Review Article, Arch Clin Pathol Vol: 1 Issue: 1
The Neural Cell Adhesion Molecule (NCAM): From Memory Formation to Cancer
Elroy Patrick Weledji*
Department of Anatomy and Surgery, Faculty of Health Sciences, University of Buea, Buea, Cameroon
*Corresponding Author : Elroy Patrick Weledji
Senior Lecturer in Anatomy and Surgery, Faculty of Health Sciences, University of Buea, Buea, Cameroon
Tel: +237699922144
E-mail: elroypat@yahoo.co.uk
Received: May 28, 2018 Accepted: July 13, 2018 Published: July 19, 2018
Citation: Weledji EP (2018) The Neural Cell Adhesion Molecule (NCAM): From Memory Formation to Cancer. Arch Clin Pathol J 1:1.
Abstract
The ubiquitous feature of NCAM highlights the importance of biological communication and recognition. Cellular recognition through the surface membrane holds the key to understanding the complexities of memory formation, motility disorders, cancer and other major human diseases. Cell-cell adhesion is a major aspect in the metastatic process of cancer. The immunoglobulin superfamily (Ig-SF) which includes NCAM and variants play a central role. NCAM can exert both a positive and a negative regulation on cancer progression, depending on the tumour context. Targeting these adherent surface glycoproteins may enhance the adjuvant treatment of cancers especially those not responsive to conventional systemic chemotherapy.
Keywords: Neural cell adhesion molecule; Synaptic plasticity; Pseudo-obstruction; Cancer; Metastasis formation
Abbreviations
NCAM: Neural Cell Adhesion Molecule; FGFR: Fibroblast Growth Factor Receptor; CaMK: Calcium Calmodulin Kinase; PKC: Protein Kinase C; Src: Src Family Kinases; MEK: Mitogen Activated Protein Kinase; ERK: Extracellular Signal-Regulated Kinase; CREB: cAMP Response Element-Binding Protein.
Introduction
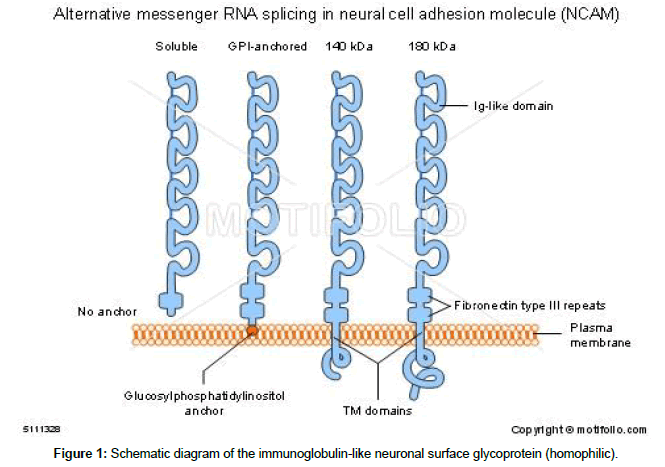
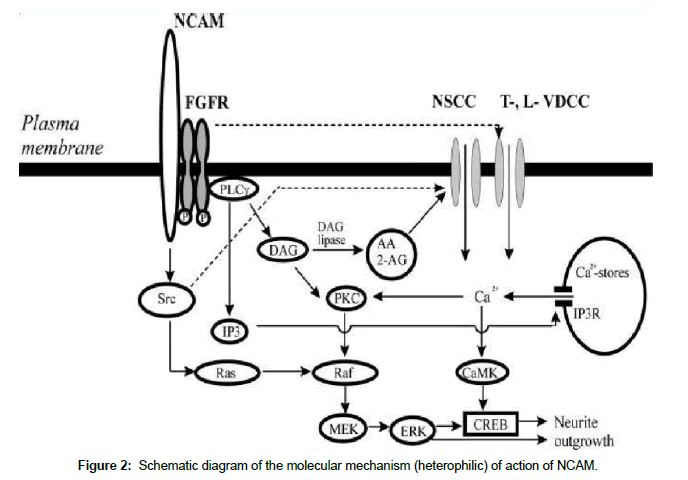
The neural cell adhesion molecule (NCAM) is an immunoglobulin-like neuronal surface glycoprotein which binds to a variety of other cell adhesion proteins to mediate adhesion, guidance and differentiation during neuronal growth (Figure 1). Four main isoforms (NCAM, PSACAM, L1CAM, receptor tyrosine kinase (RTK)) exist but there are many other variants resulting from alternative splicing and posttranslational modifications. At least 27 alternatively spliced NCAM mRNAs are produced giving a wide diversity of NCAM isoforms [1,2]. It is widely but transiently expressed in many tissues early in embryogenesis. The developmental replay of NCAM and variants, PSA CAM, L1CAM are the basis of synaptic plasticity, memory formation, central nervous system (CNS) injury repair and expression in advanced carcinomas. Their interactions with the interstitial cells of Cajal-(the pacemaker cells of the gut) is the basis of gut motility disorders, and, with the receptor tyrosine kinase is the basis of the formation of gastrointestinal stromal tumours (GIST). By mediating cell adhesion to other cells and to the extracellular matrix via the extracellular domain (NCAMNCAM homophilic interaction) and intracellular signalling (N-CAM –fibroblast