dis 6:3.
Abstract
Background: A definite and uniform standard concerning urate lowering therapy for hyperuricemic patients with different risks is lacking. It is important to identify simple and available clinical indicators to assess the risk of hyperuricemia and guide stratified treatment. The aim of the present study was to explore the association of serum Uric Acid (UA) levels with hematological parameters in healthy Chinese adults.
Case Presentation: In this retrospective study, 657 Chinese adults (447 males, 210 females) who underwent health check-up Huadong hospital affiliated to Fudan University in Shanghai, China were sampled. males, females and postmenopausal females older than 50 years (called female subgroup) were divided into three tertiles according to serum UA respectively. Clinical characteristics and hematological parameters were compared in different UA tertiles. Correlation and multiple regression analysis were used to investigate the relationship of UA with hematological parameters.
Results: After adjusting for potential confounding factors, serum UA was positively associated with RBC count, Hb and HCT in males. Serum UA was positively associated with MPV in females and postmenopausal females. RBC count, Hb and HCT were significantly higher in UA3 than that in UA1 in males. MPV was significantly higher in UA3 than that in UA1 in all females and postmenopausal females.
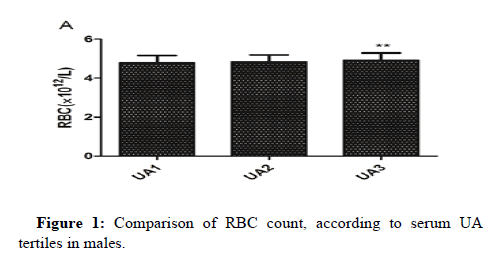
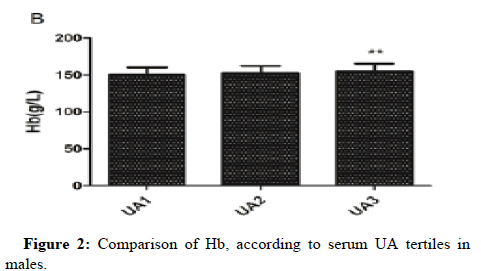
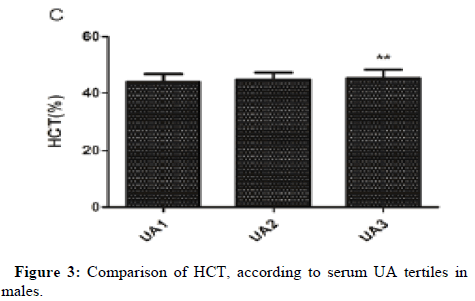
Conclusion: Our study demonstrated that serum UA was positively associated with RBC count, Hb and HCT in Chinese male adults and positively associated with MPV in Chinese female adults and postmenopausal females. Gender differences in the association between serum UA and MPV may not be related to estrogen levels. RBC count, Hb, HCT and MPV might assess the risk of hyperuricemia and guide stratified treatment.
Keywords: Serum Uric Acid; Hematological Parameters;
Chinese; Hyperuricemia; Hypertension
Introduction
In recent decades, the prevalence of hyperuricemia has gradually increased with the change of living habits and diet styles, which is almost 13.3% in China [1], 11.9% in Western countries [2] and 21.4% in US at present [3]. Hyperuricemia has become one of the major problems that endanger global public health. A large scale of studies have indicated that hyperuricemia is a risk factor for the incidence and development of obesity, hypertension, diabetes, dyslipidemia, cardiovascular disease, kidney disease and stroke [4-7]. However, it was also evidenced that asymptomatic hyperuricemia was not an independent risk factor for cardiovascular disease or mortality. Hyperuricemia did associate with an increased risk of cardiovascular death only in participants with gout and existing cardiovascular disease [8]. It is quite possible that different patients with hyperuricemia may have different risks for gout or renal and cardiovascular complications. Thus, Japanese society of Gout and nucleic acid metabolism established a "Guideline for the Management of hyperuricemia and Gout" in 2002, a revised version in 2010, so as to manage hyperuricemia according to the presence or absence of gouty arthritis or tophi or complications [9]. However, this guideline is according to the already existed complications of hyperuricemia but not to potential risks. A definite and uniform standard concerning urate lowering therapy for hyperuricemic patients with different risks is lacking. It is important to identify simple and available clinical indicators to assess the risk of hyperuricemia and guide stratified treatment.
Hematological parameters that mainly include peripheral blood cell count and its related functional parameters are accessible in clinical practice. Many evidences reveal that several hematological parameters are associated with a variety of diseases, especially cardiovascular disease and kidney disease. Elevated White Blood Cell (WBC) count might predict the odds of future kidney function decline in Chinese population with normal basic kidney function [10]. Hemoglobin (Hb) is reported to be related to hypertension and arterial stiffness. Red blood cell volume Distribution Width (RDW) could be an effective predictive index in evaluating diabetes nephropathy [11] and earlystage renal function damage in essential hypertensive patients [12]. MPV is a predictive factor of stroke, Acute Myocardial Infarction (AMI) and restenosis of coronary angioplasty [13]. Uric acid stimulation might influce peripheral blood cell number and function to a certain extent. However, the relationship of serum UA with hematological parameters and gender differences have not been investigated in Chinese adults. Therefore, in this present study, we included healthy Chinese participants to explore the association between serum UA and hematological parameters in different genders.
