Case Report, Clin Oncol Case Rep Vol: 6 Issue: 3
A Case Report of a Massive Adrenocortical Carcinoma, When The Small Dreams Become Big Nightmares
Makram Khoury1*, Rhea Akel2 , Lea Khoury3 , Viviane Trak Smayra3 , Fady Nasr1
1Department of Hematology-Oncology, Saint Joseph University, Hotel Dieu de France Hospital, Beirut, Lebanon.
2Department of Radiology, Saint Joseph University, Hotel Dieu de France Hospital, Beirut, Lebanon.
3Department of Pathology, Saint Joseph University, Hotel Dieu de France Hospital, Beirut, Lebanon.
*Corresponding Author: Makram Khoury
Department of Hematology-Oncology
Saint Joseph University, Hotel Dieu de France Hospital, Beirut, Lebanon.
E-mail: Makram.kh23@gmail.com
Received: March 09, 2023; Manuscript No: COCR-23-92947;
Editor Assigned: March 11, 2023; PreQC Id: COCR-23-92947 (PQ);
Reviewed: March 20, 2023; QC No: COCR-23-92947 (Q);
Revised: March 24, 2023; Manuscript No: COCR-23-92947 (R);
Published: March 29, 2023; DOI: 10.4172/cocr.6(3).283
Citation: Khoury M, Akel R, Khoury L, Smayra VT, Nasr F (2023) A Case Report of a Massive Adrenocortical Carcinoma, When the Small Dreams become Big Nightmares. Clin Oncol Case Rep 6:3
Abstract
Background: Adrenocortical carcinoma is a very rare and aggressive tumor of the adrenal gland. It has a poor prognosis even in the form of localized disease. It presents mainly with signs of hormonal excess but it can present with metastases.
Case presentation: We report the case of a young woman who presented a Cushing syndrome with virilization. The clinical presentation, the biochemical tests, the imaging findings and the pathology studies altogether establish the diagnosis of a huge adrenocortical carcinoma at advanced stage with identified pulmonary lesions. The patient was started on mitotane and has received chemotherapy.
Conclusion: This case is presented because of its rarity, and to highlight the importance of combining the different aspects of the presentation mode of the disease in order to establish the diagnosis.
Keywords: Adrenocortical carcinoma, Cushing, Virilization, Mitotane, Case report
Introduction
Adrenocortical Carcinoma (ACC) is a very uncommon tumor of the adrenal gland, less common than adrenal adenomas and pheochromocytoma, with an annual worldwide incidence of 0.5 to 2 per million people [1, 2]. It accounts for far less than 0.1% of all carcinomas and represents 2% to 5% of diagnosed incidentaloma [3]. This tumor, however, accounts for approximately 0.02% to 0.2% of all cancer-related deaths [4, 5]. We report a rare case of a young woman diagnosed of an advanced stage adrenocortical carcinoma, discovered on the occasion of Cushing syndrome with virilization.
Case Presentation
A 19-year-old young woman presented with progressively increasing bilateral lower extremity edema with abdominal pain and novel hypertension and hyperglycemia since two months. She is a non-smoker patient who had no past medical or surgical history and she was taking no medications or oral steroids or oral contraceptives.
She has no personal or family history of cancers.
It has been two months ago when she first started noticing a progressively increasing abdominal “ballooning” with aggravating unspecific abdominal pain, especially nocturnal, for which she is actually taking paracetamol, codeine, COX-2 inhibitor nonsteroidal anti-inflammatory drug and gabapentin, in addition to a lower limbs edema of progressive onset. This was accompanied by newly installed hypertension for which she is treated by Lisinopril and Bisoprolol, and a newly installed diabetes treated with metformin. She has gained weight throughout the last two months; her current BMI is 27.87 kg/ m2 . Menstrual irregularities are also experienced and particularly an oligospaniomenorrhea is described. In addition to these findings, the patient reports an increasing hirsutism with downy facial lanugo hair appearing mostly on her cheeks and upper lips during the last two months, a noticeable hair loss, a newly developed facial acne and a thin easy bruising skin. She reports no sleep disturbances, no emotional or cognitive problems, no appetite alteration, and no changes in stool bowel habits.
