Case Report, Clin Oncol Case Rep Vol: 3 Issue: 3
Α Rare Solitary Spinal Cord Metastasis after Epithelial Ovarian Cancer Diagnosis
Adamantia Nikolaidi1*, Kyriaki Papadopoulou2, Kalliopi Tsakiri2, Elizabeth Psoma3, Dimitris Zevgaridis4, Christos Konstantaras5, Ilias Athanasiadis1, Florentia Fostira6 and George Fountzilas2,7,8
1Oncology Clinic, Mitera Hospital, Athens, Greece
2Laboratory of Molecular Oncology, Hellenic Foundation for Cancer Research, Aristotle University of Thessaloniki, Thessaloniki, Greece
3Department of Radiology, AHEPA Hospital, Aristotle University of Thessaloniki, School of Health Sciences, Thessaloniki, Greece
4Neurosurgical Department, Kianous Stavros Euromedica, Thessaloniki, Greece
5Department of Surgery, St. Luke’s Hospital, Thessaloniki, Greece
6Molecular Diagnostics Laboratory, InRASTES, National Centre for Scientific Research ‘Demokritos’, Athens, Greece
7Aristotle University of Thessaloniki, Thessaloniki, Greece
8German Oncology Center, Limassol, Cyprusy
*Corresponding Author : Adamantia Nikolaidi, Oncology Clinic
Mitera Hospital,
Athens, Greece
Tel: 00306944883898
E-mail: mantonikolaidi@gmail.com
Received: April 06, 2020 Accepted: April 17, 2020 Published: May 04, 2020
Citation: Nikolaidi A, Papadopoulou K, Tsakiri K, Psoma E, Zevgaridis D, et al. (2020) Α Rare Solitary Spinal Cord Metastasis after Epithelial Ovarian Cancer Diagnosis. Clin Oncol Case Rep 3:3.
Abstract
Ovarian cancer is frequently diagnosed at an advanced stage, with an unfavourable five-year survival. The standard therapeutic approach includes cytoreductive surgery and platinum-based chemotherapy. Relapse and metastasis, frequently occurring within the first two years after diagnosis involves the abdominal cavity. Herein, a patient with stage IIIc, high grade serous peritoneal carcinomatosis, who was diagnosed with a secondary, solitary intramedullary lesion seventeen months after the initial diagnosis and treatment, is presented. The patient underwent R1 operation to remove the mass, with cerebrospinal fluid being tested positive for the presence of tumor cells, while the serous origin of the tumor was confirmed by the histology report. Testing of both the primary and the secondary tumors was performed, where a pathogenic TP53 variant was identified. In the absence of an actionable variant, she subsequently received intrathecal methotrexate. Six months later, an MRI showed residual intramedullary disease and she received systemic chemotherapy but deceased two months later. Intramedullary cord involvement as a metastatic lesion following an ovarian cancer diagnosis remains rare. The multidisciplinary approach through which tumor molecular profiling will be performed and assessed may significantly increase both progression-free survival and quality of life of cancer patients.1
Keywords: Ovarian cancer; Intramedullary lesion; Molecular profile
Introduction
Ovarian cancer is the leading cause of death from gynaecologic cancers. Most patients with ovarian cancer present with advancedstage disease and require adjuvant treatment. However, even in these patients, the disease is usually confined to the peritoneal cavity, while it can also lead to intra-abdominal metastasis. Unlike breast and lung cancer, ovarian carcinoma can rarely metastasize to the spinal cord, with only seven cases being reported in the literature, up to now [1].
Herein, a case of an ovarian cancer patient is presented who was admitted with an intramedullary lesion as the first localization of metastatic disease, 17 months after completion of the first line of chemotherapy.
Case Presentation
A 71-year old woman was diagnosed in May 2015 with high grade serous peritoneal carcinomatosis stage IIIc (FIGO classification). She received three cycles of neoadjuvant chemotherapy with carboplatin 6AUC and paclitaxel 175 mg/m2, every 21 days. Subsequently, she had a total abdominal hysterectomy with bilateral salpingo-oophorectomy and omentectomy and a pathological complete response was confirmed. She received three additional cycles of the same systemic chemotherapy which she tolerated well. The only adverse event reported during her course of treatment was a grade 3 myelotoxicity.
