Case Report, Clin Oncol Case Rep Vol: 4 Issue: 1
A Case Report of Acute Myeloid Leukemia in a Patient Treated with Pembrolizumab
John Chazhoor1, Jodie A Barr2 and Gary C Doolittle3*
1University of Kansas School of Medicine, Kansas City, KS, USA
2Lawrence Memorial Hospital Oncology and Hematology Center, Lawrence, KS, USA
3Division of Clinical Oncology, Department of Internal Medicine, University of Kansas Cancer Center, Kansas City, KS, USA
*Corresponding Author:
Gary C Doolittle
Division of Clinical Oncology
Department of Internal Medicine
University of Kansas Cancer Center
Kansas City, 2330 Shawnee Mission Parkway
KS 66205, KS, USA
E-mail: gdoolitt@kumc.edu
Received: September 02, 2020 Accepted: December 15, 2020 Published: January 15, 2021
Citation: Chazhoor J, Barr JA, Doolittle GC (2021) A Case Report of Acute Myeloid Leukemia in a Patient Treated with Pembrolizumab. Clin Oncol Case Rep 4:1
Abstract
We report the case of a 68-year old male who presented with worsening epigastric pain and complaints of early satiety. CT abdomen indicated widespread hepatic metastatic disease. The primary malignancy was diagnosed as unresectable cholangiocarcinoma. The patient underwent various conventional chemotherapy and localized radiotherapy, all of which proved ineffective in reducing disease burden. Checkpoint inhibitor pembrolizumab (Keytruda®) was eventually administered. While treatment was effective in decreasing disease burden, fourteen months into treatment with the anti-PD-1 therapy, signs of bone marrow insufficiency became apparent. Bone marrow biopsy revealed leukemic blast cells leading to a suspicion of therapy-related Acute Myeloid Leukemia (AML). We suspect pembrolizumab resulted in the development of leukemia, recognizing this patient received various chemotherapy agents, but only one dose of a drug irinotecan that is known to be associated with therapy-induced AML, In this case report, we suspect AML developed as an adverse event related to immunotherapy.
Keywords: Epigastric; CT; Metastatic disease; Inhibitor; Acute myeloid leukemia
Keywords
Epigastric; CT; Metastatic disease; Inhibitor; Acute myeloid leukemia
Introduction
We present the case of a 68-year old male who presented with worsening epigastric pain and complaints of early satiety. A Computed Tomography (CT) scan of the abdomen performed in November 2016, showed widespread hepatic metastatic disease with a dominant mass in the left hepatic lobe measuring 18.3 cm × 10.6 cm. Tumor markers, CEA and CA19-9, were both elevated. Based on tumor markers, pathology, and diagnostic imaging, a diagnosis of cholangiocarcinoma was made. Chemotherapy was initiated in December, 2016, with completion of 3 cycles of gemcitabine 1000 mg/m2 and cisplatin 25 mg/m2, based on the ABC-02 trial [1]. CT scans performed in February 2017 indicated disease progression. The patient then proceeded with the 3 cycles of FOLFOX (folinic acid, fluorouracil, and oxaliplatin), with further progression of disease observed. Next, the patient underwent local radiotherapy with Y-90 with no appreciable effect on disease burden. Following Y-90 treatment, the patient completed one cycle of FOLFIRI (Folinic Acid, Fluorouracil, and Irinotecan Hydrochloride) but further treatment was withheld due to intolerance. Foundation one testing showed mutations in TP53, BRCA1, BRCA2, and loss of MLH1. Loss of MLH1 impairs DNA mismatch repair, resulting in subsequent microsatellite instability leading to the accumulation of mutational load of oncogenes and increased expression of tumor neoantigens [2]. Given these findings, checkpoint inhibitor pembrolizumab (Keytruda®) 200 mg, was initiated and administered intravenously every 3 weeks. CT scan performed in December 2017, indicated partial disease response to the checkpoint inhibitor treatment, with significant disease regression. Pembrolizumab was continued and by December 2018, the dominant left hepatic lobe mass measured 8.6 cm × 4.6 cm.