growth factor receptor heterophilic interaction) [2-5], NCAM influences cell migration, neurite extension, fasciculation and formation of synapses in the brain. NCAM being a specific neural cell surface glycoprotein that mediates neural cell adhesion consists of (1) an extracellular N-terminal binding domain, (2) an extracellular sialic acid binding domain and (3) a membrane associated domain (Figure 2). Each molecule is heavily glycosylated by units of 10-12 sialic residues in alpha 2-8 linkages. The strength of the interaction between two molecules of NCAM would be dependent on the amount of negatively-charged sialic acid present. The more extracellular sialic acid binding domain present, the greater the repulsion, and thus less adhesion. The embryonic form of NCAM is highly glycosylated. This would be expected since there would be more repulsion than adhesion concurrent with the idea that not many neuronal pathways would have been made permanent. The sialic acid content, which is controlled by a sialy transferase enzyme, has been found to decrease during development from 30% (w/w) to 10% in the adult, thereby leading to an increase in adhesion and stabilization of the synaptic network [6,7]. Thus, the manifestation of neuronal plasticity (axonal sprouting and formation of new synapses) in memory formation and nerve regeneration through a neurodevelopmental replay. The ubiquitous FGFR is a member of the receptor tyrosine kinase (RTK) family and inhibitors of both RTKs and the Src (protein tyrosine kinases) family such as lavandustin A affects NCAM-induced signalling and thus neurite outgrowth [7]. In Alzheimer’s disease where plaque deposits affect the neurofibrillary protein network, a replay of NCAM neurodevelopmental events in memory formation may be inhibited, and, in ageing the loss of neurons may limit the synaptic plasticity associated with memory acquisition and consolidation. L1CAM glycoprotein alteration may cause a qualitative defect in the differentiated Cajal’s (pacemaker) cells in the anterior part of the gut causing a variable clinical spectrum of motility disorders ranging from the chronic intestinal pseudo-obstruction of the newborn (CIPO) to the qualitative defect in the migration of the neural crest cells in the distal segment of the gut in Hirschsprung’s disease. The NCAM cellular activities are re-expressed in tumour angiogenesis. A summary of the ubiquitous feature of NCAM are shown in Table 1.
| NCAM/PSA-NCAM | L1 CAM | KIT receptor tyrosine kinase |
|---|---|---|
| Plasticity of synaptic connections | Gut motility: | Gut smooth muscle function and fibroblast growth factor |
| I. Memory formation | Gastrointestinal pacemaker cells (interstitial cells of Cajal). | |
| II. Learning | ||
| III. CNS injury repair | ||
| Disorders: Alzheimer’s | L1CAM mutation: | c-KIT gene mutation: |
| DEPRESSION | CIPO | Gastrointestinal stromal tumours (GISTS) |
| Tumour angiogenesis | Hirschsprung’s | Gastrointestinal autonomic nerve tumour (GANT) |
| Obstructive hydrocephalus | ||
| Carcinomas |
Table 1: Summary of neural cell adhesion molecules.