She was then diagnosed of a Cushing syndrome with a serum cortisol level of 628.2 mmol/L. An abdominal CT-scan is done one month before the patient has presented to our institution. The scan showed a large well-defined right adrenal mass composed almost completely of soft tissue component with heterogeneous enhancement with no calcifications. The mass measures 23×14 cm in greatest dimensions, and it displaces the liver up and the right kidney more caudally and medially without invading the liver or liver metastases and without invasion of the inferior vena cava. The images of the lung bases show multiple well-defined enhancing soft tissue nodules suggesting lung metastases.
An US guided core biopsy of the adrenal mass is done and is in favor of adrenocortical tumor. She was then transferred to our institution.
On physical examination, her blood pressure is 160/108 mmHg, other vital signs are normal. The patient has a round moon face. She has acne vulgaris on her forehead, cheeks and chin. Hirsutism is seen on her cheeks and upper lips. Temporal alopecia corresponding to male pattern baldness is present. The examination of the cervical region reveals an aspect of buffalo hump with thickened dark discoloration corresponding to acanthosis nigricans. There is a cutaneous hyperpigmentation on her upper back. The abdominal examination reveals a distended abdomen with multiple wide purplish striae. A firm large mass is palpable on the right side of the abdomen extending from the right upper to the right lower quadrant. There is no jaundice or pallor and no collateral veins or other signs of hepatic impairment. There are no signs of ecchymosis. Pitting edema is noticed in both lower limbs reaching the thighs bilaterally.
We completed the laboratory tests that reveals a blood glucose level of 168 mg/dL with HbA1c at 6.5%, hypercortisolemia with a cortisol level of 269 ng/mL on 8 a.m. (Reference Range (RR) : 50 ng/mL - 250 ng/mL) and 251 ng/mL on 11 p.m. (RR : <50 ng/mL), an ACTH level of 6.3 pg/mL (RR : 0 pg/mL - 46 pg/mL), a testosterone level of 310 ng/ dL (RR : 0 ng/dL -73 ng/dL), a DHEAS level higher than 1000 µg/dL (RR : 35 µg/dL -430 µg/dL) and an active renin of 59.4 µUI/mL (RR : 2.8 µUI/mL-39.9 µUI/mL). There is no thyroid dysfunction (TSH level of 0.55 mUI/L [RR : 0.3 mUI/L -4 mUI/L] and FT4 of 1.18 ng/dL [RR : 0.78 ng/dL -1.76 ng/dL]). There is no anemia (hemoglobin of 13.1 g/ dL), no hypokalemia or hypernatremia with sodium and potassium levels of 139 mmol/L and 4 mmol/L respectively. The creatinine was normal with a level of 35µmol/L, correspondingto an estimated glomerular filtration rate of 152 ml/min/1.73m2. The serum albumin level (34 g/L) and the serum calcium level (2.35 mmol/L) are normal. The LDH level is high, 1031 U/L. Coagulation tests are normal with an INR of 1.09 and aPTT of 37 seconds.
