Case Report, Clin Oncol Case Rep Vol: 6 Issue: 2
A Case of Variant Hairy Cell Leukaemia in Durable Complete Remission on Combination Therapy
Arif Khan1*, Vamshi Krishna2, D N Reddy2
1Department of Medical Oncology, Asian Institute of Gastroenterology, Hyderabad, India
2Department of Gastroenterology, Asian Institute of Gastroenterology, Hyderabad, India
*Corresponding Author: Arif Khan
Department of Medical Oncology,
Asian Institute of Gastroenterology, Hyderabad, India.
E-mail: arif.dr.khan.1981@gmail.com
Received: February 01, 2023; Manuscript No: COCR-23-88392;
Editor Assigned: February 03, 2023; PreQC Id: COCR-23-88392 (PQ);
Reviewed: February 12, 2023; QC No: COCR-23-88392 (Q);
Revised: February 15, 2023; Manuscript No: COCR-23-88392 (R);
Published: February 18, 2023; DOI: 10.4172/cocr.6(2).274
Citation: Khan A, Krishna V, Reddy DN (2023) A Case of Variant Hairy Cell Leukaemia in Durable Complete Remission on Combination Therapy. Clin Oncol Case Rep 6:2
Abstract
A 50-year-old female presented with complaints of abdominal fullness, night sweats and loss of weight over 6 months with massive splenomegaly and pallor. Peripheral blood and bone marrow imprint smear revealed atypical lymphoid cells. Bone marrow biopsy showed a ‘fried egg appearance’ suggestive of hairy cell leukaemia. Bone marrow flow cytometry favoured the diagnosis of Variant hairy cell leukaemia. She was treated with standard 5-day course of Cladribine along with weekly rituximab for 8 weeks. There was regression of splenomegaly with improvement in anemia and sustained complete remission at 36 months. This case highlights the importance of distinguishing variant hairy cell leukaemia from the classical variety as it is more aggressive and needs addition of Rituximab to purine analogues to improve outcomes. Morphological similarity between these entities may lead to delay in diagnosis and inferior treatment. High index of suspicion is needed to initiate proper treatment and maximise outcomes.
Keywords: Cell leukaemia; Combination therapy; Bone marrow; Cladribine; Rituximab
Introduction
Hairy Cell Leukemia (HCL) is a rare, indolent, low grade, B cell non Hodgkin lymphoma which comes under chronic lymphoproliferative disorders [1]. HCL comprises of classical HCL (HCL-c) and HCL like disorders which includes aggressive entity of variant HCL (HCL-v) and Splenic Diffuse Red Pulp Lymphoma (SDRPL). Distinction of these disorders is important because these entities differ genomically, in treatment approach and prognosis [2]. Morphologically these disorders look similar and immunophenotyping helps to differentiate between these entities. The four immunophenotyping markers which help in differentiation are CD11C, CD103, CD25 and CD123. Each marker is assigned a score of 1 if expressed and 0 if not expressed. Classical HCL has score of 3 to 4 whereas the HCL like disorders have low immunological score of 0 to 1 [3].
Case Report
A 50 year old female presented with complaints of heaviness in abdomen, night sweats and loss of weight over a period of 6 months. On examination she had pallor with Hemoglobin level of - 6.9 gm/dl, Total white blood cell count - 4600 cells/mm3, platelets - 1.5 lakhs / mm3 and with mean corpuscular volume of 72 fl. Peripheral smear showed occasional atypical cells with dense chromatin prominent nucleoli and cytoplasmic projections. Peripheral blood smear was negative for malaria parasite, ESR was 16mm/hr, ferritin - 68.2 ng/ ml, LDH - 511 U/L and Coomb’s test was negative. Contrast Enhanced CT scan of abdomen revealed gross splenomegaly with para aortic, aortocaval and mesenteric lymphadenopathy. A bone marrow examination was planned in view of atypical cells on peripheral smear and splenomegaly. Bone marrow aspiration and imprint smears revealed atypical lymphoid cells comprising 6% to 8% of all nucleated cells. There was no evidence of Leishmania Donovani (LD) bodies or granulomas. The bone marrow biopsy revealed a focal atypical lymphoid aggregate with areas showing fried egg appearance (Figure 1A to D).