Discussion
NCAM, memory formation and central nervous system (CNS) inury
Synaptic plasticity and clinical implications: It is the plasticity of neuronal networks that form the basis of short-term memory formation learning, and functional recovery following CNS injury. The NCAM glycoforms can be post translationally modified by the addition of polysialic acid (PSA). This decreases its homophilic binding properties that would lead to reduced cell adhesion and facilitate cell migration and invasion. As the CNS glial cells (e.g. oligodendrocytes) inhibit regeneration of CNS axons following injury unlike the facilitating Schwann cells of peripheral nerves, synaptic plasticity renders some functional recovery following CNS injury such as brain injury. There is re-expression of molecular cues normally seen in development (a localized neurodevelopmental replay), for the guidance of collateral sprouts to the vacated synaptic sites, according to a hierarchy of specificities. Functional recovery is more favourable in the younger animal with greater plasticity of synaptic connections [8]. Learning refers to the processes whereby new knowledge about events in the surroundings are acquired and memory refers to the processes through which knowledge is retained. In memory formation, there is an increased resialylation of NCAM molecules leading to new neuronal connections [9]. The memory trace is manifested through a replay of NCAM neurodevelopmental events which involves an initial proliferation and stabilization of some of these new synapses. The negative charge of the sialic acid components repels each other, giving rise to less adhesion and stability until the memory trace is consolidated. In about the first hour post-passive avoidance training in rats, there is an over-production of synapses, NCAM becomes heavily sialylated at 12 h and remain so until 24 h during which time synapse selection is believed to occur, thereafter, NCAM sialylation is gradually lost (desialylation) [9]. Memory is consolidated by gene transcription>4 hs later [10,11]. The effects of protein synthesis inhibitors suggest that protein synthesis is not necessary for the initial stages of memory formation but is required for the later events leading to memory consolidation [11]. The use of an antibody specific for the alpha-2, 8 sialic acid linkages of resialylated NCAM may prove useful in demonstrating the expression of the polysialylated PSA-NCAM which contributes more specifically and dynamically to the structural plasticity in the brain. The anti-PSA-NCAM antibody works well in the staining of rat brains [10]. Removal of polysialic acid (PSA) from NCAM by the enzyme endoneuraminidase (EndoN) has been shown to abolish long-term potentiation (LTP) and longterm depression [5], and intraventricular anti-NCAM decrease memory formation [9]. Plaque deposits in Ahlzeimer’s disease affect the neurofibrillary protein network inhibiting the replay of NCAM. The NCAM being cleaved by beta-site amyloid precursor protein cleaving enzyme (BACE) [12]. In ageing, the loss of neurons limit the synaptic connectivity changes and the ‘neuronal plasticity’ theory may indicate the potential role of NCAM/PSA-NCAM proteins in clinical depression [13].
NCAM and motility disorders
L1CAM and chronic intestinal pseudo-obstruction (CIPO): Congenital pseudo-obstruction is a broad clinical spectrum of motility disorders of closely similar aetiology that extends from the chronic intestinal pseudo-obstruction of the newborn (CIPO) to Hirschsprung’s disease [14-17]. It is commonly neuropathic than myopathic in origin and, may be primary (familial) or secondary to in-utero insults e.g. foetal-alcohol syndrome or post-natal injuries such as ischaemic events or viral infections. Recently, abnormalities of the gastrointestinal pacemaker cells (the interstitial cells of Cajal) have been described in patients with motility disorders [18,19]. Alterations of the neural cell adhesion molecule L1CAM glycoprotein may cause a qualitative defect in the differentiated Cajal’s cells in the anterior part of the gut [20]. L1CAM mutations cause a variable clinical spectrum. This gene is located at Xq28 and encodes a transmembrane glycoprotein involved in neurite outgrowth and neuronal migration [21,22]. Hirschsprung’s disease has been reported to involve an L1CAM mutation that manifests as a quantitative defect in the migration of neural crest cells in distal segments of the gut [17]. Hirschsprung’s disease is due to the absence of intramural ganglia in the distal bowel with the commonest site is in the recto-sigmoid (95%) and rarely in small bowel. Meconium is not passed by 24 h after birth. Plain abdominal X-ray shows no gas in rectum and a contrast enema X-ray shows a collapsed rectum (aganglionic segment in spasm) with a tapering transition zone to grossly dilated and hypertrophied bowel (megacolon) above [17]. The presentation of chronic intestinal pseudo-obstruction (CIPO) in the newborn period is no longer a rare event and the prognosis appears worse than that presenting in childhood or adulthood. Many are born prematurely and the symptoms resolve with time as the interstitial cells of Cajal fully develop. Thus, it may be self-limiting in some neonates [14-16,18-21]. Many others have associated abnormalities such as urological disorders, dysautonomia, and structural gastrointestinal abnormalities such as malrotation and gastroschisis [21]. L1CAM has a role in the developmental regulation of multiple systems as seen in the association of a congenital idiopathic intestinal pseudoobstruction and hydrocephalus from stenosis of the aqueduct of Sylvius [22]. Further clinical descriptions of gastroenterological and neuropathological data are required to extend our understanding of the mechanisms underlying L1CAM function.