Unfortunately, fourteen months into treatment with pembrolizumab, signs of bone marrow insufficiency became evident. The patient presented with worsening dysgranulopoiesis, leukopenia, thrombocytopenia, and neutropenia, as shown in Table 1. A bone marrow biopsy collected in February 2019, indicated grade 2 hypocellular marrow for age (20% cellularity) with left-shifted myeloid precursors, decreased granulopoiesis, decreased megakaryocytes, and 35% atypical monocytes cells of uncertain lineage (increased monocytes). A peripheral blood smear confirmed absolute leukopenia (1,600/μL), absolute neutropenia (720/μL) with, dysgranulopoiesis, absolute lymphopenia (800/μL), decreased absolute eosinophils (48/μL), absolute monocytopenia (16/μL) and thrombocytopenia (133,000/μL). Flow cytometry was diagnostically negative for a neoplastic disorder. The patient’s chromosome analysis was negative for FLT3 mutation and positive for chromosome 8 polysomy. Further, flow cytometric analysis of the bone marrow specimen indicated T-cells with a decreased CD4+/CD8+ ratio of 1:1.2, indicating insufficient immunologic capacity necessary for an immune surveillance and response to malignancy [3]. A repeat peripheral smear showed progressive bone marrow failure with pancytopenia [leukopenia (1,570/μ), thrombocytopenia (137,000/μ), and anemia]. Bone marrow aspirate indicated hypocellular marrow with increased myeloid blasts (57% by immunophenotyping) as shown in Figure 1. This blast population expressed CD4, CD11c, bright CD33 (79.97%), dim CD45, bright CD64 (75.83%), dim CD71, partial CD117, dim CD123, and bright HLA-DR (63.80%). Following additional testing, a diagnosis of Therapy-Related Acute Myeloid Leukemia (t-AML) was reached (Figure 1). Pembrolizumab was discontinued on February 2019, due to the development of bicytopenia. Treatment with azacitidine 75 mg/m2 was initiated in March 2019, in response to the development of t-AML. Upon follow up, patient reported significant nausea, coupled with the loss of appetite, and an inability to eat and drink. Patient eventually elected to forgo azacitidine treatment after 1 cycle (3/25/19 to 6/2/19) and proceeded with hospice care.
Table 1: Blood count results over the course of immunotherapy pembrolizumab (Keytruda).
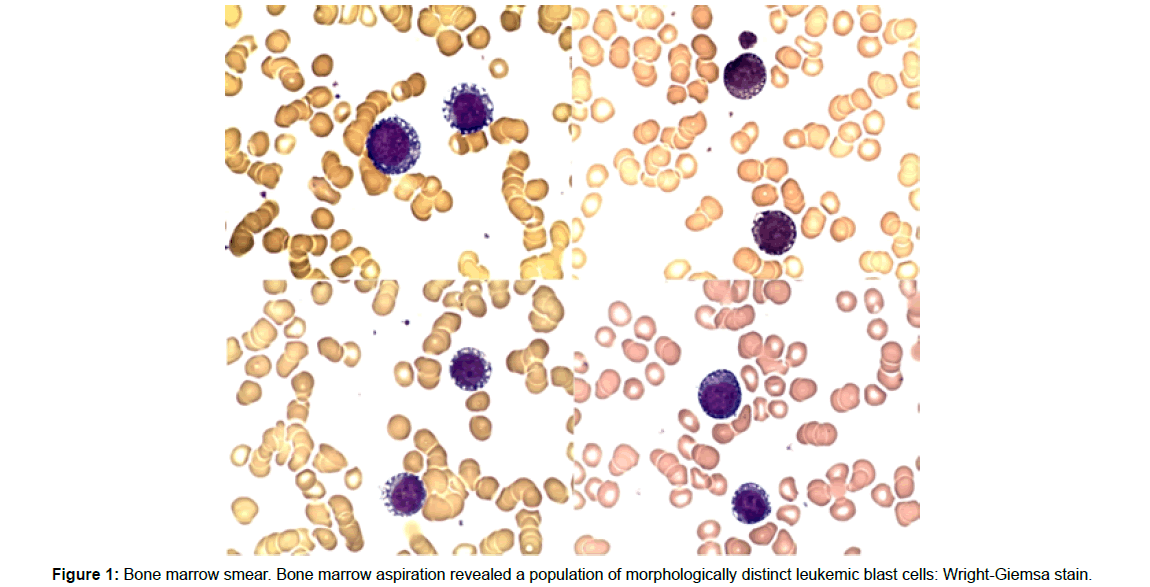
Figure 1: Bone marrow smear. Bone marrow aspiration revealed a population of morphologically distinct leukemic blast cells: Wright-Giemsa stain.