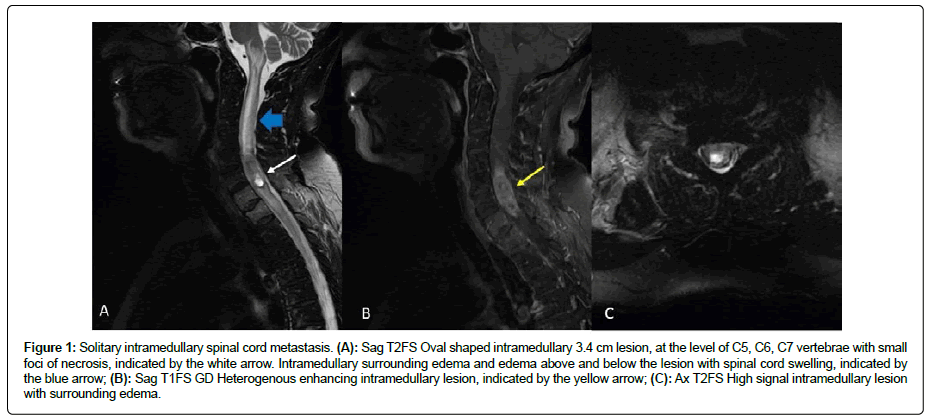
Since then she has been in regular follow up with imaging and laboratory examinations. Seventeen months later, there were no signs of disease recurrence in the scheduled CT of the abdomen. However, the lab tests were significant of slightly elevated CA 125 and CA 15-3 levels and she complained for atypical muscle pain in the area of neck and shoulders. Within the month she reported weakness in the right arm and leg, as well as incomplete loss of muscle strength. Magnetic resonance imaging of the brain and the cervical part of the spine revealed lytic lesions in C7, T1 and an intramedullary lesion at the level C5-C7 approximately 3, 4 cm in the vertical extent which appeared partially cystic. Surrounding edema above and below the lesion with spinal cord swelling was also noticed (Figure 1). Differential diagnosis included ependymoma, astrocytoma, metastasis from the known ovarian cancer or metastasis from an undiscovered second primary tumor.
Figure 1: Solitary intramedullary spinal cord metastasis. (A): Sag T2FS Oval shaped intramedullary 3.4 cm lesion, at the level of C5, C6, C7 vertebrae with small foci of necrosis, indicated by the white arrow. Intramedullary surrounding edema and edema above and below the lesion with spinal cord swelling, indicated by the blue arrow; (B): Sag T1FS GD Heterogenous enhancing intramedullary lesion, indicated by the yellow arrow; (C): Ax T2FS High signal intramedullary lesion with surrounding edema.
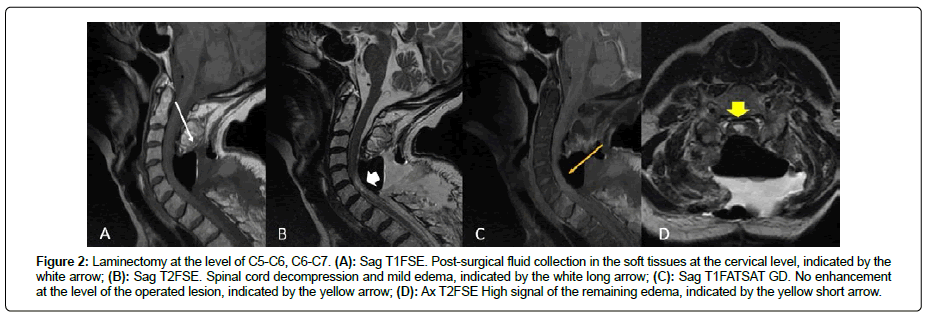
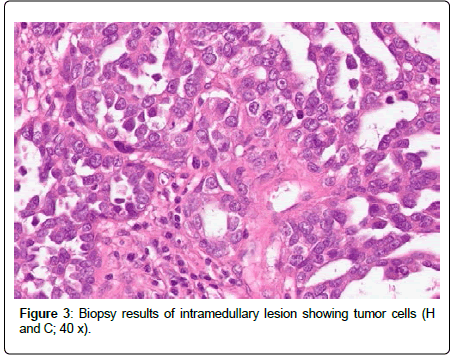
The patient received immediately corticosteroids to reduce the edema and 72 hours later she underwent an operation to remove the mass (R1 as by neurosurgeon’s impression). MRI after surgery showed significant improvements of the spinal cord edema and showed no contrast enhancement. Laminectomy was performed and post-surgical fluid collections at the level of C5, C6, C7 were noticed in the soft tissues (Figure 2). The pathological examination of the mass was indicative of metastasis from serous carcinoma (Figure 3). The cerebrospinal fluid was positive for tumor cells. In addition, intrathecal methotrexate (12 mg) was administered and the patient was transferred in the rehabilitation center to improve mobilization.