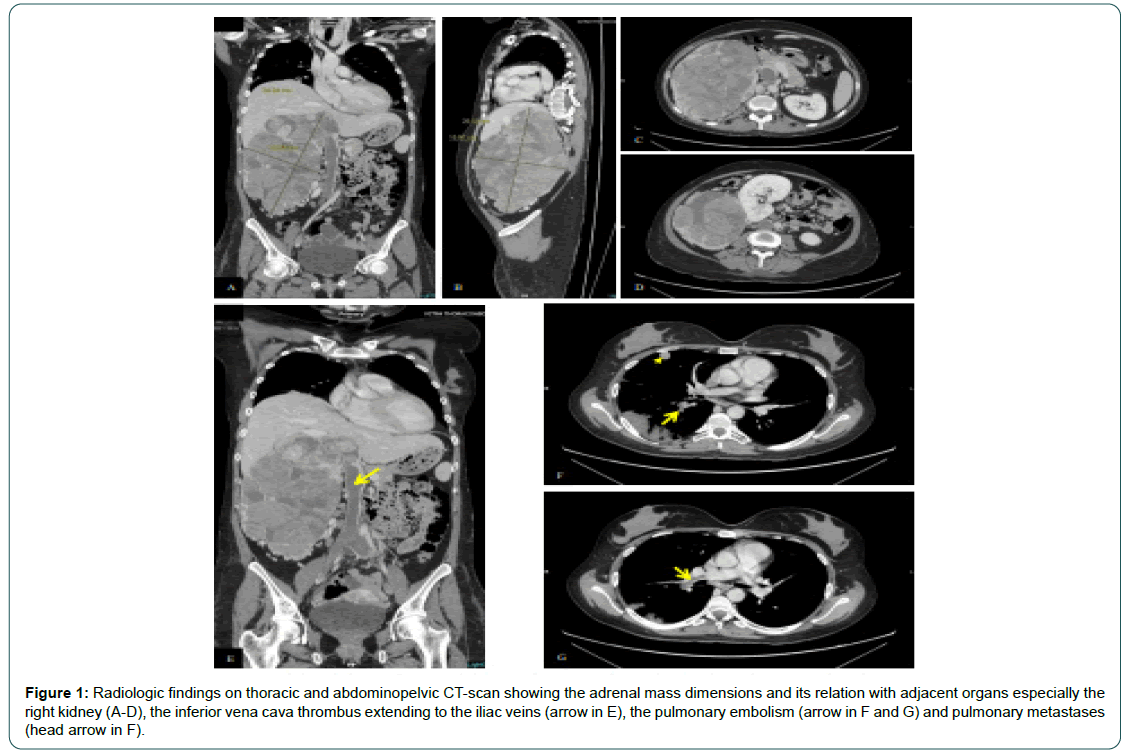
We repeated a thoracic and abdominopelvic CT-scan with IV contrast (Figure 1 A-G). The thoracic CT shows a dozen of welldefined peripheral lung nodules compatible with secondary lesions, with a mild right pleural effusion. The scan reveals also a thrombus of the right main pulmonary artery and the right lower lobe segmental branches. A large right adrenal mass measuring 25 cm ×20 cm ×15 cm, located in the right upper quadrant is seen, with heterogeneous enhancement on the arterial phase and rapid washout on the portovenous phase, displacing the right kidney more caudally and disrupting in some places the interface with the liver, suggesting millimetric liver infiltration. The mass completely infiltrates the retro-hepatic portion of the inferior vena cava with a slight supradiaphragmatic extension associated more distally to an extensive thrombus of the inferior vena cava reaching the common iliac veins and extending to the left and right renal veins. There is a peripheral triangular hypodensity in the left renal parenchyma evoking a small ischemia and an important collateral circulation in the retroperitoneum and within the mesenteric folds.
Figure 1: Radiologic findings on thoracic and abdominopelvic CT-scan showing the adrenal mass dimensions and its relation with adjacent organs especially the right kidney (A-D), the inferior vena cava thrombus extending to the iliac veins (arrow in E), the pulmonary embolism (arrow in F and G) and pulmonary metastases (head arrow in F).
