Case Report, Clin Oncol Case Rep Vol: 2 Issue: 1
A Case of Plasmacytoid Bladder Cancer Relapsing with Linitis Plastica of the Colon and Rectum
Tom Young* and Deborah Enting
Department of Medical Oncology, Guy’s Hospital, London, United Kingdom
*Corresponding Author : Tom Young
Medical Oncology Consultant, Department of Medical Oncology, Guy’s Hospital, Great Maze Pond, SE1 9RT, London, United Kingdom
Tel: 07515828749
E-mail: tom.young1989@gmail.com
Received: September 10, 2018 Accepted: February 06, 2019 Published: February 13, 2019
Citation: Young T, Enting D (2019) A Case of Plasmacytoid Bladder Cancer Relapsing with Linitis Plastica of the Colon and Rectum. Clin Oncol Case Rep 2:1.
Abstract
Plasmacytoid urothelial carcinoma is a rare and poorly understood variant of urothelial bladder cancer, which is believed to carry a worse prognosis. A 69 year old male presented with urinary symptoms and was diagnosed with plasmacytoid urothelial carcinoma of the bladder. He was found to also have rectal thickening on initial imaging, which was investigated extensively but no malignant pathology was identified. He underwent radical cystoprostatectomy then adjuvant chemotherapy. 5 months after completion of treatment he developed abdominal discomfort with Computerised Tomography scan showing previously investigated rectal thickening to have progressed to involve the whole colon, in keeping with metastatic disease in the form of linitis plastica of the large bowel. He also developed left thigh pain and was found to have a soft tissue metastasis of the left adductor muscle. Despite treatment with immunotherapy his condition worsened and he developed large bowel perforation and passed away. To our knowledge this is the first report of plasmacytoid urothe
Keywords: Plasmacytoid bladder cancer; Linitis plastica
Introduction
Bladder cancer is the tenth most common cancer in the United Kingdom and the seventh most common cause of cancer death [1]. Most patients present with superficial tumours, which remain indolent after initial treatment [2]. Muscle-invasive bladder cancers carry a much worse prognosis, with metastatic disease carrying a median overall survival of 12-14 months [3]. Bladder cancer most commonly metastasises to lymph nodes, bone and lung [4].
Around 75% of bladder cancers are classified as pure urothelial carcinomas, with the remaining 25% comprised of other histological variants in pure form (such as small cell or squamous cell carcinoma) or as differentiation associated with urothelial carcinoma [5]. The importance of variant histologies has become increasingly understood and thus relevant to clinical practice.
Plasmacytoid urothelial carcinoma (PUC) is a variant histology first described in a case report in 1991 [6]. A small number of case series and retrospective cohort studies have subsequently described this rare variant, thought to comprise 1% of urothelial carcinoma as a more aggressive form of bladder cancer.
PUC has been described to display several histological and clinical characteristics distinguishing it from pure urothelial carcinoma and other variant histologies [7]. PUC tumour cells demonstrate eccentrically placed nuclei with abundant eosinophilic cytoplasm [8]. They typically express the marker CD138 and are highly proliferative with most cells positive for Ki-67. E-cadherin, a cell adhesion marker, is often downregulated or negative, which has been hypothesised to explain the ‘coat sleeve pattern’ of local growth described.
Linitis plastica is characterised by circumferential intramural tumour infiltration of a hollow viscus [9]. It can occur secondary to metastasis. Linitis plastica affecting the large bowel is extremely rare in bladder cancer [10]. Here we present a case of PUC relapsing, after initial radical treatment, with linitis plastica of the colon and rectum. To our knowledge this is the first case to be reported of linitis plastica of the large bowel secondary to PUC.
Case History
A 69 year old male presented to his General Practitioner with urinary frequency and urgency. He had no significant past medical history and was a never smoker. Ultrasound scan revealed bilateral hydronephrosis, and a Computerised Tomography (CT) urogram was therefore performed, which showed bladder wall thickening. He subsequently underwent Transurethral Resection of Bladder Tumour, with simultaneous insertion of bilateral ureteric stents. Histology revealed high-grade (Grade 3) muscle-invasive urothelial cell carcinoma of plasmacytoid variant, with focal signet ring appearances.
