Case Report, Clin Oncol Case Rep Vol: 6 Issue: 3
A Case Report: A Step ahead in EGFR Resistance Mechanism, Non-Small Cell Lung Cancer (NSCLC).
Beatriz Alonso de Castro1*, Martin I Gomez-Randulfe Rodriguez1 , Francesco Fachinetti2 , Sofia Silva Diaz1 , Iria Parajo Vázquez1 , Joaquin Mosquera Martinez1 , Patricia Cordeiro Gonzalez1 , Rosario Garcia Campelo1 , Manuel Fernandez-Bruno1
1Department of Oncology, University Hospital A Coruna, Spain
2Department of Oncology, Institut Gustave Roussy, Villejuif, France
*Corresponding Author: Beatriz Alonso de Castro
Department of Oncology
University Hospital A Coruna, No. 2, Xubias de Arriba, 84 -15006 -A Coruna, Spain.
E-mail: baealonso_93@hotmail.es
Received: March 07, 2023; Manuscript No: COCR-23-91219;
Editor Assigned: March 09, 2023; PreQC Id: COCR-23-91219 (PQ);
Reviewed: March 17, 2023; QC No: COCR-23-91219 (Q);
Revised: March 20, 2023; Manuscript No: COCR-23-91219 (R);
Published: March 22, 2023; DOI: 10.4172/cocr.6(3).280
Citation: Castro BA,Rodriguez MG, Fachinetti F, Diaz SS,Vázquez IP, et al. A Case Report: A Step ahead in EGFR Resistance Mechanism, Non-Small Cell Lung Cancer (NSCLC) Clin Oncol Case Rep 6:3
Abstract
15% of NSCLC patients in Western countries have a mutation in Epidermal Growth Factor Receptor (EGFR). These mutations confer patients susceptibility to EGFR-Tyrosine Kinase Inhibitors (TKIs). Nevertheless, acquired resistances and secondary progressions will appear. We present a case report of a woman who had never smoked and was diagnosed of a non operable lung cancer adenocarcinoma EGFR L858R mutated treated by first line osimertinib. After progression MET amplification was found and the patient received Savolitinib added to osimertinib that had failed in the context of a C797S resistance mutation found in liquid and tissue biopsy. In this context, the patient start treatment with gefitinib with improvement of symptoms and without toxicity. This case highlights the importance of molecular testing at diagnose and after progression to TKIs, in order to improve survival in a group of patients with initial poor prognosis without target therapies.
Keywords: EGFR, Lung cancer, Kinase inhibitor, Target therapy, Case report
Introduction
Despite tumor biology and precision medicine development, treatment in lung cancer is one of the most challenging health issues. 15% of NSCLC patients in Western countries have a mutation in Epidermal Growth Factor Receptor (EGFR), a tyrosine kinase receptor, that activates cellular signaling pathway, by the disregulation of oncogenic mechanisms, with the consequent cell proliferation. Deletions in exon 19 and exon 21 L858R mutations account for 90% of “classical” EGFR mutations in NSCLC, highly prevalent in female, nonsmokers and asian patients [1, 2]. These activating mutations confer patients susceptibility to EGFR - Tyrosine Kinase Inhibitors (TKIs). Nevertheless, acquired resistances and secondary progressions will appear after a median of 18 months with third generation drugs like osimertinib [2, 3]. These resistances can be grouped into EGFR independent or dependent mechanisms. First ones are acquired by activation of alternative bypass pathways or aberrant downstream signaling: MET amplification, the most frequent EGFR resistance mechanism to first line osimertinib, encountered in 15% of patients by Next-Generation Sequence (NGS) ctDNA analyses; HER2 amplification; PI3K pathway activation; RAS-MAPK pathway aberrations; PI3K activation; cell-cycle gene alterations; oncogenic fusions; or histologic transformation [4]. On the other hand, EGFR dependent mechanisms are based on secondary receptor mutations, like C797S in exon 20, the second more frequent resistance mechanism (7%) [4]. In plasma NGS of the AURA3 study, MET amplification co-occurred with C797S insertion in 7% of cases [4]. EGFR mutations can be assessed using many methodologies in tissue and plasma. One of these technologies is NGS, a molecular profiling test that analyzes relevant genes, including small mutations, and detects more EGFR alterations compared to Polymerase Chain Reaction (PCR) [5]. Adding plasma test increases the detection of therapeutically targeteable mutations that can rescue patients when tissue is not available, insufficient or we do not find a mutation, obviating the need of repeat an invasive biopsy [6]. In our case we used a real-time PCR test for initial diagnose, COBAS 4800®. To evaluate acquired resistance mechanisms we performed AmoyDx® Pan Lung Cancer test, a real-time PCR panel assay for qualitative detection of 167 hotspot; FoundationOne®CDx NGS, analyzes entire regions of 324 cancer-relevant genes (DNA) in tissue [7]; and Guardant 360®CDx NGS, a 73-gene panel utilizing digital sequencing of ctDNA plasma (Table 1) [6].
