Review Article, Arch Clin Pathol Vol: 1 Issue: 1
The Diagnosis and Investigation of Multiple Myeloma in 2018
Janin Alant1*, Roger Pool2, Jackie Thomson3 and Vanessa Moodley1
1Department of Haematology, Sefako Makgatho Health Sciences University and NHLS, Pretoria, South Africa
2Department of Haematology, University of Pretoria and NHLS, Pretoria, South Africa
3Medical Division, South African National Blood Service, Johannesburg, South Africa
*Corresponding Author : Janin Alant
Department of Haematology, Makgatho Health Sciences University and NHLS, Pretoria, South Africa
Tel: +27125214442
E-mail: janin.alant@smu.ac.za
Received: June 25, 2018 Accepted: August 03, 2018 Published: August 10, 2018
Citation: Alant J, Pool R, Thomson J, Moodley V (2018) The Diagnosis and Investigation of Multiple Myeloma in 2018. Arch Clin Pathol J 1:1.
Abstract
Abstract:
Background and objectives: Multiple myeloma is a complex neoplastic plasma cell disorder which necessitates a high index of suspicion and a rational approach to diagnosis. The diagnosis requires meeting specific criteria, which were revised in 2014 by the International Myeloma Working Group to enable earlier diagnosis and initiation of treatment. The aim of this article is to update medical and laboratory professionals in the approach to the diagnosis and investigation of multiple myeloma, as it is currently defined.
Method: A systematic review was conducted of the latest literature published on multiple myeloma, its diagnosis and investigation and its associated complications.
Results: A streamlined and step-wise approach to the investigation and diagnosis of multiple myeloma is proposed starting with initial screening tests, followed by diagnostic investigations and tests to rule out complications of multiple myeloma, and lastly investigations for staging and prognostication.
Conclusion: This approach will hopefully improve and standardise the current work-up of patients with multiple myeloma by detecting disease and complications earlier and avoiding inappropriate referrals and costly repeated or unnecessary investigations.
Keywords: Multiple myeloma; Diagnostic criteria; Investigation; Screening; Prognosis; Staging
Abbreviations
Computed Tomography (CT); Electrocardiography (ECG); Erythrocyte Sedimentation Rate (ESR); Fluorescent in situ Hybridization (FISH); Human Immunodeficiency Virus (HIV); Interleukin- 6 (IL-6); International Myeloma Working Group (IMWG); Lactate Dehydrogenase (LDH); Magnetic Resonance Imaging (MRI); Monoclonal Gammopathy of Undetermined Significance (MGUS); Multiple Myeloma (MM); Overall Survival (OS); Positron Emission Tomography- Computed Tomography (PET-CT); Pro-Brain Natriuretic Peptide (Pro-BNP); Receptor Activator of Nuclear Factor-κ B (RANK); Receptor Activator of Nuclear Factor-κ B ligand (RANKL); Revised International Staging System (R-ISS); Serum free light chains (SFLC) Serum free light chains (SFLC) Smouldering Multiple Myeloma (SMM); Tumour Necrosis Factor α (TNFα).
Introduction
The diagnosis of multiple myeloma (MM) has evolved since the major and minor criteria of Durie and Salmon, which were first published in 1985 [1]. A number of important revisions have been made to the diagnostic criteria since then, the latest being in 2014, yet many reference articles and websites still refer to the major and minor criteria for diagnosis [2]. Employing these outdated criteria might lead to an avoidable delay in treatment which greatly impacts on the quality of life and overall survival of these patients. The aim of this article is to provide an update on and a streamlined approach to the diagnosis and investigation of MM, as it is currently defined.
What is MM?