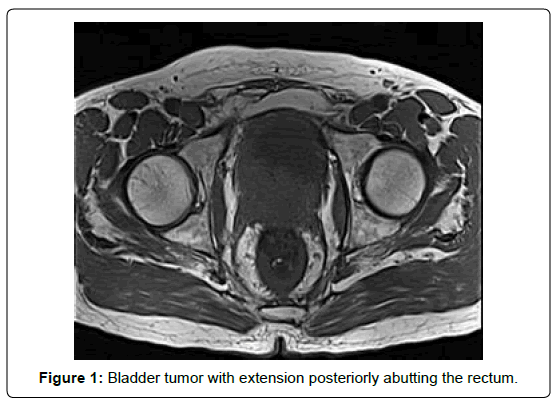
Whilst awaiting further management, the patient suffered increasing constipation and weight loss. A further CT scan showed rectal thickening and he therefore underwent Magnetic Resonance Imaging (MRI) of the pelvis, which showed significant bladder wall thickening extending through the right seminal vesicle and abutting the rectum with rectal wall thickening seen superior to this point. Flexible sigmoidoscopy was performed but biopsies of the rectum did not reveal malignant pathology. Full staging, completed with CT chest, showed no evidence of distant metastatic disease (Figure 1).
After discussion at the Bladder Cancer Multidisciplinary Meeting (MDM) and Pelvic Cancer MDM, recommendation was made for total pelvic exenteration to maximise possibility of clear surgical margins, followed by adjuvant systemic treatment. On exploration intra-operatively there was no apparent involvement of the rectum and therefore instead a cystoprostatectomy with ileal conduit urinary diversion was performed. The patient recovered well post-operatively.
Final histopathology showed Grade 3 pT4 N0 R0 urothelial carcinoma with plasmacytoid differentiation, and several foci of lymphovascular invasion. The tumour infiltrated around the seminal vesicle and the ejaculatory ducts in the prostate gland. No formal lymph node dissection was performed, but one incidentally resected lymph node was negative for cancer involvement. The patient subsequently received four cycles of adjuvant gemcitabine and cisplatin chemotherapy. Post-chemotherapy surveillance imaging showed no evidence to suggest local recurrence, but persisting mid-rectal thickening. He therefore underwent further flexible sigmoidoscopy, which was macroscopically and microscopically normal.
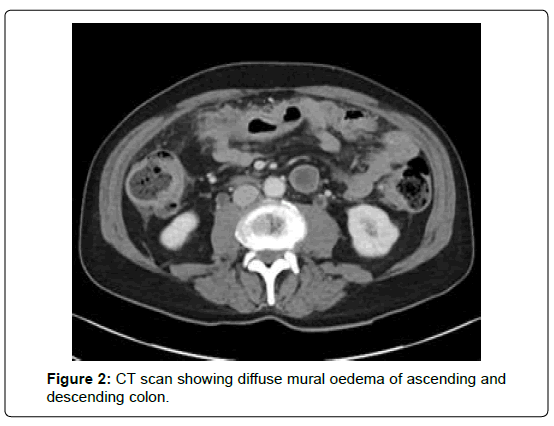
Five months after completion of the chemotherapy, the patient complained of left sided groin pain causing significant discomfort on mobilisation, as well as bloating. Repeat imaging was organised; CT scan of the chest, abdomen and pelvis showed no confirmatory features of disease relapse, but revealed diffuse mural oedema of the colon and associated inflammatory pericolic change, suggestive of a colitic process (Figure 2). MRI pelvis showed no evidence of local recurrence but demonstrated marked oedema within the left adductor magnus muscle.
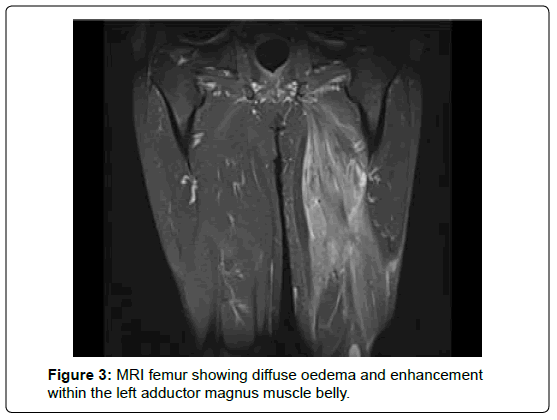
As a result of the CT findings, colonoscopy was attempted but narrowing prevented progress beyond the rectosigmoid junction. MRI femur was performed, confirming unusual changes with diffuse oedema and enhancement seen within the left adductor magnus muscle belly (Figure 3). These findings were reviewed at the regional soft tissue cancer meeting, which recommended interval imaging to follow-up the abnormalities seen.