NCAM and cancer
Recent reports have shown that L1CAM is aberrantly expressed in several different types of cancers, including colon carcinoma, ovarian and uterine carcinomas, malignant gliomas, recurrent neuroblastoma, cutaneous malignant melanoma, renal cell carcinoma, extrahepatic cholangiocarcinoma (ECC) and gallbladder carcinoma [23]. Its expression correlates with more advanced stages of tumour progression [24-26]. L1CAM promotes cellular activities through L1 homophilic interaction, as well as heterophilic interaction with other neuronal members of the immunoglobulin (Ig) superfamily, integrins, cadherins, selectins, extracellular matrix proteins and cell surface receptors [26,27]. The Ig superfamily which includes MCAM (CD146) is involve in melanoma, breast and prostate carcinoma; L1CAM (CD171), NCAM (CD56), leucocyte CAM (ALCAM, CD166), intercellular CAM-1 (ICAM1, CD54), platelet endothelial CAM-1 (PECAM-1, CD31) are associated with metastatic progression in melanoma, glioma, breast, ovarian, endometrial, prostate, colon cancer, cholangocarcinoma and hepatocellular carcinoma. In addition, ectopic L1CAM expression in carcinoma cells enhances their migration, invasion, and tumorogenesis [28-31]. In addition to functioning as a cell surface adhesion molecule, the extracellular domain of L1CAM can be shed from the cell surface via proteolytic cleavage and can stimulate the migration and survival of tumour cells through autocrine/paracrine binding to integrins [32]. Developmental replay is manifested as post translational modification to polysialylated forms (PSA-NCAM) in certain tumour cell lines. PSA-NCAM correlates with survival by reducing tumour growth through decreasing cell-cell and cell matrix adhesions in colon and pancreatic carcinomas. However, the aggressiveness of neuroblastomas, glioblastomas and certain neuroendocrine tumours correlates to the extensive polysialylation [27]. PSA-NCAM is known to induce neural invasion in pancreatic carcinoma [23].
NCAM and metastases: NCAM and variants are involved in all the steps of tumour metastasis; (1) apoptotic evasion and angiogenesis, (2) local invasion and matrix degradation (cell-cell adhesions, collective cell migration, expression of MMP genes), (3) dissemination and anoikis (loss of cell-matrix attachment), (5) extravasation (mechanical entrapment and specific cancer-cell adhesion) and (6) colonisation and proliferation (entrapment and specific adhesion, growth and adaptation to the new environment) [33]. The benefits of collective cell migration are (a) the production of relatively high local concentrations of growth factors, (b) the protection of cells in the centre of a group from immunological attack, (c) the survival advantage of a mixed population of cells, (d) the mechano-transducing force of a migrating cell group [23,33].