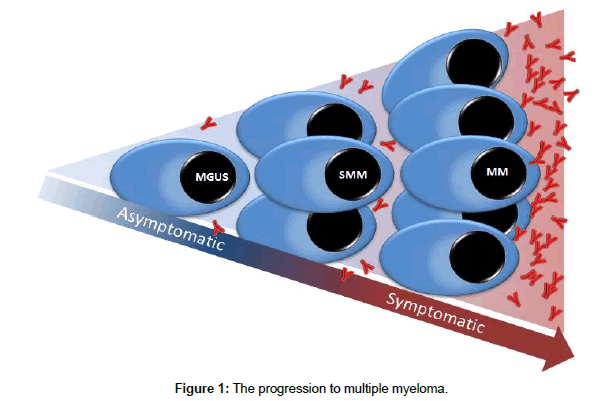
MM is a haematological malignancy characterized by the abnormal proliferation of clonal plasma cells in the bone marrow [3]. The clonal plasma cells secrete large amounts of partial or complete monoclonal immunoglobulins into the serum and/or urine (except in cases of non-secretory myeloma), also referred to as M-protein or paraprotein. MM derives from an asymptomatic pre-malignant condition known as monoclonal gammopathy of undetermined significance (MGUS) at a rate of approximately 1% per year. An intermediate stage between MGUS and MM, known as smouldering multiple myeloma (SMM), has a higher risk of progression to MM of approximately 10% per year [4] (Figure 1).
Epidemiology
Worldwide, MM is the second most common haematological malignancy after non-Hodgkin lymphoma [4]. Recent statistics on the incidence of MM in South Africa are lacking. According to the latest 2013 South African National Cancer Registry report the total number of patients diagnosed with MM in 2013 was 297, comprising 0.41% of all cancers diagnosed in South Africa in that year [5]. MM is twice as common in the South African black population as in the white population [6]. The peak age of onset is in the 6th to 7th decade, however patients in South Africa often present at a much younger age (4th decade) [7].
Pathogenesis
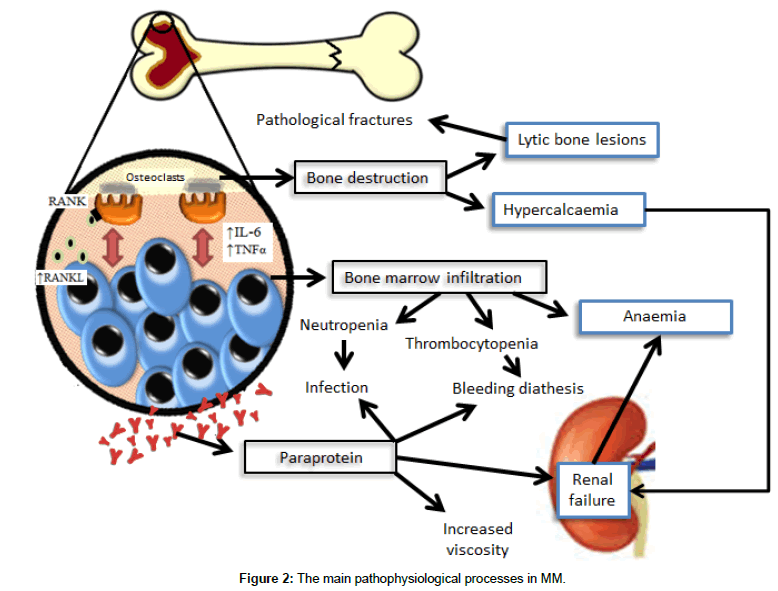
Clonal plasma cells in the bone marrow interact with stromal cells leading to the release of cytokines, such as IL-6 and TNFα, which promote myeloma cell survival and migration. Myeloma cells produce RANKL which binds to RANK receptors on osteoclasts leading to osteoclast activation. This increased osteoclast activity results in bone destruction, lytic bone lesions and hypercalcaemia. Renal impairment is a direct result of tubular damage by paraprotein and hypercalcaemia with intra-tubular calcium deposition and/or amyloidosis. Causes of anaemia in MM include bone marrow infiltration, decreased erythropoietin production and renal failure. MM patients have an increased risk of infection due to hypogammaglobulinaemia and an increased incidence of neutropenia [4]. The main pathophysiological processes in MM are illustrated in Figure 2.
Clinical features
MM is often diagnosed incidentally during routine check-up or when a flag is raised by a large gap between the total protein and albumin levels or a markedly raised erythrocyte sedimentation rate (ESR). The most common presenting symptoms are bone pain and fatigue. Osteolytic lesions and anaemia are present in about three quarters of patients with MM and renal failure and hypercalcaemia in 20% and 15% respectively [8]. Other clinical features include pathological fractures, spinal cord compression, peripheral neuropathy, weight loss, recurrent infections and, less commonly, features of amyloidosis, hyperviscosity and extramedullary plasmacytomas [4]. As a result of the non-specific range of clinical manifestations, myeloma patients present to general practitioners as well as a wide range of specialist disciplines.