Immunotherapy with checkpoint inhibitors such as anti-CTLA-4 and anti-PD-1 monoclonal antibodies provides an exciting and promising frontier for oncology. Many malignancies previously thought to be unresponsive to treatment now show appreciable response when administered with pembrolizumab (Keytruda®) and other immunotherapeutics [4]. Furthermore, an ever-growing number of oncologic patients may require such therapy for prolonged periods of time. This enthusiasm for immunotherapy must be balanced with an element of caution, as there may be secondary adverse effects yet to be fully identified. With this report, we demonstrate the importance of remaining vigilant in the care of patients and staying informed of the growing list of possible adverse effects one may encounter as the indications for immunotherapy grows. The mechanism of immunotherapy rests on its ability to upregulate the immune response, specifically T-cells, to recognize and clear malignancies that would have otherwise evaded the host response. Current generation immunotherapies target two receptors on T-cells named Cytotoxic T-Lymphocyte-Associated Antigen 4 (CTLA-4) and Programmed Cell Death-1 (PD-1) receptors, pathways exploited by malignant cells to downregulate the host’s immune response to cancer.
As tumor cells evolve via immunoediting and clonal selection via therapy-related selective pressure, cancer cells expressing PD-L1 ligands are able to avoid such immune recognition and the subsequent activation of cytotoxic T-cells leading to their unfettered proliferation. This downregulation of the immune system manifests via the coupling of the PD-L1 ligand on cancerous cells to the PD-1 receptor on activated T-cells. This coupling forces activated T-cells into anergy, allowing cancer cells to proliferate, undeterred by any meaningful activation of the immune system. This forced anergy represents the exploitation of a regulatory mechanism normally utilized by the body as a means to modulate and avoid both immune system exhaustion and autoimmunity related bodily damage. It is now postulated that in periods of chronic inflammation, T cells upregulate a variety of inhibitory receptors as a means to reduce their own effectiveness or potency and avoid deleterious effects. This immune exhaustion is normally seen in cases in which the patient is no longer able to clear a pathogen. However, this has also been displayed and associated with the chronic inflammation seen in malignant states [5,6]. Once this T-cell regulatory mechanism is targeted and inhibited by immunotherapy, a hyperreactive response by T-cells is now possible. With immunotherapy, we are able to upregulate T-cell function, but upon activation we are unable to control the selectivity of which cell lines T-cells wage their attack. This hyperreactive immune response constitutes the bulk of adverse events linked to checkpoint inhibitor therapy.
The majority of adverse effects related to immunotherapy are fatigue, dermatologic toxicity, gastrointestinal toxicity, hepatotoxicity, pulmonary toxicity, and various endocrinopathies [7,4]. Upon an exhaustive review of literature, we are now aware of a subset of hematologic toxicities related to immunotherapy [8]. These hematologic effects alter various cell lineages, causing various forms of bone marrow insufficiency [9]. To our knowledge, this is the first case reporting on suspected checkpoint inhibitor pembrolizumab involvement in therapy-induced AML. In our case, the patient developed t-AML approximately 17 months after initiation with pembrolizumab. This induction time is on par with times related to t-AML arising from treatment with conventional chemotherapy agents such as alkylating agents and epipodophyllotoxin agents [10]. However, as this patient only received a single cycle of irinotecan (as part of a regimen that also included 5FU and oxaliplatin, FOLFIRI), we believe it is reasonable to postulate a link between immunotherapy and the subsequent development of AML.
Discussion
Currently, there is not a clear understanding of the pathogenesis of immunotherapy-related acute myeloid leukemia. However, research shows t-AML and MDS following conventional chemotherapy is correlated with having a high prevalence of TP53 mutations thought to be caused by the mutagenic effects of therapy. It is believed that loss of function mutations in the tumor suppressor gene TP53 of these clonal populations leads to chemoresistance and the selective pressure of chemotherapy-induced DNA damage acting on pre-existing preleukemic cells, which allows for transformation to leukemic cells. Thus, chemotherapy contributes to the formation of myeloid tumors by promoting the expansion of pre-existing preleukemic clones under the selective pressure of genotoxic stress. However, the role checkpoint inhibitors and the subsequent hyperactivation of cytotoxic T cells occupy in this paradigm, is yet to be understood.