Table 1: Molecular testing during course disease, PCR: Polymerase Chain Reaction. NGS: Next-Generation Sequence.
| Molecular Testing | Timing of test | Type of test | Sample | Result |
| Diagnosis stage IIIB | Real time PCR: COBAS 4800® | Tumor | EGFR L858R detected | |
| Progression during osimertinib | NGS, FoundationOne®CDx | Tumor | MET amplified | |
| Progression during osimertinib + savolitinib | Real time PCR: AmoyDx® Pan Lung Cancer | Tumor | C797S | |
| Progression during atezolizumab + bevacizumab + pemetrexed | NGS, Guardant360®CDx | Plasma | EGFR C797S (63.7% of variant allele fraction of cfDNA); EGFR L858R (68.3% of variant allele fraction of cfDNA); EGFR L833V (68.2% of variant allele fraction of cfDNA); EGFR Amplification high (+++). |
Case Presenatation
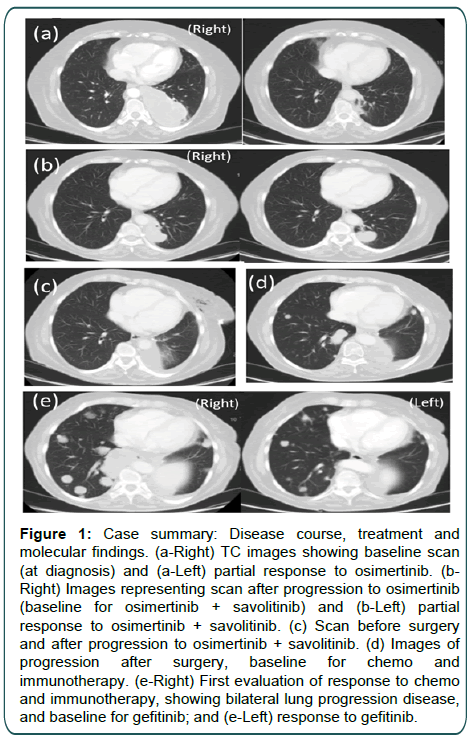
We present a 64-year-old woman who had never smoked, former drinker, with alcoholic cirrhosis Child-Pugh A5 and family background of cancer, mother deceased by ovarian carcinoma and father by pancreatic adenocarcinoma. In March 2019 a pneumologist consultation was performed because of left hemithorax pain, asthenia and dyspnea New York Heart Association (NYHA) degree II. In relation to these symptoms a Computed Tomography (CT) of the chest was carried out and revealed a lung mass in the left lung with ipsilateral mediastinal lymph nodes (Figure 1a Right), last ones with a SUVmax of 8.3 in 18fluorodeoxyglucose-positron tomography scan. A bronchoscopy and a videomediastinoscopy allowed the diagnosis of lung adenocarcinoma from the neoplasic mass in the left sixth subsegmental bronchus and in the ipsilateral mediastinal lymph nodes. Finally, the pathology report revealed a lung adenocarcinoma, EGFR L858R mutated by COBAS 4800®, with ipsilateral mediastinal lymph nodes involvement and baseline staging IIIB, cT4N2M0, (AJCC 8th Edition).