NCAM and gastrointestinal stromal tumours (GISTS): Although rare (0.1-3%) they are the most common mesenchymal malignancies of the GI tract [34]. The discovery of CD34 expression in many GISTs suggested that they were a specific entity, distinct from smooth muscle tumours [34,35]. Electron microscopy and immunohistochemical studies indicated that only a minority of stromal tumours had the typical features of smooth muscle, with some having a more neural appearance and others appearing undifferentiated. Gastrointestinal autonomic nerve tumour (GANT) now recognized as a variant of GIST was also introduced to describe sarcomas with ultrastructural evidence of autonomic system differentiation [36,37]. Polysialic acid and mucin type o-glycans on NCAM differentially regulate myoblast fusion through the ubiquitous FGFR, a member of the receptor tyrosine kinase (RTK) family [2]. It was observed that GISTS and the interstitial cells of Cajal, the pacemaker cells of the gut, expressed the receptor tyrosine kinase KIT (CD117) and, more recently, DOG1[38,39]. The immunophenotype (CD117 positive) and ultrastructural features of GISTS suggest that they arise from a precursor of interstitial cells of Cajal. This hypothesis is supported by a report that an embryonic form of smooth myosin in GISTS is similar to that found in interstitial cells of Cajal [40]. The principal function of the interstitial cells of Cajal is to serve as pacemaker cells controlling gut motility, coordinating waves of peristalsis. KIT, the product of the KIT proto-oncogene is a member of the receptor tyrosine kinase family, closely related to the receptors for plateletderived growth factor (PDGF), macrophage colony-stimulating factor (MCSF), and FMS-like receptor tyrosine kinase (FLT3) ligand [4]. Expression of the KIT protooncogene is considered essential for the development of the interstitial cells of Cajal and also for its slow wave activity. In addition KIT is functionally important and is widely expressed for example in germ cells, mast cells, some epithelial cells and in haemopoietic stem cells. KIT is a transmembrane receptor fora growth factor (stem cell factor (SCF)) or mast cell growth factor. Extracellular binding of SCF to the receptor results in dimerization of adjacent KIT molecules with concomitant activation of the intracellular KIT kinase domain, leading to activation of intracellular signalling cascades controlling cell proliferation, adhesion, and differentiation. Kinases are regulatory enzymes (dephosphorylases) of the intracellular cascades which transmit regulatory signals both from outside and inside the cell. They are often controlled by the level of phosphorylation of the signalling molecules [4]. Alteration in the level of these regulatory enzymes is a common occurrence in cancerous cells and is implicated in the development of many types of cancer. Thus activation of the KIT receptor tyrosine kinase involved in a mutation within the ckit gene is integral to the development of many GISTS [41]. The tyrosine kinase inhibitor imatinib mesylate (Gilvec) thus, represents a major breakthrough in the treatment of GISTS [42]. The oncological implications are that the tyrosine kinase inhibitor (Imitanib mesylate) as an adjuvant treatment after GIST resection increases recurrent-free survival of these patients [41]. Another tyrosine kinase inhibitor (Sorafenib) significantly improves median survival in patients with advanced hepatocellular carcinoma [43].
NCAM and oncological implications
Molecular imaging: There have been reported expression of neural cell adhesion molecule (NCAM) in the immature and tumour endothelial cells of human carcinomas [44]. Molecular imaging in the detection of neoangiogenesis in tumours using highly efficient MRI contrast agents combined with the use of specific vectors (doxorubicin-containing liposomes) targeting NCAM expression would guide anti-angiogenic therapy of Kaposi sarcoma [45,46]. Radioimmunolocalisation of metastases is efficient and accurate with injections of NCAM binding 123J-UJ13a or 131J-UJ13a to children with neuroblastoma [47].
Antibody-based immunotherapy: L1 cell adhesion molecule is a novel therapeutic target in intrahepatic cholangiocarcinoma (ICC) and a novel target of beta-catenin signaling in invasive colon cancers [48]. A functional study of L1CAM suppression or over expression in intrahepatic cholangiocarcinoma (ICC) tumour cells indicated that L1CAM plays an important role in tumour progression of ICC by promoting cell proliferation, migration, and survival [31]. These results suggested that L1CAM may serve as a therapeutic target in ICC and that anti-L1CAM mAb may have potential as diagnostic and therapeutic agents for the treatment of ICC. The prognosis of ICC is very poor, and effective therapeutic strategies are urgently needed [49-51]. Complete surgical resection is currently the only chance of cure [49,50]. Molecular therapy may be useful as an adjunct especially as ICC is known to be refractory to conventional chemotherapy and radiation treatment [51]. L1CAM is an independent poor prognostic factor of extrahepatic cholangiocarcinoma [50]. A monoclonal antibody (mAb) against L1CAM reportedly inhibits the growth and dissemination of ovarian carcinomas in nude mice [52]. Experimental antibody-based ++ anti-NCAM immunotoxin huN901-DM1 in two different clinical studies, revealed acceptable toxicity and signs of clinical response [52]. Small cell lung cancer is treated with the anti- NCAM immunotoxin huN901-DM1.