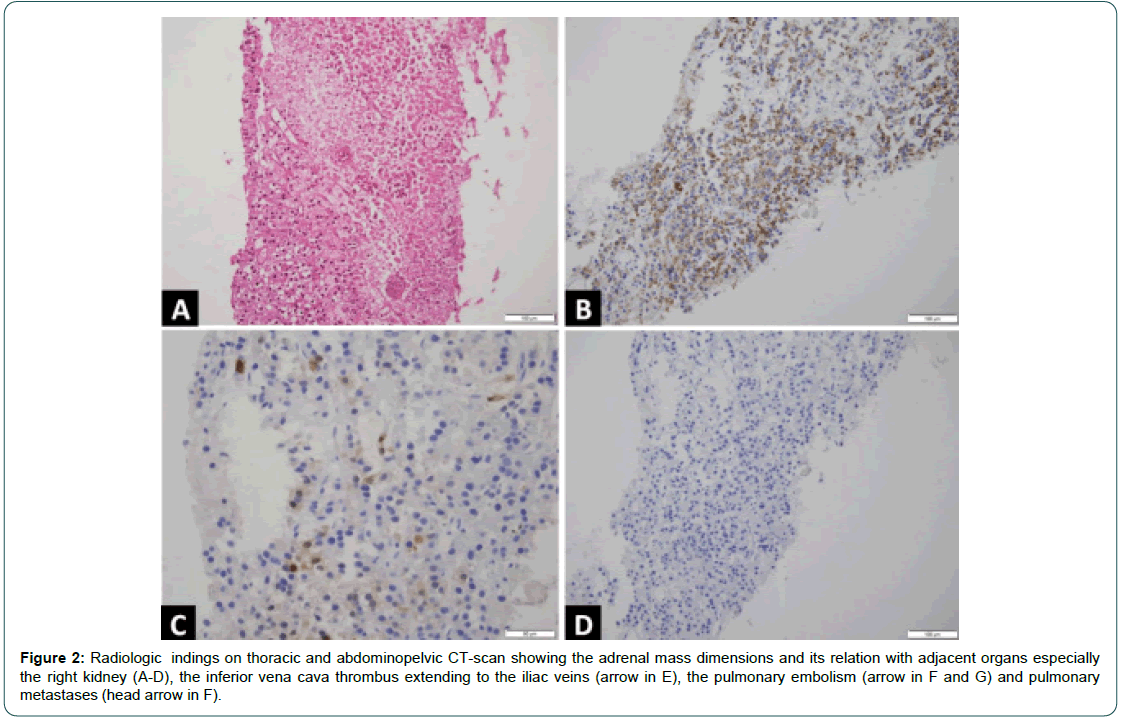
A second reading of the pathology specimen in our pathology laboratory (Figure 2 A-D) shows cells with eosinophilic cytoplasm and a slightly atypical nucleus, fragmented reticulinic network, some areas of tumor necrosis and exceptional mitoses.
Figure 2: Radiologic indings on thoracic and abdominopelvic CT-scan showing the adrenal mass dimensions and its relation with adjacent organs especially the right kidney (A-D), the inferior vena cava thrombus extending to the iliac veins (arrow in E), the pulmonary embolism (arrow in F and G) and pulmonary metastases (head arrow in F).
In immunohistochemistry, these cells express Melan A and focal calretinin, pancytokeratin marks rare cells and chromogranin A is negative. These findings favor the presence of an adrenocortical carcinoma. The immunohistochemical study shows also a conservation of the expression of DNA mismatch repair proteins (MLH1, MSH2, MSH6 and PMS2) by the tumor cells. The final diagnosis was a metastatic adrenocortical carcinoma with pulmonary lesions and invasion of the inferior vena cava, presenting with a clinical Cushing syndrome and signs of virilization. A surgical debulking of the present mass was discussed but was not feasible because of the bulky disease, the infiltration of the liver and of the inferior vena cava and the intra-luminal thrombus of the inferior vena cava and renal veins, besides having pulmonary secondary lesions. Therefore, we opted for a medical treatment. The patient received a dose of carboplatin at the dose of 450 mg. Mitotane is concomitantly initiated at 0.5 mg twice daily and increased progressively over six weeks to reach 6g/ day divided into four doses, in conjunction with replacement therapy with oral prednisone, and metoclopramide to palliate nausea and vomiting, the most common potential side effects of mitotane. The patient was instructed to perform serum mitotane dosage after three weeks of the beginning of the treatment to adjust the dose according to serum mitotane level with a blood test including thyroid function test and lipid profile.
