Recent structure relationship studies (SAR) on natural occurring sulfonium salts as potent α-glucosidase inhibitors
Weijia Xie, Genzoh Tanabe, Toshio Morikawa, Osamu Muraoka and XiaomingWu
China Pharmaceutical University, China
Kinki University, Japan
: Expert Opin Environ Biol
Abstract
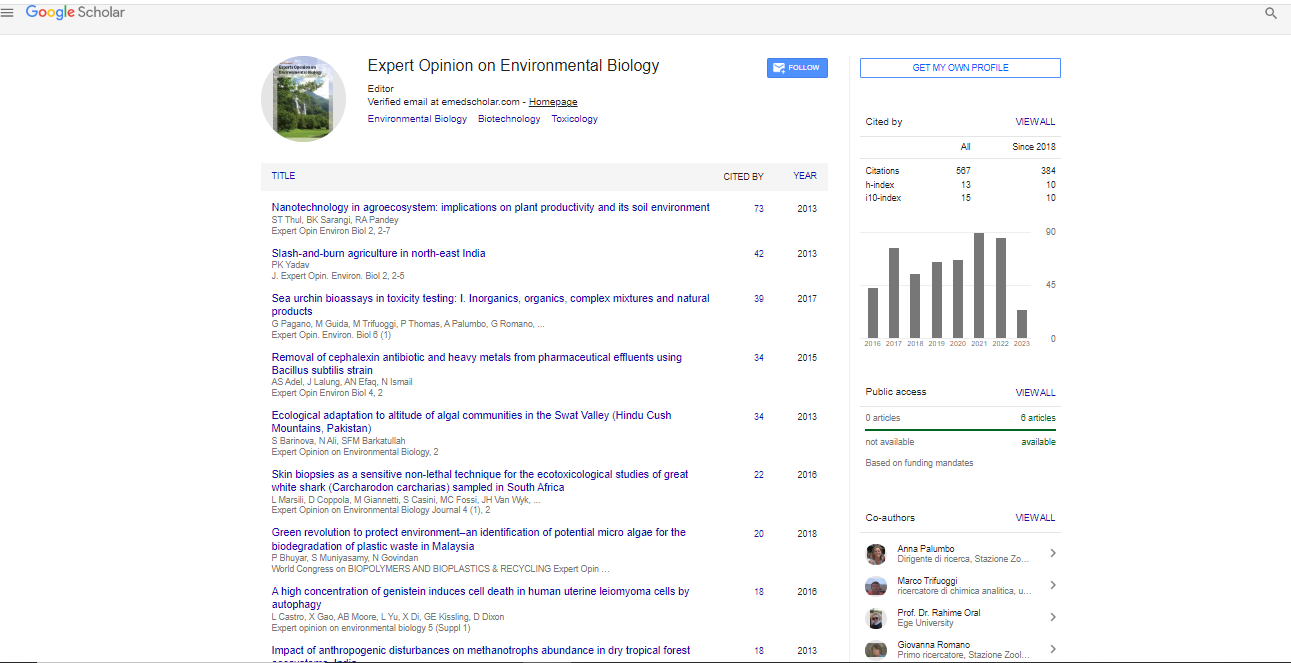
Diabetes is now becoming a global epidemic disease with its prevalence estimated to affect over 550 million people worldwide in 2030. Type 2 diabetes mellitus, previously known as non-insulin dependent diabetes mellitus, accounts for 75-90% diabetes cases. One of the practical therapeutic approaches for treating type 2 diabetes is to delay the digestion of carbohydrates, which could be achieved by the inhibition of carbohydrate hydrolyzing enzymes such as pancreatic α-amylase and α-glucosidases. In late 1990’s, a naturally occurring α-glucosidase inhibitor salacinol (1) was isolated from Salaciareticulata, which was used to treat type II diabetes in Ayurvedic system of Indian traditional medicine. The structure of (1) was quite unique, bearing the permanent positive charge as the thiosugarsulfonium sulfate inner salt comprised of 1-deoxy-4-thio- D-arabinofranosyl cation and 3’-sulfate anion. Its α-glucosidase inhibitory activity was revealed to be as potent as those of voglibose and acarbose. Shortly thereafter, its side chain analogs such as kotalanol (2), ponkoranol (3) and salaprinol (4), as well as their de-O-sulfonated versions termed neosalacinol (5), neokotalanol (6), neoponkoranol (7) and neosalaprinol (8) were subsequently isolated from the same genus plants. All these sulfonium salts showed potent α-glucosidase inhibitory activities, composing a new class of potent α-glucosidase inhibitors which could be further developed as a new class of hypoglycemic drug candidates. Thus, ever since the isolation and identification of these natural products, great efforts have been made on the synthesis, structural modification and biological activities studies on this group of sulfonium salts. This presentation mainly addresses our recent research progress of organic synthesis, SAR studies and structural modification of Salacia originated sulfonium type natural products as follows: 1. Synthetic and biological studies on (7) and its side chain analogs; 2. First total synthesis of (6), the structure of which is the most complicated one among this group of natural products; 3. Synthetic and biological studies on (2) and its side chain epimers.
Biography
Weijia Xie has completed his Pharmacy PhD at the age of 30 years from Kinki University in Japan. During his study and research period in Kinki University, he was also hired by High-tech research center of Kinki University as Assistant Researcher. He came back to China in 2011 and since then worked in the Department of Medicinal Chemistry of China Pharmaceutical University as a Lecturer. He is specialized in synthesis, SAR studies on natural products with potent biological activities and has published more than 20 research papers in reputed journals such asTetrahedron,TetrahedronLett.,Bioorg. Med. Chem.,Bioorg. Med. Chem. Lett. andChem. Commun.
Email: henry1202x@hotmail.com
 Spanish
Spanish  Chinese
Chinese  Russian
Russian  German
German  French
French  Japanese
Japanese  Portuguese
Portuguese  Hindi
Hindi