Conclusion
The complexities of memory formation, motility disorders, cancer metastases are dependent on cellular recognition and adhesion. As cell adhesion is an important component in cancer metastases, NCAM can exert both a positive and a negative regulation on cancer progression, depending on the tumour context. Targeting these adherent surface glycoproteins may enhance adjuvant treatment of cancers especially those not responsive to conventional systemic chemotherapy.
References
- Reyes AA, Small SJ, Akeson R (1991) At least 27 alternatively spliced forms of the
neural cell adhesion molecule mRNA are expressed during rat heart development. Mol Cell Biol 11: 1654-1661. - Weledj EP, Assob JC (2014) The ubiquitous neural cell adhesion molecule (N-CAM). Ann Med Surg 3: 77-81.
- Soroka V, Kiryushko D, Novitzkaya V, Ronn CB, Poulson FM, et al. (2003) Structure and interactions of NCAM Ig1-2-3 suggest a novel zipper mechanism for homophilic adhesion. Structure 11: 1291-1301.
- Kiryushoko D, Korshinova I, Berezius V, Bock E (2006) Neural cell adhesion molecule induces intracellular signalling via multiple mechanisms of Ca2+ homeostasis. Mol Biol Cell 17: 2278-2286.
- Kiselyov V, Soroka V, Berezin V, Bock E (2005) Structural biology of NCAM homophilic binding and activation of FGFR. J Neurochem 94: 1169-1179.
- Breen KC, Kelly PG, Regan CM (1987) Postnatal D2-CAM/N-CAM sialylation state is controlled by a developmentally regulated Golgi sialyltransferase. J Neurochem 48: 1486-1493.
- Edelman GM, Chuong CM (1982) Embryonic to adult conversion of neural cell adhesion molecules in normal and “staggerer” mice. Proc Natl Acad Sci 79: 7036-7040.
- Greenough WT, Chang FLF (1985) Synaptic structural correlates of information storage in mammalian nervous systems. Synaptic plasticity, Guildford Press, New York, USA.
- Nolan PM, Bell R, Regan CM (1987) Acquisition of a brief behavioural experience in the presence of neurone-specific and D2-CAM/N-CAM specific antisera. Neurochem Res 12: 619-624.
- Becker CG, Artola A, Gerardy-Schahn R, Becker T, Welzl H, et al. (1996) The polysialic acid modification of the neural cell adhesion molecule is involved in spatial learning and hippocampal long-term potentiation. J Neurosci Res 45: 143-152.
- Weledji EP, Regan CM (2014) The neuroanatomical localisation of a specific memory. Int J Brain and Cogn Sci 3: 44-49.
- Nizzari M, Thellung S, Corsaro A (2012) Neurodegeneration in Alzheimer disease: Role of amyloid precursor protein and presenilin 1 intracellular signaling. J Toxicol 187297-187306.
- Wainwright SR, Galea LAM (2013) The neuroplasticity theory of depression: assessing the roles of adult neurogenesis and PSA-NCAM within the hippocampus. Neural Plast 805497.
- Weledji EP, Nonga NB (2013) Pseudo-obstruction in the neonate: a difficult diagnosis in a poor-resourced area. J Pediatr Surg Case Reports 1: 258-259.
- Lamireau J, Millon A, Sarlange J (1993) Transient intestinal pseudo-obstruction syndrome in premature infants. Arch Fr Pediatr 50: 301-306.
- Huang YC, Lee HC, Huang FY, Kaoh A, Yeh ML, et al. (1995) Neonatal-onset chronic intestinal pseudo-obstruction syndrome. Clin Pediatr (Phila) 34: 241-247.