Discussion
This case describes the presentation of a rare tumor, the adrenocortical carcinoma [1, 2]. Although very rare, bilateral ACC is possible in 2% to 10% of the cases [6]. ACC are very aggressive tumors and have three different ways to present. They may be functional in approximately 50%-60% of the cases and therefore have symptoms related to hormonal excess which can be due to hypercortisolism (Cushing syndrome), hyperandrogenism (virilization) or both (in about 50% of the cases), or non-functional and present as an abdominal mass or other non-specific symptoms in 30% to 40% of the patients (such as abdominal or flank pain, early satiety, and abdominal fullness), or they can be diagnosed coincidentally in 10%-15% of the cases [7]. ACC are more often functional in children (90% of cases) compared to adults and most of them present with hyperandrogenism, hypercortisolism, rapidly progressing muscle weakness and pronounced muscle weakness [8]. As with all adrenal tumors, the presence of local or distant spread defines their malignancy [9]. Approximately 30% of the patients have distant metastasis at the presentation [10]. Our patient presents with a large abdominal mass responsible for an abdominal fullness, with several clinical features of hormonal excess defining a Cushing syndrome (plethora, diabetes mellitus, hypertension, purple striae on the abdominal wall, truncal obesity, etc.) and virilization (male pattern baldness, hirsutism, menstrual abnormalities, etc.) highlighting cortisol and androgen secretion by the tumor.
A clear etiology of ACC is not well established. They can arise sporadically or be a part of familial cancer syndrome such as LiFraumeni syndrome or Familial Adenomatous Polyposis (FAP) or MEN1 or Beckwith-Wiedemann Syndrome (BWS), but most cases of ACC appear to be sporadic [10]. A familial or hereditary etiology is less likely in our case, taking into consideration the patient age and knowing the lack of significant family history and the absence of other features characterizing these syndromes [11]. On the other hand, cigarette smoking and oral contraceptives use were identified as potential risk factors for ACC, but were not found in our patient history.
By sex, women are more affected than men with female-to-male ratio of 1.5-2.5:1 [12]. Although ACC can develop at any age, there is usually a bimodal age distribution and the 6 disease peaks before the age of 5 and in the fourth to fifth decade of life [13]. However, our patient does not fit any of the 2 major age category. At the time of diagnosis, the average size of the tumor is approximately 10 cm to 13 cm and a minority of patients present with tumors less than 6 cm with larger tumors at presentation being typically nonfunctional tumors. At the contrast, our patient presented with an unpredictable tumor size for a functional malignant tumor.
In concordance with the National Comprehensive Cancer Network (NCCN) guidelines, the European Network for the Study of Adrenal Tumors (ENSAT) recommends performing the following tests to determine the secretory activity of an adrenal tumor: fasting blood glucose, serum potassium, cortisol, corticotropin, 24-hour urinary free cortisol, fasting serum cortisol at 8 AM following a 1 mg dose of dexamethasone at bedtime, adrenal androgens (Dehydroepiandrosterone Sulfate (DHEAS), and rostenedione, testosterone, 17- hydroxyprogesterone), and serum estradiol in men and postmenopausal women. Furthermore, it is recommended to exclude pheochromocytoma by obtaining plasma metanephrines or urinary metanephrines. However, given the clinical presentation and the prior imaging and biopsy performed before the patient has presented to our institution, the diagnosis of pheochromocytoma was already ruled out and the 24-hour urinary free cortisol and the dexamethasone suppression test were omitted, and not all the androgens were tested.
Diagnosis and staging of ACC by imaging modalities are crucial for preoperative planning and prognostication [13]. Imaging findings of ACC on CT consist of a well-defined mass usually larger than 6 cm with inhomogeneous density due to internal hemorrhage and necrosis, associated to the presence of microcalcification or macrocalcification that can be seen in about 30% of the cases. The diagnostic work-up for our patient was completed by a CT-scan of the abdomen and the pelvis. This imaging modality rules out the presence of the disease in the contralateral adrenal gland and shows malignancy criteria, orienting therefore the diagnosis.