In April 2019 the patient came to the oncology department with the same symptoms, ECOG - Performance Status (PS) 1 and a normal exploration. After discussing different options of treatment, including chemotherapy or chemoradiotherapy before surgery; radical chemoradiotherapy; or systemic treatment, the patient decided to participate in clinical trial ELIOS (NCT03239340) iniciating osimertinib 80 mg orally once daily.
Osimertinib induced rapid clinical improvement, with diarrhea degree 1 and erythematous rash degree 1 (Common Terminology Criteria for Adverse Events, CTCAE, v5.0) toxicity. At follow-up, 12 weeks after starting osimertinib, she had a partial response in the CT evaluation by RECIST version 1.1. (Figure 1a Left). Progressive disease in the lung was noted in April 2020, after 12 months of treatment (Figure 1b Right). At this moment, the patient was screened in the ORCHARD trial (NCT03944772) and a new biopsy with FoundationOne®CDx NGS was performed in the lung revealing MET amplification. Savolitinib 600 mg orally once daily was added to osimertinib 80 mg orally once daily in ORCHARD Trial.
Figure 1: Case summary: Disease course, treatment and molecular findings. (a-Right) TC images showing baseline scan (at diagnosis) and (a-Left) partial response to osimertinib. (bRight) Images representing scan after progression to osimertinib (baseline for osimertinib + savolitinib) and (b-Left) partial response to osimertinib + savolitinib. (c) Scan before surgery and after progression to osimertinib + savolitinib. (d) Images of progression after surgery, baseline for chemo and immunotherapy. (e-Right) First evaluation of response to chemo and immunotherapy, showing bilateral lung progression disease, and baseline for gefitinib; and (e-Left) response to gefitinib.
Combination treatment was well tolerated with an asymptomatic elevation of Alanine Aminotransferase (ALT) and Aspartate Aminotransferase (AST) degree 1 and amylase degree 2 (CTCAE, v5.0) that did not require therapy modifications. CT evaluation in the 6th week revealed a partial response by RECIST version 1.1. (Figure 1b Left), maintained until March 2021, when a progression of the lung mass was detected, after 10 months of therapy (Figure 1c).
The case was discussed on tumor board and due to exclusive lung progression with nodal sustained response, without metastases, a left lower lobectomy and lymph node dissection (ypT4pN2M0) was performed in May 2021. Surgery was followed by Post-Operative Radiotherapy (PORT) in hiliomediastinal lymph node areas and surgical margin, 50Gy in 25 fractions of 2Gy. AmoyDx® Pan Lung Cancer was performed in surgical specimen showing a C797S resistance mutation of EGFR in exon 20, without any other mutation, neither MET amplification.
One month after PORT, September 2021, CT- scan showed new bilateral pulmonary nodules (Figure 1d). The patient was included into ABC-Lung trial (NCT04245085) receiving combination therapy with atezolizumab 1200 mg/Kg plus bevacizumab 15 mg/Kg plus pemetrexed 500 mg/Kg intravenous each 21 days. In November 2021, she started complaining about lumbar pain and the first follow-up imaging showed disease progression in lung and bone (Figure 1e Right). At this moment, Guardant 360®CDx NGS was performed in plasma and revealed: EGFR C797S (63.7% of variant allele fraction of cfDNA), EGFR L858R (68.3% of variant allele fraction of cfDNA), EGFR L833V (68.2% of variant allele fraction of cfDNA), EGFR Amplification high (+++). Notably, T790M mutation was not detected.
The case was commented on the molecular tumour board that decided to start Gefitinib 250 mg orally once daily. Treatment was started with improvement of symptoms and without toxicity. A partial response was seen in CT - scan in January 2022 (Figure 1e Left). After 4 months of therapy, the patient was hospitalized with dyspnea and asthenia degree 3 (CTCAE, v5.0), ECOG -PS 3, due to pulmonary progression and finally died in March 2022.