Case Presentation
Study participants
In this retrospective study, 657 subjects (447 males, 210 females) who underwent routine health examinations from January 1, 2010 to September 30, 2015 at Huadong hospital affiliated to Fudan University (Shanghai, P.R.China) were included. Those who had cardiovascular disease (coronary artery disease, congestive heart failure and atrial fibrillation), renal dysfunction (eGFR<60 mL/min/1.73 m2), hepatic dysfunction, malignant tumour, hematologic disease, rheumatic disease and recent infection (pneumonia, urinary tract infection and gastrointestinal infection) were excluded. Those who had taken UA-lowering drugs, such as benzbromarone, allopurinol or febuxostat were also excluded from this study. Our study was consented by the ethical review board of Huadong hospital affiliated to Fudan University and conformed to the ethics guidelines.
Biochemical measurements
Blood samples were collected in the morning after 12 hours of fasting and were then analyzed in the laboratory of Huadong hospital. Hematological parameters mainly include WBC count, neutrophil%, eosinophil%, basophil%, lymphocyte%, monocyte%, Red Blood Cell (RBC) count, Hb, HCT, Mean Corpuscular Volume (MCV), Mean corpuscular Hemoglobin (MCH), Mean Corpuscular Hemoglobin Concentration (MCHC), RDW, Platelet (PLT) count, MPV, Plateletcrit (PCT), Platelet Distribution Width (PDW). The serum levels of UA, Serum Creatinine (Scr), Erythrocyte Sedimentation Rate (ESR), High Sensitive C-Reactive Protein (hsCRP), Fasting Blood Glucose (FBG), postprandial 2-Hours Blood Glucose (2HBG), Fasting C-peptide (FC), postprandial 2-Hours C-Peptide (2HC), Fasting Insulin (FINS), postprandial 2-Hours Insulin (2HINS), Glycosylated Hemoglobin (HbA1c), Alkline Phosphatase (ALP), Total Cholesterol (TC), Triglycerides (TG), High-Density Lipoprotein (HDL), Low-Density Lipoprotein (LDL), Apolipoprotein A (ApoA), Apolipoprotein B (ApoB) and Estradiol (E2) were also measured. Both serum UA and Scr were measured by enzymatic methods. The estimated glomerular filtration rate (eGFR: milliliters per minute per 1.73 m2), an index of renal function, was calculated using the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) formula [14]. Insulin Resistance (IR) was assessed by Homeostasis Model of Assessment-Insulin Resistance (HOMA-IR) that was calculated as fasting glucose (mmol/L) × fasting insulin (mIU/L) /22.5. Basic features, including body weight and body height, were also recorded. Body mass index (BMI) was calculated as the ratio of weight (kg) divided by height square (m2).
Statistical analysis
Statistical analysis was performed using software SPSS version 17.0. The continuous clinical data were expressed as means ± Standard Deviation (SD). Males, females and postmenopausal females older than 50 years (called female subgroup) were divided into three tertiles respectively according to serum UA levels (UA1:UA<5.4 mg/dL, UA2:5.4 ≤ UA<6.7 mg/dL and UA3:UA ≥ 6.7 mg/dL for males; UA1:UA<4.5 mg/dL, UA2:4.5 ≤ UA<5.4 mg/dL and UA3:UA ≥ 5.4 mg/dL for females; UA1:UA<4.5 mg/dL, UA2:4.5 ≤ UA<5.5 mg/dL and UA3:UA ≥ 5.5 mg/dL for female subgroup). The clinical characteristics and hematological parameters among each of the three UA tertiles were compared using one-way Analysis of Variance (ANOVA) if the data were distributed normally. If the data were non-normally distributed, we used rank-sum test among groups. In ANOVA, we used Least Significance Difference (LSD) test if the variance is homogeneous, and used Tamhane's T2 test if not. Pearson correlation or Spearman rank correlation were used to acquire hematological parameters and biochemical factors associated with serum UA levels. Multiple linear regression analysis was subsequently performed to analysis the relationship between serum UA levels and hematological parameters by adjusting for potential confounding variables. P<0.05 was considered to be statistically significant for all analyses.
Results
Clinical characteristics in three UA turtles in both genders
To exclude the confounders that might interact the effect of UA on hematological parameters, we firstly explored the effect of UA on the clinical characteristics. Clinical characteristics and comparisons of characteristics were detailed. BMI, Scr, eGFR, UA, FC, 2HC, FINS, 2HINS, HOMA-IR, TC, TG and ApoB differed significantly among three UA turtles in males (Table 1).