The extension of the tumor is essentially evaluated according to the imaging results. The abdominopelvic CT-scan confirms or not the extension into the renal vein and/or inferior vena cava or adjacent organs particularly the liver, and chest CT-scan searches for metastases to the lungs especially if the primary tumor size is greater than 4 cm. The lungs were the site of metastases in our patient. In fact, the most common metastases sites are lungs (30% to 80%), liver (40% to 90%), lymph nodes (7% to 20%), and bones (5% to 20%) [14].
Although the diagnosis of ACC is based on combination of clinical, biological and imaging findings, the diagnosis of certainty is histological. However, the ENSAT recommends against the use of an adrenal biopsy in the diagnostic work-up of patients with suspected ACC unless there is evidence of metastatic disease that precludes surgery and histopathologic proof is required to inform oncological management. The latter was the case of our patients who presented with lung metastases at diagnosis. The diagnosis can be made on the basis of hematoxylin and eosin-stained specimens, but some markers such as SF1, inhibin-alpha, melanA, and calretinin can help in establishing the diagnosis, and the last 2 markers were available on the immunohistochemistry staining in our case. Despite only having the necrosis in our specimen without having the remaining criteria, the diagnosis of ACC is kept because of the association of clinical presentation, the biological tests and the radiologic findings, especially the presence of pulmonary metastases
The prognosis of ACC is however poor and it depends on several factors including the tumor stage, resection status, Ki67 index (or mitotic count), autonomous cortisol secretion and the patient’s general condition. The median overall survival of all ACC patients is about 3 years to 4 years. Among the prognostic factors cited earlier, the tumor stage is the most important reported factor. Even for early stages, the cure rate is estimated to be about only 30% with a 5 year survival of 60% to 80% for tumors confined to the adrenal space and 35% to 50% for locally advanced disease. This highlights the strong pejorative value of metastatic disease, with a median survival of less than one year, and a five-year survival ranging from 0% to 28%. Particularly, for the metastatic stage, high tumor burden, high tumor grade, high Ki67 index, and uncontrolled symptoms are major factors associated with worse prognosis [12]. Solak et al. demonstrated in a recent study that a higher Neutrophil-Lymphocyte Ratio (NLR) level in ACC patients is a single, inexpensive and readily available prognostic marker as it was associated with a more advanced stage of the disease and a higher Ki-67 index. Therefore, a NLR >3.9 is associated with worse DiseaseSpecific Survival (DSS) and Overall Survival (OS) compared to a NLR ≤3.9. The calculated NLR in our case is equal to 5.7, corresponding to a worse prognosis according to the above-mentioned study, without forgetting the advanced stage at diagnosis, the bulkiness of the disease and the presence of hypercortisolism.
The management of ACC is multidisciplinary requiring the cooperation of the oncologist along with the pathologist, the radiologist, the radiotherapist and the surgeon. The mainstay of the treatment of early stage ACC is complete resection (R0) with adjuvant therapy with mitotane and irradiation because of risk of recurrence. Although surgery is the treatment recommended for localized ACC, the resection of the primary tumor in stage IV disease remains controversial and requires individualized decision. Several parameters must be taken into consideration including the tumor volume, the number of metastatic organs, and the progression rates. Some authors concluded that radical adrenalectomy combined with systemic chemotherapy may improve survival outcomes in carefully selected patients with synchronous metastatic ACC but without liver metastases. As per NCCN guidelines, resection may be considered if greater than 90% of the tumor and metastases can be removed . In other words, debulking surgery only benefits in patients with a limited number of involved organs (≤ 2), resectable tumoral mass (which make it at least technically feasible), with a light progression and when severe hormone excess is not manageable by medical treatment.