The investigation of MM
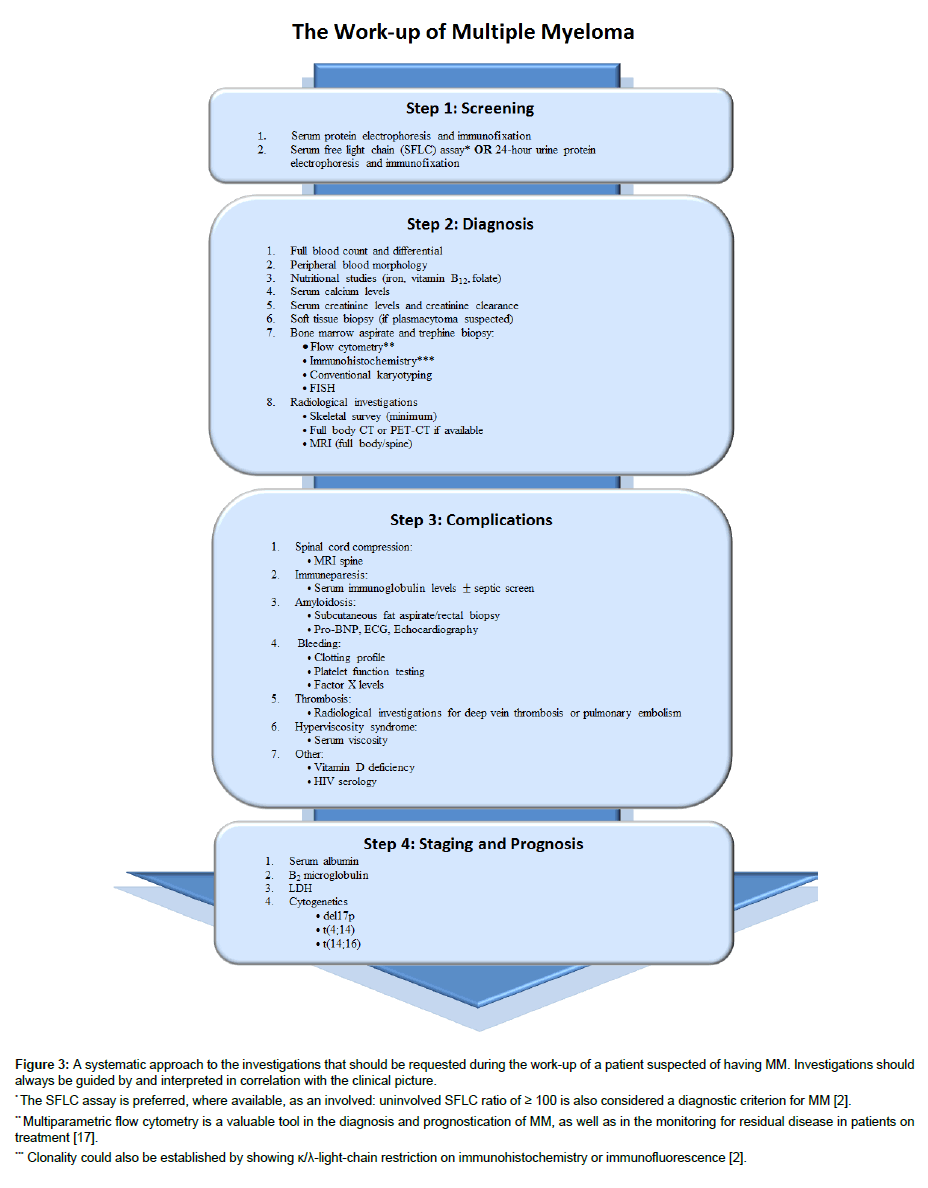
The work-up and investigation for suspected MM should be approached systematically, as illustrated in Figure 3.
Figure 3: A systematic approach to the investigations that should be requested during the work-up of a patient suspected of having MM. Investigations should always be guided by and interpreted in correlation with the clinical picture.
* The SFLC assay is preferred, where available, as an involved: uninvolved SFLC ratio of ≥ 100 is also considered a diagnostic criterion for MM [2].