| Variables | All males | UA1 | UA2 | UA3 | P value |
|---|---|---|---|---|---|
| (n=447) | (n=89) | (n=161) | (n=197) | ||
| UA<5.5 mg/dL | 5.5 ≤ UA<6.7mg/dL | UA ≥ 6.7 mg/dL | |||
| Age (years) | 54.36 ± 8.12 | 56.13 ± 8.61 | 54.46 ± 7.90 | 53.48 ± 7.96 | 0.053 |
| BMI (kg/m2) | 25.41 ± 3.00 | 25.11 ± 3.32 | 24.94 ± 3.08 | 25.93 ± 2.71* | 0.001 |
| Scr (µmol/L) | 78.11 ± 10.93 | 75.30 ± 11.17 | 77.43 ± 9.84 | 79.98 ± 11.37** | 0.002 |
| eGFR (mL/min/1.73m2) | 92.27 ± 16.02 | 95.86 ± 17.11 | 92.70 ± 14.43 | 90.25 ± 16.48** | 0.022 |
| UA (mg/dL) | 6.53 ± 1.27 | 4.81 ± 0.52 | 6.06 ± 0.33** | 7.69 ± 0.79** | <0.001 |
| ESR(mm/h) | 10.09 ± 8.23 | 9.85 ± 9.22 | 9.94 ± 7.93 | 10.31 ± 8.07 | 0.677 |
| hsCRP (mg/L) | 1.43 ± 3.21 | 1.11 ± 1.09 | 1.31 ± 2.37 | 1.62 ± 4.09 | 0.233 |
| FBG (mmol/L) | 5.64 ± 1.30 | 5.78 ± 1.93 | 5.67 ± 1.29 | 5.55 ± 0.90 | 0.684 |
| 2HBG (mmol/L) | 8.80 ± 3.25 | 9.20 ± 4.30 | 8.76 ± 3.11 | 8.64 ± 2.73 | 0.983 |
| FC (ng/ml) | 2.33 ± 1.01 | 2.11 ± 1.24 | 2.17 ± 0.82 | 2.56 ± 0.99** | <0.001 |
| 2HC (ng/ml) | 8.22 ± 4.05 | 7.24 ± 4.03 | 8.35 ± 3.87* | 8.60 ± 4.16** | 0.018 |
| FINS (mIU/L) | 11.21 ± 6.26 | 10.42 ± 4.94 | 10.50 ± 7.02 | 12.21 ± 6.09* | 0.001 |
| 2HINS (mIU/L) | 58.24 ± 43.07 | 49.12 ± 39.77 | 59.93 ± 40.15* | 61.29 ± 46.57* | 0.048 |
| HbA1c (%) | 5.75 ± 0.68 | 5.91 ± 1.04 | 5.72 ± 0.61 | 5.69 ± 0.51 | 0.609 |
| HOMA-IR | 2.95 ± 2.36 | 2.81 ± 1.80 | 2.79 ± 2.89 | 3.15 ± 2.10 | 0.012 |
| ALP (U/L) | 65.33 ± 18.67 | 64.37 ± 16.46 | 66.14 ± 17.72 | 65.11 ± 20.37 | 0.362 |
| TC (mmol/L) | 4.66 ± 1.03 | 4.46 ± 1.02 | 4.59 ± 1.06 | 4.81 ± 0.99** | 0.018 |
| TG (mmol/L) | 1.87 ± 1.35 | 1.48 ± 0.87 | 1.81 ± 1.51* | 2.10 ± 1.35** | <0.001 |
| HDL (mmol/L) | 1.17 ± 0.27 | 1.17 ± 0.26 | 1.19 ± 0.27 | 1.15 ± 0.28 | 0.479 |
| LDL (mmol/L) | 2.71 ± 0.82 | 2.62 ± 0.81 | 2.70 ± 0.80 | 2.75 ± 0.84 | 0.437 |
| ApoA (g/L) | 1.24 ± 0.25 | 1.22 ± 0.24 | 1.25 ± 0.25 | 1.24 ± 0.25 | 0.616 |
| ApoB (g/L) | 1.35 ± 8.02 | 2.77 ± 17.82 | 1.03 ± 1.54 | 0.96 ± 0.23** | 0.03 |
| E2 (pmol/L) | 159.41 ± 137.83 | 127.20 ± 74.47 | 172.79 ± 170.10 | 147.75 ± 71.98 | 0.786 |
Note: * P <0.05, ** P <0.01 vs UA1.
Table 1: Clinical characteristics and comparison of characteristics according to serum UA tertiles in males.
Note: BMI: Body Mass Index; Scr: Serum Creatinine; eGFR: Estimated Glomerular Filtration Rate; ESR: Erythrocyte Sedimentation Rate; hsCRP: High sensitive C-Reactive Protein; FBG: Fasting Blood Glucose; 2HBG: 2-Hours Blood Glucose; FC: Fasting C-peptide; 2HC: 2-Hours C-peptide; FINS: Fasting Insulin; 2HINS: 2-Hours Insulin; HbA1c: Glycosylated hemoglobin; HOMA-IR: Homeostasis Model of Assessment-Insulin Resistance, which was calculated as fasting glucose (mmol/L) × fasting Insulin (mIU/L)/22.5; ALP: Alkline Phosphatase; TC: Total Cholesterol; TG: Triglycerides; HDL: High-Density Lipoprotein; LDL: Low-Density Lipoprotein; ApoA: Apolipoprotein A; ApoB: Apolipoprotein B; E2: Estradiol.
Age, BMI, Scr, eGFR, UA, ESR, FBG, FC, 2HC, FINS, 2HINS, HbA1c, HOMA-IR, ALP, TG, HDL and ApoA differed significantly among three UA turtles in females (Table 2).