Once the surgical management is no longer possible, a medical treatment is the remaining choice. The choices of systemic therapy for metastatic disease include mitotane as monotherapy, or combination of cisplatin or carboplatin with etoposide with or without doxorubicin and/or mitotane, or streptozocin with or without mitotane, or pembrolizumab with or without mitotane [11].
Mitotane is considered to be the only adrenolytic drug approved by the FDA for treating ACC, and it is recommended to start mitotane as soon as clinically possible. Partial responses with mitotane are reported to be 10% to 30% at its best. The response to therapy was shown to be greater when mitotane is combined to other cytotoxic agents such as Etoposide, Doxorubicin and Cisplatin (EDP) or streptozocin reporting an overall response rate of 36 to 49% with a better advantage in rates of response and Progression-Free Survival (PFS) with EDP plus mitotane (EDP-M) as confirmed in the FIRMACT trial. Considering the high tumor burden of our patient and the presence of metastases, a more aggressive treatment than mitotane monotherapy was decided. Given the presence of a bilateral renal vein thrombus and an area of renal parenchymal ischemia, and in order to preserve the renal function, carboplatin is chosen instead of cisplatin. Nevertheless, because of the patient concerns about hair loss subsequent to therapy, and the patient received a cycle of carboplatin alone with concomitant treatment by mitotane.
Mitotane has a very long half-life with high toxicity profile and a narrowed therapeutic window (between 8 mg/mL and 25 mg/mL). Therefore, the dose of mitotane needs to be monitored every 3 to 4 weeks by measurements of serum levels of the drug with the aim of reaching a mitotane blood level above 14 mg/L. Due to the adrenolytic activity of mitotane, glucocorticoids need to be administered to avoid acute adrenal insufficiency. Common reported side effects of mitotane are fatigue, diarrhea, nausea, and vomiting and epigastric discomfort. The gastrointestinal side effects are mainly addressed by a prophylactic treatment with metoclopramide. Besides, mitotane may have many metabolic, hematologic and endocrine effects whereas it can affect the lipid profile and the thyroid function, it can cause hepatotoxicity and blood disorders such as leucopenia, anemia, thrombocytopenia and prolonged bleeding time, as well as it may have central nervous system toxicity manifesting by ataxia, vertigo, somnolence, etc. This justifies the need for routine clinical visits and blood tests checking for these possible disorders.
On the other hand, because of the risk of immunosuppression due to chemotherapy and corticosteroid supplementation, a higher frequency of symptomatic SARS-CoV-2 infection in ACC patients was reported in Italy with a frequency reaching 10.9% compared to a frequency of 2.5% for other cancers [15]. Counseling about precaution measures for coronavirus infection was reinforced.
Conclusion
We reported a rare case of a huge adrenocortical carcinoma that is not manageable to surgery, presenting with signs of hormonal excess due to secretions by the tumors and with metastases at diagnosis. The diagnosis w as made a fter ta king into co nsideration cl inical, biological, radiological and pathological findings together. Th e therapeutic decision was based essentially on the tumor burden and the stage of the disease. The medical management relies mainly on mitotane in adjunction with chemotherapy. The potential toxicities of the treatment must be controlled by routine clinical and blood tests surveillance.
Acknowledgements
We would like to thank the patient for accepting to be the subject of this report.
Competing Interests
The authors declare that they have no competing interests.
Funding
The authors received no financial support for the authorship, and/ or publication of this article.
Consent for Publication
The patient has accepted to be the subject of this case report and has fully accepted the photos to be taken and included in the manuscript.