** Multiparametric flow cytometry is a valuable tool in the diagnosis and prognostication of MM, as well as in the monitoring for residual disease in patients on treatment [17].
*** Clonality could also be established by showing κ/λ-light-chain restriction on immunohistochemistry or immunofluorescence [2].
Screening investigations: MGUS is estimated to be present in 3.2% of the white population and is approximately twice as high in the black population [9]. Population screening programmes for MGUS are however not currently recommended due to the low risk of progression, cost and potential patient anxiety [10]. However, all patients who present with either back pain, unexplained anaemia, renal impairment, hypercalcaemia, age-inappropriate osteopenia, or osteolytic lesions should be screened for the presence of an M-protein [9]. The Mayo clinic reports the combination of a serum protein electrophoresis and immunofixation and either a serum free light chain assay or a 24-hour urine protein electrophoresis and immunofixation as being able to detect 97% of patients with MM [8].
Diagnostic investigations: Previously, the diagnosis of multiple myeloma required laboratory and/or radiological evidence of endorgan damage, the so called CRAB criteria (hypercalcaemia, renal failure, anaemia and/or lytic bone lesions), in addition to the presence of an M-protein in serum/urine and clonal bone marrow plasma cells or plasmacytoma [3]. Patients who did not fulfil at least one of the CRAB criteria were observed until such evidence was detected, thereby qualifying them for the diagnosis of MM and the initiation of therapy. These criteria were used because, at the time, treatment options for MM were very limited and the potential side-effects did not justify early intervention. However, recent dramatic advances in myeloma treatment have resulted in a substantial improvement in the survival of myeloma patients. Delaying treatment until the onset of end-organ damage is therefore no longer justifiable. As a result, the diagnostic criteria for MM were revised by the International Myeloma Working Group (IMWG) in 2014 in order to facilitate earlier diagnosis and initiation of treatment, even before end-organ damage occurs [2] (Table 1).
| Diagnostic criteria for multiple myeloma | ||
|---|---|---|
| 1 | Clonal bone marrow plasma cells ≥ 10% or a biopsy-proven bony or extramedullary plasmacytoma | |
| 2 | Any one or more of the following myeloma defining events: | |
| a. Evidence of end-organ damage that can be attributed to the underlying plasma cell proliferative disorder: | Hypercalcaemia: Serum calcium>0.25mmol/L above the upper limit of normal or>2.75mmol/L | |
| Renal insufficiency: Creatinine clearance<40ml/min or serum creatinine>177umol/L | ||
| Anaemia: Haemoglobin>2g/dL below the lower limit of normal or haemoglobin<10g/dL | ||
| Bone lesions: One or more osteolytic lesions on skeletal radiography, CT or PET-CT | ||
| b. Any one or more of the following biomarkers of malignancy: | Clonal bone marrow plasma cells percentage ≥ 60% | |
| Involved: Uninvolved serum free light chain ratio ≥ 100 | ||
| >1 focal lesions on MRI | ||
Table 1: 2014 IMWG Diagnostic criteria for multiple myeloma [2].
A notable change in the new diagnostic criteria is the absence of an M-protein requirement. This modification was made in order to include those 3% of myeloma cases known as non-secretory myeloma where no M-protein can be detected but all other features of MM are present [2].
All CRAB features used for diagnosis must be attributable to the underlying MM. Megaloblastic anaemia should be excluded, especially if a macrocytic anaemia (not uncommon in MM) is present [11]. Hypocalcaemia in the absence of clear bone disease must be carefully investigated to rule out other causes such as hyperparathyroidism [2].
A plain radiograph of the entire skeleton is the minimum radiological examination required for the investigation of osteolytic lesions. Low dose full body CT or PET-CT is more sensitive but is not always readily available. An MRI is indicated in patients with suspected SMM or to exclude spinal cord compression in patients with osteolytic lesions of the spinal vertebrae (see Investigating for complications) [12].
In addition to the traditional CRAB criteria, three biomarkers of malignancy are now included in the diagnostic criteria of MM. These biomarkers accurately identify those patients, who would previously have been diagnosed as having SMM, who are at imminent risk of developing end-organ damage (>80% probability of progression within 2 years). The three myeloma biomarkers include a bone marrow aspirate/biopsy clonal plasmacytosis of ≥ 60%, a ratio of involved (clonal light chain) to uninvolved (normal residual light chain) serum free light chains of ≥ 100 and >1 focal lesion (larger than 5 mm) on MRI. The presence of any one of these, even in the absence of CRAB criteria, together with at least 10% clonal plasma cells in the bone marrow or a biopsy proven plasmacytoma, is sufficient to diagnose MM. It is likely that more such biomarkers will be added to the diagnostic criteria in future [2].