Discussion
This case highlights the importance of molecular testing at diagnose and after progression to TKIs, in order to improve survival in a group of patients with initial poor prognosis without target therapies. We were focused on searching resistance mechanisms to TKIs with the intention to carry out a target treatment sequence instead of second line chemotherapy, the current recommendation in clinical guideliness, to improve survival and decrease toxicity.
Patient´s molecular analysis showed MET amplification after first response to osimertinib. Combination therapy with EGFR and MET targeted therapies, osimertinib plus savolitinib, is an investigational option that showed clinical benefit in previously treated third-generation EGFR-TKIs in TATTON trial (NCT02143466), with a Response Rate (RR) of 33% (95% Confidence Interval [CI], 22, 46), median Progression Free Survival (PFS) of 5.5 months (95% CI, 4.1, 7.7) and a safety profile [8]. This clinical benefit requires confirmation with studies that are underway, SAVANNAH (NCT03778229) and ORCHARD (NCT03944772) [8, 9]. Preliminary results from ORCHARD trial (NCT0394472) were recently reported with a response rate of 41% in MET amplified cohort after first line osimertinib. Our patient was included in this study without notably toxicity, partial response and a PFS of 10 months [10].
In EGFR mutations previously treated with TKIs, atezolizumab plus bevacizumab plus carboplatin plus paclitaxel showed a RR of 70.6% with survival benefit in IMPOWER-150 trial [4]. Taking these results into account, we started treatment in ABC lung protocol, with atezolizumab plus bevacizumab plus pemetrexed, but the patient progressed in the first evaluation, after two cycles of treatment. Maybe this can be explained for the abcense of carboplatin in the trial, the main difference with IMPOWER-150 (NCT02366143) [11].
The patient was subsequently treated with a first generation EGFR TKI, Gefitinib, that demonstrated sensitivity to wild type T790 with C797S mutation cells in preclinical models and case reports [12, 13, 14]. Disease control with partial response was maintained for 4 months without notable toxicity. Similar results were found in a 52-years-old asian woman with lung adenocarcinoma and a EGFR exon 19 deletion treated with first line osimertinib and second line erlotinib after progression to third generation TKI in relation to a EGFR C797S mutation [12]. It seems that first generation EGFR TKIs (gefitinib or erolitinib) can achieve tumor response in patients with EGFR C797S mutation, though duration of response in the two cases is limited to 4 months. We need to carry out clinical trials to confirm this hypothesis.
Conclusion
Our case showed sequential acquisition of the two most frequent EGFR resistance mechanisms to TKIs and an overall survival benefit, reaching 35 months, with target therapies. Nowadays, one of the main challenges of precision oncology is the investigation of novel molecules that target resistance mutations and improve survival without toxicity. For this reason, is necessary to incorporate sensitive and safe tumor profiling, as plasma and tissue NGS, into routine clinical management of patients with NSCLC.
Acknowledgements
We thank all patients, caregivers and staff of the oncology service of Hospital A Coruña that participated in this case report.
Author Contributions
B.A.C., M.F.B., contributed equally to this work as co-first authors. M.I.G-R.R., F.F., S.S.D., I.P.V., J.M.M., P.C.G., M.R.G.C. contributed equally to this work as co-senior authors.
Conflicts of interests
The authors declare no conflicts of interest.
Informed Consent
The authors obtained informed consent to publish the report from patient´s legal representative.