| Variables | All females | UA1 | UA2 | UA3 | P value |
|---|---|---|---|---|---|
| (n=210) | (n=72) | (n=65) | (n=73) | ||
| UA<4.5 mg/dL | 4.5 ≤ UA<5.4 mg/dL | UA ≥ 5.4 mg/dL | |||
| Age (years) | 54.90 ± 8.53 | 51.92 ±7.97 | 55.28 ± 8.85* | 57.51 ± 7.93** | <0.001 |
| BMI (kg/m2) | 23.37 ± 3.18 | 22.22 ± 2.86 | 23.21 ± 2.77 | 24.62 ± 3.26** | <0.001 |
| Scr (µmol/L) | 58.53 ± 10.13 | 56.16 ± 11.14 | 59.90 ± 9.04* | 59.75 ± 9.66* | 0.043 |
| eGFR (mL/min/1.73m2) | 96.54 ± 19.96 | 103.33 ± 23.15 | 93.01 ± 16.36** | 92.86 ± 17.76** | 0.002 |
| UA (mg/dL) | 5.02 ± 1.06 | 3.94 ± 0.45 | 4.91 ± 0.25** | 6.17 ± 0.69** | <0.001 |
| ESR (mm/h) | 16.27 ± 12.26 | 14.54 ± 11.34 | 14.11 ± 10.76 | 19.70 ± 13.60** | 0.003 |
| hsCRP (mg/L) | 1.33 ± 2.18 | 1.10 ± 1.02 | 1.17 ± 0.94 | 1.59 ± 3.11 | 0.701 |
| FBG (mmol/L) | 5.18 ± 0.69 | 5.02 ± 0.64 | 5.05 ± 0.55 | 5.45 ± 0.77** | <0.001 |
| 2HBG (mmol/L) | 8.34 ± 6.50 | 7.81 ± 2.55 | 9.18 ± 11.09 | 8.09 ± 2.60 | 0.709 |
| FC (ng/ml) | 2.29 ± 1.32 | 2.07 ± 1.53 | 2.05 ± 0.96 | 2.64 ± 1.34** | 0.002 |
| 2HC (ng/ml) | 7.78 ± 3.80 | 6.78 ± 4.15 | 7.47 ± 3.23 | 8.87 ± 3.70** | 0.018 |
| FINS (mIU/L) | 10.17 ± 5.31 | 9.10 ± 4.48 | 9.63 ± 4.76 | 11.49 ± 6.11** | 0.024 |
| 2HINS (mIU/L) | 57.08 ± 39.91 | 51.31 ± 40.31 | 50.14 ± 35.38 | 67.87 ± 41.46* | 0.031 |
| HbA1c (%) | 5.59 ± 0.57 | 5.50 ± 0.68 | 5.53 ± 0.36 | 5.73 ± 0.59** | <0.001 |
| HOMA-IR | 2.43 ± 1.46 | 2.08 ± 1.10 | 2.22 ± 1.24 | 2.88 ± 1.76** | 0.004 |
| ALP (U/L) | 67.79 ± 20.71 | 60.04 ± 18.92 | 70.14 ± 20.70** | 73.39 ± 20.36** | <0.001 |
| TC (mmol/L) | 5.06 ± 1.03 | 5.05 ± 0.93 | 5.01 ± 0.98 | 5.12 ± 1.17 | 0.804 |
| TG (mmol/L) | 1.31 ± 0.91 | 1.16 ± 1.13 | 1.32 ± 0.70** | 1.46 ± 0.83** | <0.001 |
| HDL (mmol/L) | 1.42 ± 0.37 | 1.49 ± 0.37 | 1.48 ± 0.41 | 1.29 ± 0.31** | 0.002 |
| LDL(mmol/L) | 2.95 ± 0.86 | 2.93 ± 0.77 | 2.88 ± 0.71 | 3.02 ± 1.04 | 0.61 |
| ApoA (g/L) | 1.39 ± 0.28 | 1.43 ± 0.29 | 1.43 ± 0.31 | 1.32 ± 0.22* | 0.023 |
| ApoB (g/L) | 0.94 ± 0.24 | 0.92 ± 0.26 | 0.92 ± 0.21 | 0.97 ± 0.25 | 0.445 |
| E2 (pmol/L) | 178.01 ± 316.73 | 196.67 ± 263.90 | 267.61 ± 455.75 | 64.94 ± 128.48 | 0.133 |
Note: * P <0.05, ** P <0.01 vs UA1.
Table 2: Clinical characteristics and comparison of characteristics according to serum UA tertiles in females. Note: Abbreviations are the same as Table 1.
BMI, Scr, eGFR, UA, ESR, FBG, FC, HbA1c, HOMA-IR, ALP, TG, HDL and ApoA differed significantly among three UA tertiles in female subgroup (Table 3).
| Variables | Female subgroup | UA1 | UA2 | UA3 | P value |
|---|---|---|---|---|---|
| (n=152) | (n=38) | (n=56) | (n=58) | ||
| UA<4.5 mg/dL | 4.5 ≤ UA<5.5 mg/dL | UA ≥ 5.5 mg/dL | |||
| Age (years) | 58.73 ± 6.34 | 57.89 ± 5.64 | 58.96 ± 6.16 | 59.05 ± 6.98 | 0.688 |
| BMI (kg/m2) | 23.93 ± 3.12 | 22.93 ± 3.15 | 23.65 ± 2.61 | 24.83 ± 3.35** | 0.01 |
| Scr (µmol/L) | 58.66 ± 10.16 | 54.62 ± 10.83 | 61.14 ± 9.40** | 58.94 ± 9.72* | 0.01 |
| eGFR (mL/min/1.73m2) | 94.99 ± 20.43 | 104.39 ± 25.63 | 90.29 ± 17.01** | 93.16 ± 17.58** | 0.003 |
| UA (mg/dL) | 5.23 ± 1.05 | 3.98 ± 0.45 | 4.99 ± 0.30** | 6.29 ± 0.70** | <0.001 |
| ESR (mm/h) | 17.12 ± 13.13 | 13.11 ± 12.14 | 16.14 ± 11.95 | 20.56 ± 14.02** | 0.001 |
| hsCRP (mg/L) | 1.41 ± 2.34 | 1.34 ± 1.27 | 1.25 ± 0.92 | 1.58 ± 3.36 | 0.802 |
| FBG (mmol/L) | 5.31 ± 0.71 | 5.20 ± 0.74 | 5.13 ± 0.51 | 5.57 ± 0.79* | 0.002 |
| 2HBG (mmol/L) | 8.57 ± 7.11 | 8.10 ± 2.40 | 9.30 ± 11.46 | 8.20 ± 2.69 | 0.56 |
| FC (ng/mL) | 2.41 ± 1.37 | 2.29 ± 1.78 | 2.21 ± 1.16 | 2.65 ± 1.25* | 0.02 |