- Kessmann J (2006) Hirschsprung's disease: Diagnosis and management. Am Fam Physician 74: 1319-1322.
- Connor L.F, Dilorenzo C (2006) Chronic intestinal pseudo-obstruction: Assessment and management. Gastroenterology 130: S29–S36.
- Heneyke S, Spitz L, Miller PJ (1999) Chronic intestinal pseudo-obstruction: treatment and long term follow-up of 44 patients. Arch Dis Child 81: 21–27.
- Sanders KM (1996) A case of interstitial cells of Cajal as pacemakers and mediators of neurotransmission in the gastrointestinal tract. Gastroenterology 111: 492-515.
- Bagwell CF, Fuller RM, Cutz E, Stringer D, Ein SH, et al. (1984) Neonatal intestinal pseudo-obstruction. J Paedatr Surg 196: 732-739.
- Bott L, Boute O, Mention K, Vinchon M, Boman F, et al. (2004) Congenital idiopathic intestinal pseudo-obstruction and hydrocephalus with stenosis of the aqueduct of Sylvius. Am J Med Genet A 130: 84–87.
- Raveh S, Gavert N, Ben-Ze'ev A (2009) L1 cell adhesion molecule (L1CAM) in invasive tumors. Cancer Lett 282: 137-145.
- Li S, Jo YS, Lee JH, Min JK, Lee ES, et al. (2009) L1 cell adhesion molecule is a novel independent poor prognostic factor of extrahepatic cholangiocarcinoma. Clin Cancer Res 15: 7345-7351.
- Choi SY, Jo YS, Huang SM, Liang ZL, Min JK, et al. (2011) L1 cell adhesion molecule as a novel independent poor prognostic factor in gallbladder carcinoma. Hum Pathol 42: 1476-1483.
- Moos M, Tacke R, Scherer H, Teplow D, Fruh K, et al. (1988) Neural adhesion molecule L1 as a member of the immunoglobulin superfamily with binding domains similar to fibronectin. Nature 334: 70-73.
- Amoureux MC, Coulibaly B, Chinot O, Loundou A, Metellus P, et al. (2010) Polysialic Acid Neural Cell Adhesion Molecule (PSA-NCAM) is an adverse prognosis factor in glioblastoma, and regulates olig2 expression in glioma cell lines. BMC Cancer 10: 91.
- Silletti S, Yebra M, Perez B, Cirulli V, McMahon M, et al. (2004) Extracellular signal-regulated kinase (ERK)-dependent gene expression contributes to L1 cell adhesion molecule- dependent motility and invasion. J Biol Chem 279: 28880-28888.
- Gast D, Riedle S, Schabath H, Schlich S, Schneider A, et al. (2005) L1 augments cell migration and tumor growth but not beta3 integrin expression in ovarian carcinomas. Int J Cancer 115: 658-665.
- Gavert N, Conacci-Sorrell M, Gast D, Schneider A, Altevogt P, et al. (2005) L1, a novel target of beta-catenin signalling, transforms cells and is expressed at the invasive front of colon cancers. J Cell Biol 168: 633-642.
- Min JK, Kim JM, Li S, Lee JW, Yoon H, et al. (2010) L1 cell adhesion molecule is a novel therapeutic target in intrahepatic cholangiocarcinoma. Clin Cancer Res 16: 3571-3580.
- Gutwein P, Mechtersheimer S, Riedle S, Stoeck A, Gast D, et al. (2003) ADAM10-mediated cleavage of L1 adhesion molecule at the cell surface and in released membrane vesicles. FASEB J 17: 292-294.
- Wong CW (2012) The role of immunoglobulin superfamily cell adhesion molecules in cancer metastasis. Int J of Cell biology 2012: 471591.
- Graadt van Roggen JF, van Velthuysen MLF, Hogendoorn PC (2001) The histopathological differential diagnosis of gastrointestinal stromal tumours. J Clin Pathol 54: 96-102.
- Mikhael AI, Bacchi CE, Zarbo RJ, Ma CK, Gown AM (1994) CD34 expression in stromal tumors of the gastrointestinal tract. Appl Immunohistochemistry 2: 89-93.