References
- Hanna NH, Schneider BJ, Temin S, Baker Jr S, Brahmer J, et al. (2020) Therapy for stage IV non-small-cell lung cancer without driver alterations: ASCO and OH (CCO) joint guideline update. J Clin Oncol. [Google Scholar] [Cross Ref]
- Papini F, Sundaresan J, Leonetti A, Tiseo M, Rolfo C, et al. (2021) Hype or hope-Can combination therapies with third-generation EGFR-TKIs help overcome acquired resistance and improve outcomes in EGFR-mutant advanced/metastatic NSCLC? Crit Rev Oncol/Hematol 166: 103454. [Google Scholar] [Cross Ref]
- Soria JC, Ohe Y, Vansteenkiste J, Reungwetwattana T, Chewaskulyong B, et al. (2018) Osimertinib in untreated EGFR-mutated advanced non-small-cell lung cancer. New Eng J Med 378: 113-125. [Google Scholar] [Cross Ref]
- Leonetti A, Sharma S, Minari R, Perego P, Giovannetti E, et al. (2019) Resistance mechanisms to osimertinib in EGFR-mutated non-small cell lung cancer. Brit J Can 121: 725-737. [Google Scholar] [Cross Ref]
- Imyanitov EN, Iyevleva AG, Levchenko EV (2021) Molecular testing and targeted therapy for non-small cell lung cancer: Current status and perspectives. Crit Rev Oncol/Hematol 157: 103194. [Google Scholar] [Cross Ref]
- Aggarwal C, Thompson JC, Black TA, Katz SI, Fan R, et al. (2019) Clinical implications of plasma-based genotyping with the delivery of personalized therapy in metastatic non-small cell lung cancer. JAMA Oncol 5: 173-180. [Google Scholar] [Cross Ref]
- Milbury CA, Creeden J, Yip WK, Smith DL, Pattani V, et al. (2022) Clinical and analytical validation of FoundationOne® CDx, a comprehensive genomic profiling assay for solid tumors. PLoS One 17: e0264138. [Google Scholar] [Cross Ref]
- Hartmaier RJ, Markovets AA, Ahn MJ, Sequist LV, Han JY, et al. (2022) Osimertinib+Savolitinib to overcome acquired MET-mediated resistance in epidermal growth factor receptor mutated MET-amplified non-small cell lung cancer. TATTON Cancer Discov 13: 98. [Google Scholar] [Cross Ref]
- Lai-Kwon J, Tiu C, Pal A, Khurana S, Minchom A (2021) Moving beyond epidermal growth factor receptor resistance in metastatic non-small cell lung cancer - a drug development perspective. Crit Rev Oncol Hematol 159: 103225. [Google Scholar] [Cross Ref]
- Remon J, Hendriks LEL, Mountzios G, García-Campelo R, Saw SPL, et al. (2022) MET alterations in NSCLC-current perspectives and future challenges. J Thorac Oncol. [Google Scholar] [Cross Ref]
- Reck M, Mok TSK, Nishio M, Jotte RM, Cappuzzo F, et al. (2019) Atezolizumab plus bevacizumab and chemotherapy in non-small-cell lung cancer (IMpower150): Key subgroup analyses of patients with EGFR mutations or baseline liver metastases in a randomised, open-label phase 3 trial. Lancet Respir Med 7: 387-401. [Google Scholar] [Cross Ref]
- Rangachari D, To C, Shpilsky JE, VanderLaan PA, Kobayashi SS, et al. (2019) EGFR-mutated lung cancers resistant to osimertinib through EGFR C797S respond to first-generation reversible EGFR inhibitors but eventually acquire EGFR T790M/C797S in preclinical models and clinical samples. J Thorac Oncol 14: 1995-2002. [Google Scholar] [Cross Ref]
- Niederst MJ, Hu H, Mulvey HE, Lockerman EL, Garcia AR, et al. (2015) The allelic context of the C797S mutation acquired upon treatment with third-generation EGFR inhibitors impacts sensitivity to subsequent treatment strategiesc797s promotes resistance to third-generation EGFR inhibitors. Clin Can Res 21: 3924-3933. [Google Scholar] [Cross Ref]
- Chic N, Mayo-de-Las-Casas C, Reguart N (2017) Successful treatment with gefitinib in advanced non-small cell lung cancer after acquired resistance to osimertinib. J Thora Oncol 12: e78-e80. [Google Scholar] [Cross Ref]
 Spanish
Spanish  Chinese
Chinese  Russian
Russian  German
German  French
French  Japanese
Japanese  Portuguese
Portuguese  Hindi
Hindi