| 2HC (ng/mL) | 8.08 ± 3.79 | 7.23 ± 4.22 | 7.53 ± 3.27 | 9.07 ± 3.77 | 0.06 |
| FINS (mIU/L) | 10.64 ± 5.65 | 9.97 ± 5.17 | 9.80 ± 5.02 | 11.79 ± 6.31 | 0.113 |
| 2HINS (mIU/L) | 60.46 ± 39.81 | 58.67 ± 39.04 | 54.04 ± 40.15 | 67.31 ± 39.75 | 0.215 |
| HbA1c (%) | 5.70 ± 0.59 | 5.70 ± 0.78 | 5.59 ± 0.35 | 5.79 ± 0.63 | 0.026 |
| HOMA-IR | 2.57 ± 1.55 | 2.32 ± 1.26 | 2.27 ± 1.26 | 2.99 ± 1.85 | 0.035 |
| ALP (U/L) | 71.88 ± 21.46 | 63.15 ± 21.83 | 74.04 ± 21.17* | 75.50 ± 20.25** | 0.015 |
| TC (mmol/L) | 5.16 ± 1.03 | 5.19 ± 0.82 | 5.05 ± 1.00 | 5.24 ± 1.19 | 0.596 |
| TG (mmol/L) | 1.33 ± 0.75 | 1.05 ± 0.61 | 1.35 ± 0.64** | 1.51 ± 0.88** | 0.001 |
| HDL (mmol/L) | 1.39 ± 0.36 | 1.49 ± 0.38 | 1.45 ± 0.36 | 1.27 ± 0.31** | 0.003 |
| LDL (mmol/L) | 3.03 ± 0.88 | 3.04 ± 0.74 | 2.91 ± 0.71 | 3.15 ± 1.09 | 0.355 |
| ApoA (g/L) | 1.40 ± 0.28 | 1.46 ± 0.30 | 1.44 ± 0.31 | 1.31 ± 0.22* | 0.013 |
| ApoB (g/L) | 0.95 ± 0.24 | 0.93 ± 0.26 | 0.92 ± 0.19 | 1.00 ± 0.26 | 0.123 |
| E2 (pmol/L) | 36.49 ± 48.60 | 37.87 ± 59.36 | 37.18 ± 43.69 | 34.59 ± 45.98 | 0.772 |
Note: * P <0.05, ** P <0.01 vs UA1.
Table 3: Clinical characteristics and comparison of characteristics according to serum UA tertiles in female subgroup. Note: Abbreviations are the same as Table 1.
Relationship between the serum UA levels and hematological parameters
Pearson correlation, Spearman rank correlation analysis showed that serum UA levels were positively associated with BMI (r=0.153, P=0.001), Scr (r=0.174, P<0.001), FC (r=0.273, P<0.001), 2HC (r=0.162, P=0.002), FINS (r=0.166, P=0.002), HOMA-IR (r=0.131, P=0.013), TC (r=0.157, P=0.001), TG (r=0.281, P<0.001) and ApoB (r=0.137, P=0.004) in males. Serum UA levels were negatively associated with age (r=-0.146, P=0.002) and eGFR (r=-0.131, P=0.006) in males.
Serum UA levels were positively associated with age (r=0.250, P<0.001), BMI (r=0.380, P<0.001), ESR (r=0.238, P=0.001), FBG (r=0.306, P<0.001), FC (r=0.307, P<0.001), 2HC (r=0.267, P=0.001), FINS (r=0.262, P=0.001), 2HINS (r=0.209, P=0.012), HbA1c (r=0.332, P<0.001), HOMA-IR (r=0.313, P<0.001), ALP (r=0.282, P<0.001) and TG (r=0.281, P<0.001) in females. Serum UA levels were negatively associated with eGFR (r=-0.192, P=0.006), HDL (r=-0.240, P<0.001), ApoA (r=-0.146, P=0.038) and E2 (r=-0.225, P=0.024) in females.
Serum UA levels were positively associated with BMI (r=0.309,P<0.001), ESR (r=0.320,P<0.001), FBG (r=0.266,P=0.001), FC (r=0.289,P=0.001), 2HC (r=0.256,P=0.005), FINS (r=0.218,P=0.004), HbA1c (r=0.197,P=0.017), HOMA-IR (r= 0.258,P=0.004), ALP (r=0.199,P=0.015) and TG (r=0.283,P<0.001) in female subgroup. Serum UA levels were negatively associated with eGFR (r=- 0.195,P=0.038), HDL (r=- 0.231,P=0.004) and ApoA (r=-0.182,P=0.028) in female subgroup. These indicators were considered as confounding factors for UA on hematological parameters.
Serum UA levels were positively associated with WBC count (r=0.113, P=0.017), RBC count (r=0.154, P=0.001), Hb (r=0.161, P=0.001) and HCT (r=0.165, P<0.001) in males before adjusting for the confounding factors. Serum UA levels were positively associated with WBC count (r=0.136, P=0.049), RBC count (r=0.246, P<0.001), Hb (r=0.240, P<0.001), HCT (r=0.256, P<0.001), MPV (r=0.176, P=0.01) and negatively associated with neutrophil% (r=-0.139, P=0.045) in females before adjusting for confounders. Serum UA levels were positively associated with RBC (r=0.217,P=0.007), Hb (r=0.202,P=0.012), HCT (r=0.240,P=0.003) and MPV (r=0.253,P=0.002) in female subgroup before adjusting for confounders.