The results of the patient’s investigations were re-discussed at the Bladder Cancer MDM and felt to be highly suspicious for recurrence. Whilst awaiting scope-guided colonoscopy, he was admitted to hospital with abdominal pain and vomiting. CT scan showed small bowel obstruction, felt by the surgical team to be secondary to adhesions, and he was managed conservatively with symptoms settling without need for further intervention. After discharge he had recurrent symptoms with worsening abdominal pain and bloating. A further CT scan showed no evidence of obstruction but persistent presence of pancolitis. His case was again discussed at MDM, and the consensus was that the patient’s symptoms were likely related to metastasis in the form of linitis plastica of the large bowel. His bowel symptoms were again managed conservatively and he was started on a reducing regimen of dexamethasone for symptomatic benefit. After a prolonged admission he was discharged following improvement in his symptoms. Following discharge repeat MRI femur showed progression of the soft tissue changes in the posterior thigh consistent with a progressive soft tissue metastasis. He was reviewed in the outpatient clinic and consented for immunotherapy treatment (pembrolizumab).
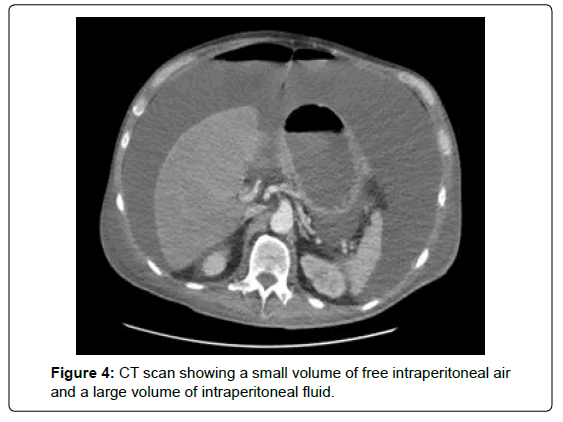
After one cycle of pembrolizumab he was admitted to hospital again with diarrhoea and abdominal pain. He was treated empirically with broad-spectrum antibiotics, as well as steroids to cover for possible immunotherapy-induced colitis. CT imaging showed persistent ongoing circumferential thickening of the whole colon and rectum. After initial improvement, he subsequently developed more severe pain and repeat CT scan showed free intra-abdominal air consistent with perforation and large volume ascites (Figure 4). As the perforation was felt to be secondary to progressive malignancy, surgical management was adjudged not to be appropriate, and the patient deteriorated quickly and passed away.
Discussion
Several studies have shown that PUC appears to present at more advanced disease stages [11]. It behave more aggressively than pure urothelial carcinoma, with poorer survival rates seen in both localized and metastatic disease described in one of the largest retrospective cohort study investigating PUC, comprising of 31 patients [12]. This study showed median overall survival in PUC patients treated with cystectomy and adjuvant chemotherapy (the same management received by the patient discussed above) to be 27.4 months compared to 62.6 months for pure urothelial carcinoma patients.
The patient discussed here did not receive neoadjuvant chemotherapy, proceeding to radical cystectomy. Available literature suggests PUC patients do not significantly benefit from neoadjuvant chemotherapy, with one small retrospective study demonstrating that although 80% of patients receiving neoadjuvant chemotherapy showed pathological downstaging, no survival benefit was seen [9]. Based on the evidence available, the current consensus is that optimal management for PUC is to proceed with early radical cystectomy for operable patients [5]. No clear evidence currently exists regarding whether adjuvant chemotherapy provides survival benefit in PUC.
This patient’s disease relapsed in an unusual manner in the form of linitis plastica affecting the whole of the large bowel. This pattern shows some degree of similarity to a previously reported case of a patient who relapsed with PUC metastasis to the duodenum after cystectomy [8]. With the benefit of hindsight, it is likely that the rectal thickening seen on this patient’s initial imaging represented metastatic disease, particularly as this thickening subsequently progressed to involve the entire colon as the patient’s clinical condition worsened. Despite extensive investigation no histological evidence of this was found prior to proceeding with surgery, a pitfall reported previously in the diagnosis of linitis plastica with endoscopic biopsies often not yielding cancer cells due to mucosal sparing [10]. Although to our knowledge this is the first case to be described of PUC metastasising as linitis plastica of the large bowel, linitis plastica within the bladder as a pattern of local invasion of PUC has been described [12]. Suggesting a distinct biology underpinning the manner in which PUC progresses compared to pure urothelial carcinoma. This patient also developed a skeletal muscle metastasis within the left adductor magnus muscle, another uncommon site of disease relapse.