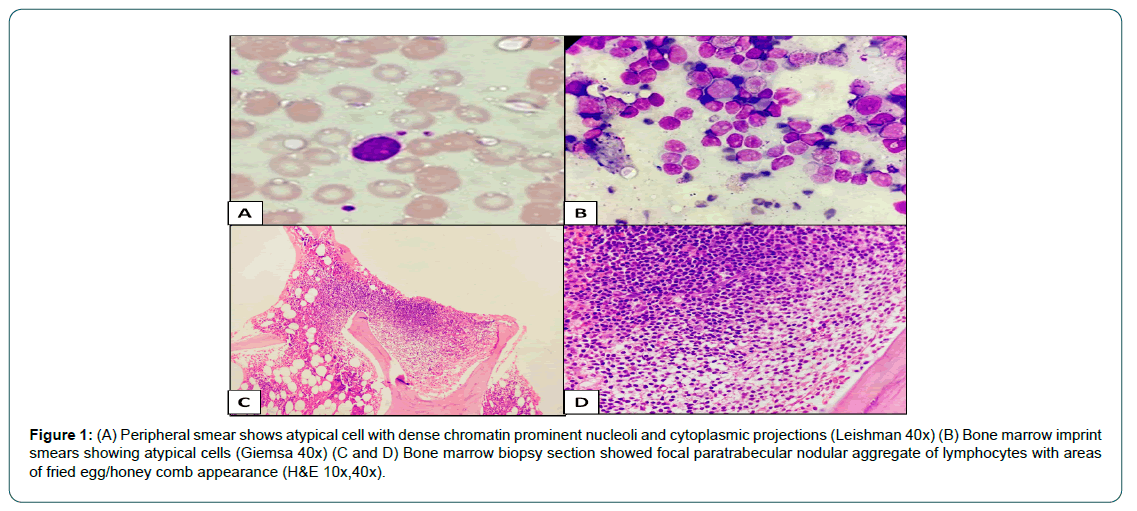
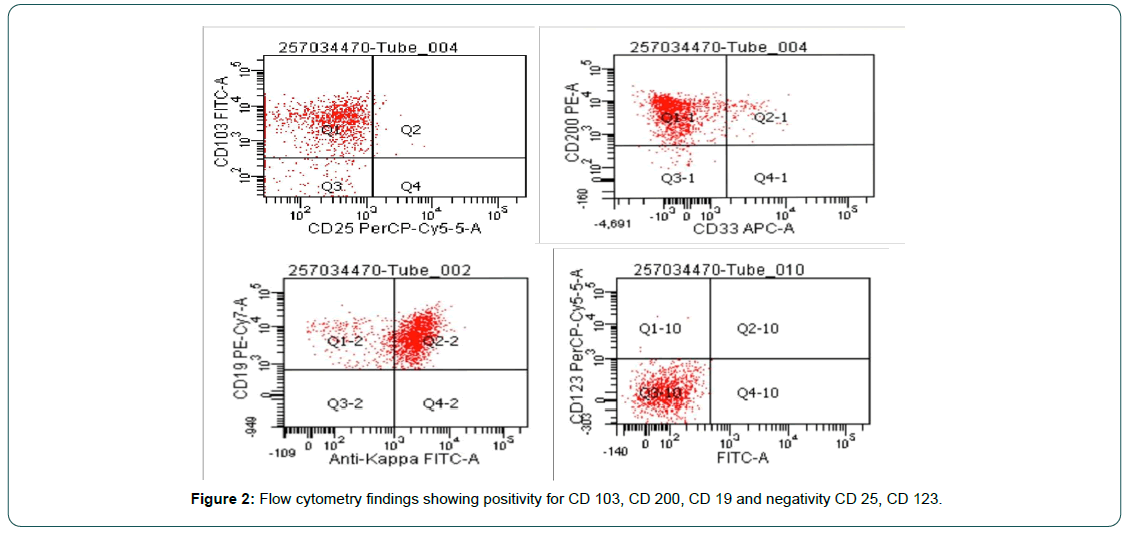
Bone marrow flow cytometry showed atypical cells positive for CD20, CD19, CD79b, FMC-7, CD200, CD103 and surface IgM with kappa light chain restriction and negative for CD5, CD10, CD25, CD11C and CD123 (Figure 2). Based on flowcytometry findings, diagnosis of HCL-v was considered and patient was started on cladribine 0.15 mg/kg/day for 5 days with 8 doses of weekly rituximab at 375 mg/m2 regimen. The splenomegaly regressed after 2 doses of rituximab with good tolerance upto 8 weeks of treatment. However, she developed neutropenia without fever which improved with growth factor support. Subsequent bone marrow examination showed complete morphological remission with no Minimal Residual Disease (MRD) on flow cytometry. She continues to be in Complete Remission (CR) after 36 months of follow up.
Discussion
This case represents a rare hematological malignancy. It is essential to distinguish HCL-c from HCL-v due to the difference in management and potential aggressiveness of HCL-v. Diagnosis of HCL requires a thorough clinical history, physical examination, and investigations including peripheral blood smear and bone marrow morphology, flow cytometry, and newer molecular diagnostics. Peripheral blood morphology of HCL-c and HCL-v typically shows small-to-medium lymphocytes with circumferential cytoplasmic projections. Splenic marginal zone lymphoma with villous lymphocytes is another indolent B-cell lymphoma that may be mistaken for HCL, however, there are unipolar or bipolar cytoplasmic projections rather than circumferential projections as seen in HCL [4]. In both HCL-c and HCL-v, the nucleus is often regular, taking up the majority of the cell. A prominent nucleolus can be seen in many cases of HCL-v [5].
Bone marrow is easily aspirable in HCL-v as opposed to HCL-c [6]. In our case immunological score is 1 in view of positivity for CD 103 and there is aberrant expression of CD 200 which is usually absent in HCL-v [7]. The CD 25 marker is almost always negative in HCL-v as compared to HCL-c [8]. The genomic profile of HCL-v differs from HCL-c in showing positivity for MAP2K1 and negativity for BRAF V600 E [9]. In our case BRAF V 600E was negative. There should be high index of suspicion to diagnose this entity as presentation is not straight forward and it closely mimics HCL-c.