After adjusting for confounding factors in three models in males (model 1 adjusted for age and BMI; model 2 adjusted for age, BMI, ESR, hsCRP, eGFR, FC, 2HC, FINS and HOMA-IR; model 3 adjusted for age, BMI, ESR, hsCRP, eGFR, FC, 2HC, FINS, HOMA-IR, TC, TG and ApoB), the association between serum UA and RBC count, Hb, HCT were still statistically significant, while the relationship of UA with WBC count disappeared (Table 4).
| Model 1 | Model 2 | Model 3 | |||||||
|---|---|---|---|---|---|---|---|---|---|
| B | SE | P | B | SE | P | B | SE | P | |
| WBC | 0.119 | 0.049 | 0.015 | 0.165 | 0.075 | 0.03 | 0.118 | 0.076 | 0.121 |
| RBC | 0.033 | 0.013 | 0.013 | 0.052 | 0.018 | 0.005 | 0.052 | 0.019 | 0.007 |
| Hb | 1.052 | 0.377 | 0.005 | 1.428 | 0.532 | 0.008 | 1.406 | 0.544 | 0.01 |
| HCT | 0.308 | 0.106 | 0.004 | 0.382 | 0.148 | 0.011 | 0.386 | 0.153 | 0.012 |
Table 4: Multiple linear regression analysis for the associations between serum UA (independent variable) and hematological parameters (dependent variables) in different models in males.
Note: Model 1 adjusted for age and BMI; model 2 adjusted for age, BMI, ESR, hsCRP, eGFR, FC, 2HC, FINS and HOMA-IR; model 3 adjusted for age, BMI, ESR, hsCRP, eGFR, FC, 2HC, FINS, HOMA-IR, TC, TG and ApoB. B, unstandardized coefficient that means the degree of change in hematological parameters per 1 mg/dL of serum UA increase; SE, standard error.
After the adjustment of confounders in females (model 1 adjusted for age and BMI; model 2 adjusted for age, BMI, ESR, hsCRP and eGFR; model 3 adjusted for age, BMI, ESR, hsCRP, eGFR, FC, HbA1c, HOMA-IR, ALP, TG, HDL, ApoA and E2), the association between serum UA and MPV remained statistically significant (Table 5).
| Model 1 | Model 2 | Model 3 | |||||||
|---|---|---|---|---|---|---|---|---|---|
| B | SE | P | B | SE | P | B | SE | P | |
| WBC | 0.169 | 0.089 | 0.06 | - | - | - | - | - | - |
| NEUT% | -1.134 | 0.613 | 0.065 | - | - | - | - | - | - |
| RBC | 0.068 | 0.022 | 0.002 | 0.087 | 0.03 | 0.004 | 0.005 | 0.072 | 0.945 |
| Hb | 1.583 | 0.63 | 0.013 | 2.096 | 0.832 | 0.013 | -0.968 | 1.995 | 0.631 |
| HCT | 0.506 | 0.179 | 0.005 | 0.612 | 0.246 | 0.015 | -0.11 | 0.586 | 0.853 |
| MPV | 0.21 | 0.076 | 0.006 | 0.25 | 0.093 | 0.008 | 0.435 | 0.181 | 0.023 |
Table 5: Multiple linear regression analysis for the associations between serum UA (independent variable) and hematological parameters (dependent variables) in different models in females.
Note: Model 1 adjusted for age and BMI; model 2 adjusted for age, BMI, ESR, hsCRP and eGFR; model 3 adjusted for age, BMI, ESR, hsCRP, eGFR, FC, HbA1c, HOMA-IR, ALP, TG, HDL, ApoA and E2. B, unstandardized coefficient that means the degree of change in hematological parameters per 1 mg/dL of serum UA increase; SE, standard error.
After the adjustment of confounders in female subgroup (model 1 adjusted for age and BMI;model 2 adjusted for age, BMI, ESR, hsCRP and eGFRmodel 3 adjusted for age, BMI, ESR, hsCRP, eGFR, FC, HbA1c, HOMA-IR, ALP, TG, HDL, ApoA and E2), the relationship between serum UA and MPV remained (Table 6).
Note: Model 1 adjusted for age and BMI; Model 2 adjusted for age, BMI, ESR, hsCRP and eGFR; Model 3 adjusted for age, BMI, ESR, hsCRP, eGFR, FC, HbA1c, HOMA-IR, ALP, TG, HDL, ApoA and E2. B, unstandardized coefficient that means the degree of change in hematological parameters per 1 mg/dL of serum UA increase; SE, standard error.
Hematological parameters in different UA tertiles
One-way ANOVA analysis revealed that RBC count, Hb and HCT in UA3 were significantly higher than that in UA1 in males. MPV in UA3 was significantly higher than that in UA1 in females and female subgroup (Figures 1-3).
Discussion
In this retrospective study, we explored the relationship of serum UA with hematological parameters and gender differences in healthy Chinese adults. The results indicated that serum UA was positively associated with RBC count, Hb and HCT in males and positively associated with MPV in females and postmenopausal females after adjusting for a variety of potential confounding factors. Gender differences in the association between serum UA levels and MPV may not be related to estrogen levels. RBC count, Hb, HCT and MPV might assess the risk of hyperuricemia and guide stratified treatment.