- Lee JR, Joshi V, Griffin Jr JW, Lasota J, Miettinen M (2001) Gastrointestinal autonomic nerve tumour: immunohistochemical and molecular identity with gastrointestinal stromal tumour. Am J Surg Pathol 25: 979-987.
- Suzuki M, Angata K, Nakayama J, Fukuda M (2003) Polysialic acid and mucin type oglycans on the neural cell adhesion molecule differentially regulate myoblast fusion. J Biol Chem 278: 49459-49468.
- Romert P, Mikkelsen HB (1998) C-kit immunoreactive interstitial cells of Cajal in the human small and large intestine. Histochem Cell Biol 109: 195-202.
- Kindblom LG, Remotti HE, Aldenborg F, Meis-Kindbolm JM (1998) Gastrointestinal pacemaker cell tumour (GIPACT): Gastrointestinal stromal tumours show phenotypic characteristics of the interstitial cells of Cajal. Am J Pathol 152: 1259-1269.
- Dei TA, Rossi S, Flanagan A, Hogendoornn PC, Novelli M (2008) The diagnostic utility of DOG1 expression in KIT negative GIST. J Clin Oncol 26. No 15 S10551.
- Rubin BP, Singer S, Tsao C, Duensing A, Lux ML, et al. (2001) KIT activation is a ubiquitous feature of gastrointestinal stromal tumours. Cancer Res 61: 8118-8121.
- Heinrich MC, Blanke CD, Druker BJ, Corless CL (2002) Inhibition of KIT tyrosine kinase activity, a novel molecular approach to the treatment of KIT-positive malignancies. J Clin Oncol 20: 1692-1703.
- Cheng AL, Kang YK, Chen Z, Tsao CJ, Qin S, et al. (2009) Efficacy and safety of sorafenib in patients in the Asia-Pacific region with advanced hepatocellular carcinoma: A phase III randomised, double- blind, placebo-controlled trial. Lancet Oncol 10: 25-34.
- Bussolati B, Grange C, Bruno S, Buttiggieri S, Deregibus MC, et al. (2006) Neural cell adhesion molecule (NCAM) expression by immature and tumor-derived endothelial cells favors cell organization into capillary-like structures. Exp Cell Res 312: 913-924.
- Senapati S, Mahanta AK, Kumar S, Maiti P (2018) Controlled drug delivery vehicles for cancer treatment and their performance. Signal Transduct Target ther 3: 7.
- Grange C, Geninatti-Crich S, Esposito G, Alberti D, Tei L, et al. (2010) Combined delivery and magnetic resonance imaging of neural cell adhesion molecule-targeted doxorubicin-containing liposomes in experimentally induced Kaposi's sarcoma. Cancer Res 70: 2180-2190.
- Klehr M, Koehl U, Mühlenhoff M, Tawadros S, Fischer T, et al. (2009) The novel chimeric anti-NCAM (neural cell adhesion molecule) antibody ch.MK1 displays antitumor activity in SCID mice but does not activate complement-dependent cytolysis (CDC). J Immunother 32: 442-451.
- Khan SA, Thomas HC, Davidson BR, Taylor-Robinson SD (2005) Cholangiocarcinoma. Lancet 366: 1303-1314.
- Weledji EP, Enoworock G (2014) How grim is cholangiocarcinoma. Int J Tumor Ther 3: 10-16.
- Sirica AE (2005) Cholangiocarcinoma: molecular targeting strategies for chemoprevention and therapy. Hepatology 41: 5-15.
- Wolterink S, Moldenhauer G, Fogel M, Kiefel H, Pfeifer M, et al. (2010) Therapeutic antibodies to human L1CAM: functional characterization and application in a mouse model for ovarian carcinoma. Cancer Res 70: 2504-2515.
- Kwa HB, Wesseling J, Verhoeven AH, Zandwijk NV, Hilkens J (1996) Immunoscintigraphy of small-cell lung cancer xenografts with antineural cell adhesion molecule monoclonal antibody, 123C3: Improvement of tumour uptake by internalisation. Br J Cancer 73: 439-446.
 Spanish
Spanish  Chinese
Chinese  Russian
Russian  German
German  French
French  Japanese
Japanese  Portuguese
Portuguese  Hindi
Hindi