Splenectomy is a potential treatment option and a study by Matutes et al showed partial response which was seen in 13 out of 19 patients. None of the patients achieved CR [10]. Typically, purine nucleoside analogues such as Cladribine and Pentostatin have an excellent effect on HCL, with high complete remission rates with long-term progression-free survival. HCL-v is inherently aggressive and does not respond to single agent purine analogues. The combination therapy of purine analogues with anti CD20 monoclonal antibody namely rituximab is first line treatment option for HCL-v. In the study by kreitman et al when rituximab was added to cladribine it increased the proportion of patients achieving MRD negative CR [11]. In our case the patient achieved MRD negative CR with combination therapy and is in remission till last follow up. Once the patient relapses amongst the several second line options available are ibrutinib and anti-CD 22 moxetumomab pasudotox which are quite effective [12]. MAP2K1 mutation if found represents an attractive target for MEK inhibitors [13].
Conclusion
HCL-v is a more aggressive mature B-cell lymphoma than HCL-c, so it is crucial to diagnose this entity due to different treatment. Early detection of this variant HCL is the key to patient management. HCL-v can be successfully treated with combination therapy with cladribine and rituximab. This regimen is quite effective in achieving MRD negative complete remission. Also it is imperative to do molecular profiling at baseline as it may have implications for subsequent targeted therapy in relapsed refractory setting.
References
- Naik RR, Saven A (2012) My treatment approach to hairy cell leukemia. Mayo Clin Proceedings Elsevier 87: 67-76 Elsevier. [Google Scholar] [Cross Ref]
- Maitre E, Cornet E, Troussard X (2019) Hairy cell leukemia: 2020 update on diagnosis, risk stratification, and treatment. Am J Hematol 94: 1413-1422. [Google Scholar] [Cross Ref]
- Matutes E, Morilla R, Owusu-Ankomah K, Houliham A, Meeus P, (1994) The immunophenotype of hairy cell leukemia (HCL) proposal for a scoring system to distinguish HCL from B-cell disorders with hairy or villous lymphocytes. Leuk Lymphoma 14: 57-61. [Google Scholar] [Cross Ref]
- Matutes E (2006) Immunophenotyping and differential diagnosis of hairy cell leukemia. Hematol Oncol Clin North Am 20: 1051-1063. [Google Scholar] [Cross Ref]
- Matutes E, Wotherspoon A, Catovsky D (2003) The variant form of hairy-cell leukaemia. Best Pract Res Clin Haematol 16: 41-56. [Google Scholar] [Cross Ref]
- Wotherspoon A, Attygalle A, Mendes LS (2015) Bone marrow and splenic histology in hairy cell leukaemia. Best Pract Res Clin Haematol 28: 200-207. [Google Scholar] [Cross Ref]
- Rudolf-Oliveira RCM, Pirolli MM, Souza FSD, Michels J, Santos-Silva MC (2015) Hairy cell leukemia variant: The importance of differential diagnosis. Revista Brasileira de Hematologia e Hemoterapia 37:132-135. [Google Scholar] [Corss Ref]
- Durham BH, Getta B, Dietrich S, Taylor J, Won H, et al. (2017) Genomic analysis of hairy cell leukemia identifies novel recurrent genetic alterations. Blood J A Soc Hematol 130: 1644-1648. [Google Scholar] [Cross Ref]
- Kreitman RJ, Wilson W, Calvo KR, Arons E, Roth L, et al. (2013) Cladribine with immediate rituximab for the treatment of patients with variant hairy cell leukemiacladribine-rituximab for variant hairy cell. Clin CanRese 19: 6873-6881. [Google Scholar] [Cross Ref]
- Matutes E, Wotherspoon A, Brito-Babapulle V, Catovsky D (2001) The natural history and clinico-pathological features of the variant form of hairy cell leukemia. Leukemia 15: 184-186. [Google Scholar] [Cross Ref]
- Kreitman RJ, Tallman MS, Robak T, Coutre S, Wilson WH, et al. (2018) Minimal residual hairy cell leukemia eradication with moxetumomab pasudotox: Phase 1 results and long-term follow-up. Blood J Am Soc Hematol 131: 2331-2334. [Google Scholar] [Cross Ref]
- Bohn JP, Wanner D, Steurer M (2007) Ibrutinib for relapsed refractory hairy cell leukemia variant. Leuk Lymphoma 58: 1224-1226. [Google Scholar] [Cross Ref]
- Andritsos LA, Grieselhuber NR, Anghelina M, Rogers KA, Roychowdhury S, et al. (2018) Trametinib for the treatment of IGHV4-34, MAP2K1-mutant variant hairy cell leukemia. Leuk Lymphoma 59: 1008-1011. [Google Scholar] [Cross Ref]
 Spanish
Spanish  Chinese
Chinese  Russian
Russian  German
German  French
French  Japanese
Japanese  Portuguese
Portuguese  Hindi
Hindi 