As this patient had progressive disease within 12 months of completing adjuvant platinum-based chemotherapy, he received immunotherapy as second-line treatment rather than second line chemotherapy. There are few published reports of PUC treatment with immunotherapy, with one report describing 2 patients who showed short-lived responses to checkpoint inhibitor treatment before disease progression [13]. As this patient only received one cycle of treatment it is difficult to draw any significance from his failure to respond.
Conclusion
There remains no level one research specifically investigating the best oncological management for PUC. Given the increasing availability of new systemic agents for the treatment of metastatic bladder cancer, such as immunotherapy, it remains to be seen whether these have a clear role in the treatment of PUC. For now consensus remains that patients should receive early radical treatment, with high vigilance required for early detection of relapse given the aggressive nature of the disease, which can relapse in atypical sites as seen in this case.
References
- Lionk S (2008) Cancer Research UK. Bladder cancer mortality statistics.
- Vincov D (2003) Advanced bladder cancer meta-analysis collaboration. Neoadjuvant chemotherapy in invasive bladder cancer: a systematic review and meta-analysis. Lancet 361: 1927–34.
- Von der MH, Sengelov L, Roberts JT, Ricci S, Dogliotti L (2005) Long-term survival results of a randomized trial comparing gemcitabine plus cisplatin, with methotrexate, vinblastine, doxorubicin, plus cisplatin in patients with bladder cancer. J Clin Oncol 23: 4602.
- Shinagare AB, Ramaiya NH, Jagannathan JP, Fennessy FM, Taplin ME (2011) Metastatic pattern of bladder cancer: correlation with the characteristics of the primary tumor. AJR Am J Roentgenol 19: 117-122.
- Moschini M, D’Andrea D, Korn S, Irmak Y, Soria F, (2017) Characteristics and clinical significance of histological variants of bladder cancer. Nat Rev Urol 14: 651-668.
- Sahin AA, Myhre M, Ro JY, Sneige N, Dekmezian RH (1991) Plasmacytoid transitional cell carcinoma. Report of a case with initial presentation mimicking multiple myeloma. Acta Cytol 3: 277-280.
- Dayyani F, Czerniak BA, Sircar K, Munsell MF, Millikan RE (2013) Plasmacytoid urothelial carcinomas – a chemo-sensitive cancer with poor prognosis, and peritoneal carcinomatosis. J Urol 189: 1656-1561.
- Brustmann H (2017) Plasmacytoid urothelial carcinoma of the urinary baldder metastatic to the duodenum: A case report diagnostic relevance of GATA3 immunohistochemistry. Case Reports in Pathology.
- Dresen RC, Beets GH, Vliegen RF, Creytens DH, Beets-Tan RG (2008) Linitis plastica of the rectum secondary to bladder carcinoma: a report of two cases and its MR features. Br J Radiol 81: 249-251.
- McPherson VA, Ott M, Tweedie EJ, Izawa JI (2012) Case report and review of the literature: Rectal linitis plastica secondary to the lipoid cell variant of transitional cell carcinoma of the urinary bladder. Can Urol Asso J 6: 431-434.
- Klaile Y, Schlack K, Boegemann M, Steinestel J, Schrader AJ (2016) Variant histology in bladder cancer: how it should change the management in non-muscle invasive and muscle invasive disease? Trans Androl Urol 5: 692-701.
- Keck B, Wach S, Stoehr R, Kunath F, Bertz S (2013) Plasmacytoid variant of bladder cancer defines patients with poor prognosis if treated with cystectomy and adjuvant cisplatin-based chemotherapy. BMC Cancer 8: 71.
- Hashemi-Sadraei N, Perrino CM, Francesca Monn F, Bandali E, Cheng L (2018) Plasmacytoid urothelial carcinoma: clinicopathological study. J Clin Oncol 36: 482.
 Spanish
Spanish  Chinese
Chinese  Russian
Russian  German
German  French
French  Japanese
Japanese  Portuguese
Portuguese  Hindi
Hindi