Figure 2: Laminectomy at the level of C5-C6, C6-C7. (A): Sag T1FSE. Post-surgical fluid collection in the soft tissues at the cervical level, indicated by the white arrow; (B): Sag T2FSE. Spinal cord decompression and mild edema, indicated by the white long arrow; (C): Sag T1FATSAT GD. No enhancement at the level of the operated lesion, indicated by the yellow arrow; (D): Ax T2FSE High signal of the remaining edema, indicated by the yellow short arrow.
At that time, a gene-panel test was ordered, where no BRCA1 and BRCA2 or any other ovarian cancer predisposing pathogenic gene variants were identified. The APC p.(Ile1307Lys) the risk allele was the only significant finding of the genetic analysis, which is known to double the lifetime risk for colorectal cancer but seems irrelevant to the patient diagnosis.
A follow-up brain MRI was clear while the cervical spine MRI showed a residual intramedullary mass at the level of C5-C7. The patient received a single cycle of systemic chemotherapy with carboplatin; she demonstrated rapid clinical deterioration and succumbed.
Methods
Both patient’s primary and metastatic tumor tissue, as well as whole blood, were collected to investigate the presence of somatic and/or germline pathogenic variants that might underlie the patient’s initial diagnosis of high grade serous ovarian carcinoma, its subsequent metastasis, as well as to guide treatment options. Informed consent for the provision of biological material for future research purposes was signed on admission.
Tissue processing and DNA extraction
Formalin-Fixed Paraffin-Embedded (FFPE) tissue samples have been evaluated for confirmation of diagnosis and Tumor Cell Content (TCC), the latter evaluated as the rate of tumor cell nuclei against total nuclei in the section and corresponding to 60% and 70% for the primary and metastatic tumor sample, respectively. Tumor dense areas were marked for macrodissection and DNA extraction followed from 10 μm tissue sections with the QIAamp DNA Mini Kit (Qiagen GmbH, Hilden, Germany). DNA was quantified with the Qubit fluorometer (ThermoFisher Scientific, Massachusetts, USA).
Genetic and bioinformatic analysis of tumor DNA
Tumor analysis was performed by a previously published custom Ampliseq panel (IAD75668_167; ThermoFisher Scientific, Massachusetts, USA), which includes 330 amplicons; 189 of these target coding regions in 40 actionable genes, including BRCA1 and BRCA2 [2]. The patient’s germline DNA was also analyzed in parallel to allow discrimination among somatic and germline variants. Genomic libraries were constructed by implementing a multiplex PCR using 20 ng of DNA using Ampliseq Library Kit v.2.0 and Ion Xpress barcodes, following manufacturer’s instructions (ThermoFisher Scientific, Massachusetts, USA).
Sample libraries, once normalized to 15 ng/ml with the Qubit fluorometer (ThermoFisher Scientific, Massachusetts, USA) and were then processed on a One-Touch-2 instrument, followed by enrichment on a One-Touch-ES station. Templating was performed with the Ion PI Template OT2-200 Kit v.3, whereas sequencing was carried out on a PI chip (ThermoFisher Scientific, Massachusetts, USA). Histological review of the patient’s tumors, sample processing, targeted NGS genotyping and initial bioinformatics analysis were implemented in the laboratory of molecular oncology (Aristotle University of Thessaloniki-Hellenic Foundation for Cancer Research).
For data retrieval, base calling was performed on the Torrent Server with Torrent Suite v5.0.2. Variants were called, annotated with Ion Reporter 5.6 and considered eligible for analysis upon further quality filtering, as before: amplicons should have >100 reads, variants should have variant position coverage >100; variant allele coverage >40; p<0.0001; Variant Allele Frequency (VAF) of >5. Indels involving GC-stretches, as well as non-annotated variants, were excluded [2].