Investigating for complications: Once the diagnosis has been confirmed, specific complications associated with MM should be excluded.
The spine is the most affected skeletal organ and spinal cord compression is reported to occur in up to 24% of patients with myeloma spinal disease. Spinal cord compression constitutes a medical emergency and requires prompt treatment to prevent permanent disability [13].
Infection is the leading cause of death in MM. Immune paresis due to hypogammaglobulinaemia makes patients especially susceptible to infection by encapsulated organisms such as Haemophilus influenzae and Streptococcus pneumonia [4].
The features of primary AL amyloidosis occur as a result of the deposition of myeloma proteins into target organs such as the heart, kidneys, gastro-intestinal tract and central nervous system. AL amyloidosis should be suspected in all myeloma patients with nephrotic syndrome, cardiomyopathy, hepatomegaly, or peripheral neuropathy. Bleeding in patients with MM can occur as a result of AL amyloidosis (amyloid deposition in small vessels or acquired factor X deficiency), uraemia, thrombocytopenia or hyperviscosity syndrome [4].
Myeloma patients are at an increased risk of thrombosis due to the malignancy associated thrombophilic state, paraprotein-related mechanisms, treatments such as thalidomide or lenalidomide and a range of other risk factors such as immobility, infections and renal failure [13].
Hyperviscosity syndrome is seen in less than 10% of patients with MM and most commonly in those with an IgA paraprotein. It should be suspected in patients presenting with cutaneous or mucosal bleeding, blurred vision, headache, neurological symptoms or deafness [4].
An association has been found between MM and vitamin D deficiency in some patients. As Vitamin D plays a critical role in calcium absorption and bone metabolism, vitamin D deficiency should also be excluded in all patients [14]. Testing for Human Immunodeficiency Virus (HIV) infection should be considered especially in younger patients with MM and patients with atypical presentations [15].
Investigations for staging and prognostication: The clinical course of MM is heterogeneous with survival ranging from a few months to more than 10 years. Prognosis is influenced by a number of factors including age, performance status, comorbidities as well as the disease stage at diagnosis. The IMWG developed the Revised International Staging System (R-ISS) in 2015 which combines important biomarkers of disease burden, tumour biology and chromosomal abnormalities in patients with newly diagnosed MM (Table 2). The prognostic impact of R-ISS on overall survival (OS) was confirmed independently of patient age and treatment regimen [16,17].
| Staging system for multiple myeloma | ||
|---|---|---|
| Stage I | All of the following: a. Serum albumin ≥ 35g/L b. Serum β2-microglobulin ≤ 3.5mg/L c. Serum LDH<upper limit of normal d. No high risk cytogenetic abnormalities |
5 Year OS of 82% |
| Stage II | Not fitting stages I or III | 5 Year OS of 62% |
| Stage III | All of the following: a. Serum β2-microglobulin ≥ 5.5mg/L b. LDH>upper limit of normal OR presence of high-risk cytogenetic abnormalities: del 17p t(4;14) t(14;16) |
5 Year OS of 40% |
Table 2: R-ISS Staging system for multiple myeloma [16].
MM and Human Immunodeficiency Virus (HIV)
Although MM is not considered an AIDS-defining condition, HIV-infected patients have been reported to have a 4.5-fold increased risk of MM [15]. HIV-associated MM typically presents in patients under the age of 40. The clinical manifestations of MM in HIV are often atypical and include large malignant effusions, hyperviscosity, extramedullary plasmacytomas in unusual locations and plasma cell leukaemia [15].
Diagnosing MM in an HIV-infected patient poses several unique diagnostic challenges. Reactive bone marrow plasmacytosis, monoclonal gammopathy and anaemia are commonly encountered in HIV-infected patients and the diagnosis of MM is therefore often delayed or overlooked [18]. In addition, attributing CRAB features such as anaemia and renal failure to myeloma as opposed to the HIV infection or its complications can be challenging. Therefore, additional work-up to exclude opportunistic infections, antiretroviral complications and other HIV-related causes of anaemia and/or renal failure is advised and evidence of lytic lesions and hypercalcaemia should be sought to support the diagnosis [19].