Hyperuricemia was positively associated with IR. IR is a prominent feature of a serious of metabolic disorders, including obesity, hypertension, diabetes mellitus, dyslipidemia and atherosclerotic cardiovascular disease. The previous study have certified that IR could increase erythropoiesis, ensuing HCT/Hb and consequently increase blood viscosity [15]. In our study, serum UA levels were positively associated with RBC, Hb and HCT in males. Higher HCT was linked to an increased risk of hyperuricemia, which has demonstrated in a recent prospective study [16]. A study showed that mean arterial blood pressure was positively associated with RBC count, Hb and HCT. Average arterial blood pressure was higher in males than in females and this was related to higher values of RBC count, Hb and HCT in males as compared to females [17]. In addition, a cross-sectional survey in Japan observed an independent positive relationship between Hb and increased arterial stiffness only in individuals with BMI <25 kg/m2. The multivariable adjusted odds ratios (OR) of 1-standard deviation increment in Hb for increased arterial stiffness were 1.40 in males and 1.19 in females [18]. A study showd that HCT was associated with prehypertension (systolic blood pressure: 120 mmHg-139 mmHg or diastolic blood pressure: 80 mmHg-89 mmHg) in people less than 60 years old [19]. A prospective study demonstrated that higher HCT, even within the normal range, was independently related to the incidence of hypertension in men [20]. Another prospective study found that HCT was independent predictors of type 2 diabetes mellitus in a graded fashion [21].
Platelet plays an important role in the formation of atherosclerotic plaque. Increased MPV was associated with shorter bleeding time and higher plasma thromboxane B2 levels, so MPV is a marker of PLT activation. A rat experiment found that serum UA inhibited Nitric Oxide (NO) production and consequently induced endothelial dysfunction [22]. IR related to reduction of NO synthesis was involved in the effect of UA on endothelial injury [23]. Local activation of oxidative stress and the renin-angiotensin system also mediated UA-induced endothelial dysfunction [24]. Cytokines released by dysfunctional endothelium might stimulate the bone marrow to produce large PLT. Besides, IR, oxidative stress and lower NO levels also directly influenced MPV [25,26]. A vitro study showed that urate crystals could induce platelet activation [27]. Our study showed a positive correlation of UA and MPV in females and postmenopausal females. Even the potential covariates of E2 was adjusted, this association remained. So we suggested that the gender-related difference in the association of serum UA with MPV might be caused by other factors but not estrogen levels. Recent studies have shown that MPV is an effective predictor and prognostic marker for cardiovascular disease. In patients with metabolic syndrome, CAD and stroke, levels of MPV increased [13]. A retrospective cohort study indicated that elevated MPV is associated with increased incidence of hypertension [28]. In addition, MPV can predict the risk of Acute Myocardial Infarction (AMI) and restenosis after coronary angioplasty [13]. A retrospective study included 553 patients with CKD, suggesting that MPV was negatively correlated with eGFR and significantly increased with progression of CKD [29]. A Chinese prospective stud showed that increased MPV was associated with all-cause mortality in patients with STEMI. MPV might be useful as a marker for risk stratification in Chinese patients with STEMI [30].
The present study possesses several noteworthy strengths. Firstly, we systematically and comprehensively explored the relationship between serum UA and hematological parameters in a healthy population, in order to rule out the influences of diseases. Secondly, we established rigorous exclusion criteria and made careful adjustments for potential confounding factors. Last and most importantly, we set up a female subgroup and adjusted for estrogen levels. The result demonstrated that gender-related difference in the association of serum UA with MPV may not be related to estrogen levels.
However, limitations should also be noticed. Firstly, this was a cross-sectional study, so we could not ascertain whether the relationship between serum UA and hematological parameters was an occasional phenomenon. Secondly, in our study, participants who underwent routine health check-up might not represent the general public, possibly leading to a selection bias. Lastly, because we did not get the information about smoking, alcohol and exercise habits, we could not adjust for these factors in multiple linear regression analysis.
Conclusion
The present study revealed the relationship of serum UA with hematological parameters in healthy Chinese adults and gender differences. The results indicated that serum UA was positively associated with RBC count, Hb and HCT in Chinese male adults, and was positively associated with MPV in Chinese female adults and postmenopausal females. Gender differences in the association between serum UA levels and MPV may not be related to estrogen levels. RBC count, Hb, HCT and MPV might assess the risk of hyperuricemia and guide stratified treatment. More work is needed to determine the cause-effect relationships between serum UA and hematological parameters.
Acknowledgement
Not applicable.
Disclosure Statement
All authors declare no conflicts of interest.
Funding Source
This research is supported by Shanghai municipal commission of health and family planning, key developing disciplines (grant number 2015ZB0501).
References
- Liu R, Han C, Wu D, Xia X, Gu J, et al. (2015) Prevalence of hyperuricemia and gout in mainland China from 2000 to 2014: A systematic review and meta-analysis. Bio Med Res Intern 1-12.
- Trifiro G, Morabito P, Cavagna L, Ferrajolo C, Pecchioli S, et al. (2013) Epidemiology of gout and hyperuricaemia in Italy during the years 2005-2009: A nationwide population-based study. Ann Rheu Dis 72: 694-700.
- Zhu Y, Pandya BJ, Choi HK (2011) Prevalence of gout and hyperuricemia in the US general population: The national health and nutrition examination survey 2007-2008. Arthr Rheu 63: 3136-3141.
- Mallat SG, Al Kattar S, Tanios BY, Jurjus A (2016) Hyperuricemia, hypertension and chronic kidney disease: An emerging association. Curr Hyperten Rep 18: 1-6.