References
- Rodgers SE, Evans DB, Lee JE, Perrier ND (2006) Adrenocortical carcinoma. Surg Oncol Clin 15: 535-553. [Google Scholar] [Cross Ref]
- Allolio B, Fassnacht M (2006) Adrenocortical carcinoma: Clinical update. J Clin Endocrin Metabol 91: 2027-2037. [Google Scholar] [Cross Ref]
- Walz MK, Metz KA, Theurer S, Myland C, Alesina PF, et al. (2020) Differentiating benign from malignant adrenocortical tumors by a single morphological parameter-a clinicopathological study on 837 adrenocortical neoplasias. Indian J Surg Oncol 11: 705-710. [Google Scholar] [Cross Ref]
- Cawood TJ, Hunt PJ, O'shea D, Cole D, Soule S. (2009) Recommended evaluation of adrenal incidentalomas is costly, has high false-positive rates and confers a risk of fatal cancer that is similar to the risk of the adrenal lesion becoming malignant; time for a rethink? Europ J Endocrinol 161: 513-527. [Google Scholar] [Cross Ref]
- Young Jr WF (2000) Management approaches to adrenal incidentalomas: A view from Rochester, Minnesota. Endocrinol Metabol Clin North Am 29: 159-185. [Google Scholar] [Cross Ref]
- Moraes RFS, Lando E, Pastorello J, Cechetti CP, do Amaral CDS., et al. (2021) Tumor adrenocortical metastatico, uma apresentacao atipica: Relato de caso. Brazil J Case Rep 1: 98-104. [Google Scholar] [Cross Ref]
- Bilimoria KY, Shen WT, Elaraj D, Bentrem DJ, Winchester DJ, et al. (2008) Adrenocortical carcinoma in the United States: Treatment utilization and prognostic factors. Cancer: Interdis Internat J Am Can Soc 113: 3130-3136. [Google Scholar] [Cross Ref]
- Bharwani N, Rockall AG, Sahdev A, Gueorguiev M, Drake W, et al. (2011) Adrenocortical carcinoma: The range of appearances on CT and MRI. Am J Roentgenol 196: 706-714. [Google Scholar] [Cross Ref]
- Claimon A, Tantranont N, Claimon T (2020) 18F-fluoro-2-deoxy-D-glucose positron emission tomography/computed tomography for preoperative planning in a rare case of hyperfunctional bilateral adrenocortical carcinoma and review of literatures. World J Nuclear Med 19: 301-305. [Google Scholar] [Cross Ref]
- Amodru V, Garcia ME, Libe R, Brue T, Reznik Y, et al. (2021) Medical management of adrenocortical carcinoma: Current recommendations, new therapeutic options and future perspectives. In Ann D'endocrinologie 82: 52-58. [Google Scholar] [Cross Ref]
- Fassnacht M, Dekkers OM, Else T, Baudin E, Berruti A, et al. (2018) European society of endocrinology clinical practice guidelines on the management of adrenocortical carcinoma in adults, in collaboration with the european network for the study of adrenal tumors. Europ J Endocrinol 179: G1-G46. [Google Scholar] [Cross Ref]
- Paragliola RM, Corsello A, Locantore P, Papi G, Pontecorvi A, et al. (2020) Medical approaches in adrenocortical carcinoma. Biomedicines 8: 551. [Google Scholar] [Cross Ref]
- Ribeiro RC, Sandrini Neto RS, Schell MJ, Lacerda LSGA, Sambaio GA, et al. (1990) Adrenocortical carcinoma in children: A study of 40 cases. J Clin Oncol 8: 67-74. [Google Scholar] [Cross Ref]
- Martins-Filho SN, Almeida MQ, Soares I, Wakamatsu A, Alves VAF, et al. (2021) Clinical impact of pathological features including the Ki-67 labeling index on diagnosis and prognosis of adult and pediatric adrenocortical tumors. Endocrine Pathology, 32: 288-300. [Google Scholar] [Cross Ref]
- Sidhu S, Sywak M, Robinson B, Delbridge L (2004) Adrenocortical cancer: Recent clinical and molecular advances. Curr Opin Oncol 16: 13-18. [Google Scholar] [Cross Ref]
 Spanish
Spanish  Chinese
Chinese  Russian
Russian  German
German  French
French  Japanese
Japanese  Portuguese
Portuguese  Hindi
Hindi