Based on the above criteria, 93 eligible variants were retrieved for the primary tumor, 91 for the metastatic lesion and 83 for blood. Regarding NGS metrics of primary and metastatic tumor samples, average base coverage depth was 5,495 and 10, 430, uniformity 88% and 80%, whereas 95% and 97% of amplicons possessed >100 reads, respectively. For the blood sample mean depth was 1,349, uniformity 83% and amplicons with >100 reads were 92%. Eligible variants were called if these involved amino acids or splice site changes, and with no reported minor allele frequency (MAF) or with MAF <0.1% according to 5000 Exomes database if involved annotated SNPs.
Germline analysis using trusight cancer panel
To assess hereditary predisposition to ovarian cancer, germline DNA was extracted from whole blood. The patient signed informed consent prior to the analysis. Germline DNA is enzymatically fragmented, adaptor tagged, indexed and captured to target the 1736 genomic regions of 94 cancer-predisposing genes using TruSight Cancer Panel, following manufacturer’s instructions (Illumina, San Diego, USA). Amplified libraries were evaluated qualitatively and quantitatively using Fragment Analyzer (Advanced Analytical Technologies, Heidelberg, Germany). Indexed libraries were sequenced on MiSeq using the Standard V2 kit performing 150 base paired-end reads, while FASTQ, BAM and VCF files were generated through Illumina MiSeq Reporter; annotation was performed against the human reference genome GRCh38 using VariantStudio V.3 (Illumina). The mean read depth was 284x. Sanger sequencing was implemented for orthogonal validation. The procedure was performed in the Molecular Diagnostics Laboratory of National Center for Scientific Research ‘Demokritos’, in Athens.
Results
Tumor testing revealed the presence of a TP53 pathogenic variant [(c.757_758insA, p.(Thr253fs)], which was present in both the patient’s primary and metastatic tumor DNA, but not in the germline DNA. It is reported in COSMIC database (COSM45313). The TP53 pathogenic variant was detected with Variant Allele Frequencies (VAF) of 31% and 79% in the primary and metastatic tumors, respectively, indicating loss of the wild-type allele and therefore, loss of heterozygosity, in the metastatic lesion. No pathogenic or likely pathogenic variants have been identified in many genes involved in Homologous Recombination Repair (HRR) pathway targeted by our custom panel.
Germline testing revealed the presence of a PALB2 missense variant [c.1123C>A, p.(Leu375Ile), rs373298267], which has been classified as a variant of unknown significance (VUS) in ClinVar and reported in dbSNP database with MAF 0.0001 (1/12994, GOESP). This rare variant was also present in the patient’s primary and metastatic tumor samples with similar VAFs relative to blood (56%, 60%, and 59%, respectively). In addition to that, an APC missense variant has been identified [(c.3920T>A, p.(Ile1307Lys), rs1801155]. This is a relatively common, risk allele, which is known to double the lifetime risk for colorectal cancer, mostly seen in individuals of Jewish ancestry [3] but seems irrelevant to the patient’s diagnosis. No pathogenic/likely pathogenic variants were identified in BRCA1 and BRCA2 or any other ovarian cancer-predisposing genes.
Discussion
Ovarian cancer is the most fatal of gynaecological malignancies, with two-thirds of cases being presented with disseminated disease at the time of diagnosis. The lack of an anatomic barrier allows the ovarian cancer cells to very conveniently spread into the peritoneal cavity, but also there is the hypothesis that as tumor cells detach singularly or in clusters from the primary tumor, they travel by peritoneal fluid into the peritoneum and omentum [3]. Distant metastases are relatively rare and involve the lungs, liver, brain, and other sites [4]. The presence of TP53 pathogenic variants, genomic instability, and tumor vascularization is considered as possible predictors for distant metastases. More specifically, the localized intra-abdominal spread of ovarian cancer may be influenced by TP53 missense variants, whereas extra-abdominal spread can be associated to complete loss of TP53 function and subsequent inability to suppress VEGF [5], which is coherent with our case.