Conclusion
MM is a common haematological malignancy, however the diverse range of clinical manifestations, the use of outdated diagnostic criteria and/or a general lack of awareness often causes the diagnosis to be delayed or overlooked. While the outlook in these patients used to be poor, survival has improved remarkably with the development of more effective treatments, thereby emphasizing the importance of early diagnosis. A rational approach to the diagnosis and investigation of MM is recommended to avoid unnecessary delays in treatment and inappropriate referrals.
References
- Durie BG, Salmon SE (1985) Staging kinetics, and flow cytometry of multiple myeloma. Neoplastic Diseases of the Blood. Churchill Livingstone, London, UK.
- Rajkumar SV, Dimopoulos MA, Palumbo A, Blade J, Merlini G, et al. (2014) International Myeloma Working Group updated criteria for the diagnosis of multiple myeloma. Lancet Oncol 15: 538-548.
- Swerdlow SH, Campo E, Harris NL, Jaffe ES, Pileri SA, et al. (2017) WHO Classification of tumours of haematopoietic and lymphoid tissues. IARC Press, Lyon, France.
- O’Donnell E, Cottini F, Raje N, Anderson K (2016) Myeloma. Williams Hematology. McGraw-Hill Education, New York, USA.
- The Cancer Association of South Africa (2017) The Cancer Association of South Africa: 2013 National Cancer Registry. CANSA Johannesburg, South Africa.
- Blattner W, Jacobson R, Shulman G (1979) Multiple myeloma in South African blacks. Lancet 313: 928-929.
- Thomson J (2007) Management of multiple myeloma. Continuing Medical Education 25: 276-278.
- Rajkumar SV, Kumar S (2016) Multiple myeloma: Diagnosis and treatment. Mayo Clin Proc 91: 101-119.
- Kyle RA, Buadi FI, Rajkumar SV (2011) Management of monoclonal gammopathy of undetermined significance (MGUS) and smoldering multiple myeloma (SMM). Oncology (Williston Park) 25: 578-586.
- Fuerst M (2015) New data provoke screening debate about MGUS and multiple myeloma. Oncology Times 37: 1-27.
- Smith A, Wisloff F, Samson D (2006) Guidelines on the diagnosis and management of multiple myeloma. Br J Haematol 132: 410-451.
- Molloy S, Lai M, Pratt G, Ramasamy K, Wilson D, et al. (2015) Optimizing the management of patients with spinal myeloma disease. Br J Haematol 171: 332-343.
- Terpos E, Kleber M, Engelhardt M, Zweegman S, Gay F, et al. (2015) European myeloma network guidelines for the management of multiple myeloma-related complications. Haematologica 100: 1254-1266.
- Ravenborg N, Udd K, Berenson A, Costa F, Berenson JR (2014) Vitamin D levels are frequently below normal in multiple myeloma patients and are infrequently assessed by their treating physicians. Blood 124: 5769.
- Cheung MC, Pantanowitz L, Dezube BJ (2005) AIDS-related malignancies: Emerging challenges in the era of highly active antiretroviral therapy. Oncologist 10: 412-426.
- Palumbo A, Avet-Loiseau H, Oliva S, Lokhorst HM, Goldschmit H, et al. (2015) Revised International staging system for multiple myeloma: A report from international myeloma working group. J Clin Oncol 33: 2863-2869.
- Jelinek T, Bezdekova R, Zatopkova M, Burgos L, Simicek M, et al. (2017) Current applications of multiparameter flow cytometry in plasma cell disorders. Blood Cancer J 7: e617.
- Saif MW, Shannon K (2005) Multiple myeloma and HIV infection: An association or a coincidence. J Appl Res 5: 318-324.
- Coker WJ, Jeter A, Schade H, Kang Y (2013) Plasma cell disorders in HIV-infected patients: Epidemiology and molecular mechanisms. Biomark Res 1: 8.
 Spanish
Spanish  Chinese
Chinese  Russian
Russian  German
German  French
French  Japanese
Japanese  Portuguese
Portuguese  Hindi
Hindi