- Sluijs I, Beulens JW, Van Der A DL, Spijkerman AM, Schulze MB, et al. (2013) Plasma uric acid is associated with increased risk of type 2 diabetes independent of diet and metabolic risk factors. J Nutri 143: 80-85.
- Wu AH, Gladden JD, Ahmed M, Ahmed A, Filippatos G (2016) Relation of serum uric acid to cardiovascular disease. Intern J Cardiol 213: 4-7.
- Li M, Hou W, Zhang X, Hu L, Tang Z (2014) Hyperuricemia and risk of stroke: A systematic review and meta-analysis of prospective studies. Atherosclero 232: 265-270.
- Nossent J, Raymond W, Divitini M, Knuiman M (2016) Asymptomatic hyperuricemia is not an independent risk factor for cardiovascular events or overall mortality in the general population of the busselton health study. Cardiovasc Disor 16: 1-8.
- Yamanaka H (2011) Japanese guideline for the management of hyperuricemia and gout. Nucleosi Nucleoti Nucl Aci 30: 1018-1029.
- Fan F, Jia J, Li J, Huo Y, Zhang Y (2017) White blood cell count predicts the odds of kidney function decline in a Chinese community-based population. Nephrol 18: 1-9.
- Zhang M, Zhang Y, Li C, He L (2015) Association between red blood cell distribution and renal function in patients with untreated type 2 diabetes mellitus. Ren Failure 37: 659-663.
- Li ZZ, Chen L, Yuan H, Zhou T, Kuang ZM (2014) Relationship between red blood cell distribution width and early-stage renal function damage in patients with essential hypertension. J Hypertens 32: 2450-2456.
- Vizioli L, Muscari S, Muscari A (2009) The relationship of mean platelet volume with the risk and prognosis of cardiovascular diseases. Intern J Clin Prac 63: 1509-1515.
- Delanaye P, Mariat C (2013) The applicability of eGFR equations to different populations. Nat Rev Nephrol 9: 513-522.
- Barbieri M, Ragno E, Benvenuti E, Zito GA, Corsi A, et al. (2001) New aspects of the insulin resistance syndrome: Impact on haematological parameters. Diabetol 44: 1232-1237.
- Zeng C, Wei J, Yang T, Li H, Xiao WF, et al. (2015) Higher blood hematocrit predicts hyperuricemia: A prospective study of 62897 person-years of follow-up. Sci Rep 5: 1-1.
- Gobel BO, Schulte-Gobel A, Weisser B, Glonzer K, Vetter H, et al. (1991) Arterial blood pressure: Correlation with erythrocyte count, hematocrit and hemoglobin concentration. Am J Hypertens 4: 14-9.
- Shimizu Y, Nakazato M, Sekita T, Kadota K, Yamasaki H, et al. (2014) Association between hemoglobin levels and arterial stiffness for general Japanese population in relation to body mass index status: The Nagasaki Islands study. Geria Gerontol Intern 14: 811-818.
- Liu X, Liang J, Qiu Q, Sun Y, Ying P, et al. (2015) Association of hematocrit and pre-hypertension among Chinese adults: The CRC study. Cell Biochem Biophys 71: 1123-1128.
- Jae SY, Kurl S, Laukkanen JA, Heffernan KS, Choo J, et al. (2014) Higher blood hematocrit predicts hypertension in men. J Hypertens 32: 245-250.
- Tamariz LJ, Young JH, Pankow JS, Yeh HC, Schmidt MI, et al. (2008) Blood viscosity and hematocrit as risk factors for type 2 diabetes mellitus: The Atherosclerosis Risk In Communities (ARIC) study. Am J Epidemiol 168: 1153-1160.
- Khosla UM, Zharikov S, Finch JL, Nakagawa T, Roncal C, et al. (2005) Hyperuricemia induces endothelial dysfunction. Kidney Intern 67: 1739-1742.
- Choi YJ, Yoon Y, Lee KY, Hien TT, Kang KW, et al. (2014) Uric acid induces endothelial dysfunction by vascular insulin resistance associated with the impairment of nitric oxide synthesis. 28: 3197-3204.
- Yu MA, Sánchez-Lozada LG, Johnson RJ, Kang DH (2010) Oxidative stress with an activation of the renin-angiotensin system in human vascular endothelial cells as a novel mechanism of uric acid-induced endothelial dysfunction. J Hypertens 28: 1234-1242.
- Santilli F, Vazzana N, Liani R, Guagnano MT, Davì G (2012) Platelet activation in obesity and metabolic syndrome. Obes Rev 13: 27-42.
- Guven A, Tolun F (2012) Effects of smokeless tobacco “Maras Powder” use on nitric oxide and cardiovascular risk parameters. Intern J Med Sci 9: 786.
- Ginsberg MH, Kozin F, O'malley M, McCarty DJ (1977) Release of platelet constituents by monosodium urate crystals. J Clin Inves 60: 999-1007.
- Gang L, Yanyan Z, Zhongwei Z, Juan D (2017) Association between mean platelet volume and hypertension incidence. Hypertens Res 40: 779-84.
- Ju HY, Kim JK, Hur SM, Woo SA, Park KA, et al. (2015) Could mean platelet volume be a promising biomarker of progression of chronic kidney disease? Platelets 26: 143-147.
- Sun XP, Li BY, Li J, Zhu WW, Hua Q (2016) Impact of mean platelet volume on long-term mortality in Chinese patients with ST-elevation myocardial infarction. Sci Rep 6: 1-5.
 Spanish
Spanish  Chinese
Chinese  Russian
Russian  German
German  French
French  Japanese
Japanese  Portuguese
Portuguese  Hindi
Hindi