Ovarian cancer metastasis to the spinal cord is rare, i.e. can occur in 1%-2.2%, of patients, based on five reported series [6]. It is essential, that treating physicians are aware of this rare entity, especially since the clinical presentation can vary significantly and can involve from minor neurological to major symptoms. These can involve local pain, causing neck or back stiffness and can be described as burning and bilateral, sensory changes, such as paresthesia, all of which can lead to motor disturbances [7]. Beyond evidence that can be obtained through imaging techniques, an open biopsy is essential to establish a definitive diagnosis. In selected patients with well-circumscribed margins and limited primaries, gross total resection might improve the length and quality of survival, although the medium survival is described around 19.5 weeks, based on current evidence [8].
To the best of our knowledge, seven cases of intramedullary metastases from ovarian cancer have been reported to date [9-15]. Among these, in four cases the intramedullary lesion was the only metastatic site, with surgery being performed in three cases. Most importantly, molecular profiling or genetic analysis was not performed in any of these cases.
Conclusion
In our case, although both tumor and germline DNA were extensively analysed, no actionable variants have been identified in either BRCA1 and BRCA2 or any other ovarian cancer-predisposing genes. As novel therapeutic possibilities arise for ovarian cancer patients, and most importantly PARP inhibition, survival is improved and therefore, unusual metastatic lesions will arise.
References
- Safadi S, Rendon P, Rutledge T, Mayasy S (2016) Ovarian carcinoma with isolated spinal cord metastasis. J Investig Med High Impact Case Rep 4: 2324709616657644.
- Kotoula V, Lakis S, Tikas I, Giannoulatou E, Lazaridis G, et al. (2019) Pathogenic BRCA1 mutations may be necessary but not sufficient for tissue genomic heterogeneity: Deep sequencing data from ovarian cancer patients. Gynecol Oncol 152: 375-386.
- Lengyel E (2010) Ovarian cancer development and metastasis. Am J Pathol 177: 1053-1064.
- Cormio G, Rossi C, Cazzolla A, Resta L, Loverro G, et al. (2003) Distant metastases in ovarian carcinoma. Int J Gynecol Cancer 13: 125-129.
- Sood AK, Sorosky JI, Dolan M, Anderson B, Buller RE (1999) Distant metastases in ovarian cancer: Association with p53 mutations. Clin Cancer Res 5: 2485-2490.
- Gossman W, Hoang S, Mesfin FB (2020) Cancer, Intramedullary spinal cord tumors, In: StatPearls: Treasure Island (FL).
- Gershoni-Baruch R, Patael Y, Dagan, Figer A, Kasinetz L, et al. (2000) Association of the I1307K APC mutation with hereditary and sporadic breast/ovarian cancer: More questions than answers. Br J Cancer 83: 153-155.
- Isoya E, Saruhash Y, Katsuura A, Takahashi S, Matsusue Y, et al. (2004) Intramedullary spinal cord metastasis of ovarian tumor. Spinal Cord 42: 485-487.
- Huang J, Lei D (2017) Intramedullary spinal cord metastasis from ovarian cancer in a 50-year-old female. World Neurosurg 106: 1043-1049.
- Bakshi A, Biswas G, Deshmukh C, Prasad N, Nair R, et al. (2006) Successful complete regression of isolated intramedullary spinal cord metastases from epithelial ovarian carcinoma with chemotherapy and radiotherapy. Indian J Cancer 43: 136-138.
- Cormio G, Vagno G, Fazio F, Loverro G, Selvaggi L (2001) Intramedullary spinal cord metastasis from ovarian carcinoma. Gynecol Oncol 81: 506-508.
- Miranpuri AS, Rajpal S, Salamat MS, Kuo JS (2011) Upper cervical intramedullary spinal metastasis of ovarian carcinoma: A case report and review of the literature. J Med Case Rep 5: 311.
- Rastelli F, Benedetti G, Tommaso L, Mazzoli M, Calbucci F, et al. (2005) Intramedullary spinal metastasis from ovarian cancer. Lancet Oncol 6: 123-125.
- Soylemez B, Ozum U, Egilmez R (2020) Cervical spinal intramedullary metastasis of ovarian adenocarcinoma: A case report. Asian J Case Rep Surg 3: 21-24.
- Damoun S, Fattaneh T, Jabbari B (2015) Intramedullary metastasis from ovarian cancer: A systemic review of the literature. Neurology 119: 1-6.
 Spanish
Spanish  Chinese
Chinese  Russian
Russian  German
German  French
French  Japanese
Japanese  Portuguese
Portuguese  Hindi
Hindi