A comprehensive search of the literature has yielded a fair number of other reports of bone marrow insufficiency post-treatment with checkpoint inhibitors (Table 2) [11-13]. Modern immunotherapy relies on the upregulation of activated T-cells and the subsequent antitumor effect carried out by the patient’s own immune system. In the process of stimulating this activated T-cell population, immunotherapy can cause hyperactivation leading to an autoimmune barrage on host cells, including those of bone marrow cell lineages. Hematologic complications due to immunotherapy have been reported, as listed in the table. Michot JM et al. reports three patients who presented with bone marrow failure and immune-related aplastic anemia following anti-PD-1 therapy with nivolumab [9], Le Roy A et al. presents two cases with thrombocytopenia and anemia, in which both ipilimumab and pembrolizumab were administered prior to bone marrow changes developing [14], Kanameishi S et al. presents one case of Grade IV thrombocytopenia [15] Kong BY et al. reports the development of autoimmune hemolytic anemia following approximately 5 cycles of nivolumab for metastatic melanoma [16]. One phase 2 study with 41 patients with progressive metastatic melanoma with or without mismatch repair deficiency [17]. Approximately 20% (n=8) reported Grade 3-4 lymphopenia and 17% (n=7) Grade 3-4 anemia [6] and Atwal D et al, reports a metastatic melanoma patient who developed severe pancytopenia following 18 cycles of pembrolizumab treatment [18].
Table 2: Immunotherapy induced bone marrow insufficiency.
Conclusion
In this case, we reported upon a patient who developed t-AML following immunotherapy, pembrolizumab, for the treatment of metastatic cholangiocarcinoma. To our knowledge, this is the first case of acute myeloid leukemia following anti-PD-1 treatment. Further research is necessary to elucidate the mechanism for this process; nonetheless, we believe it is crucial that physicians are aware of the possible hematologic adverse effects arising from the use of pembrolizumab and other potential immunotherapies.
References
- Valle J, Wasan H, Palmer DH, Cunningham D, Anthoney A, et al. (2010) Cisplatin plus gemcitabine for biliary tract cancer. N Engl J Med 362: 1273-1281.
- Viale G, Trapani D, Curigliano G (2017) Mismatch repair deficiency as a predictive biomarker for immunotherapy efficacy. Biomed Res Int 2017: 4719194.
- Chen L, Linsley PS, Hellstrom KE (1993) Costimulation of T cells for tumor immunity. Immunol Today 14: 483-486.
- Villadolid J, Amin A (2015) Immune checkpoint inhibitors in clinical practice: Update on management of immune-related toxicities. Transl Lung Cancer Res 4: 560-575.
- Disis ML (2014) Mechanism of action of immunotherapy. Semin Oncol 5: S3-S13.
- Seidel JA, Otsuka A, Kabashima K (2018) Anti-PD-1 and anti-Ctla-4 therapies in cancer: Mechanisms of action, efficacy, and limitations. Front Oncol 8: 86.
- Naidoo J, Page DB, Li BT, Connell LC, Schindler K, et al. (2016) Toxicities of the anti-pd-1 and anti-pd-l1 immune checkpoint antibodies. Ann Oncol 27: 1362.
- Calvo R (2019) Hematological side effects of immune checkpoint inhibitors: The example of immune-related thrombocytopenia. Front Pharmacol 10: 454.
- Michot JM, Vargaftig J, Leduc C, Quere G, Burroni B, et al. (2017) Immune-related bone marrow failure following anti-PD1 therapy. Eur J Cancer 80: 1-4.
- Van Leeuwen FM (1996) Risk of acute myelogenous leukaemia and myelodysplasia following cancer treatment. Baillieres Clin Haematol 9: 57-85.
- Jan M, Majeti R (2013) Clonal evolution of acute leukemia genomes. Oncogene 32: 135-140.
- Walter MJ, Shen D, Ding L, Shao J, Koboldt DC, et al. (2012) Clonal architecture of secondary acute myeloid leukemia. N Engl J Med 366: 1090-1098.
- Wong TN, Ramsingh G, Young AL, Miller CA, Touma W, et al. (2015) Wilson, role of TP53 mutations in the origin and evolution of therapy-related acute myeloid leukaemia. Nature 518: 552-555.
- Le Roy E, Kempf F, Ackermann E, Routier C, Robert A, et al. (2016) Two cases of immune thrombocytopenia associated with pembrolizumab. Eur J Cancer 54: 172-174.
- Kanameishi S, Otsuka A, Nonomura Y, Fujisawa A, Endo Y, et al. (2016) Idiopathic thrombocytopenic purpura induced by nivolumab in a metastatic melanoma patient with elevated PD-1 expression on B cells. Ann Oncol 27: 546-547.
- Kong BY, Micklethwaite KP, Swaminathan S, Kefford RF, Carlino MF (2016) Autoimmune hemolytic anemia induced by anti-PD-1 therapy in metastatic melanoma. Melanoma Res 26: 202-204.
- Le DT, Uram JN, Wang H, Bartlett BR, Kemberling H, et al. (2015) PD-1 blockade in tumors with mismatch-repair deficiency. N Engl J Med 372: 2509-2520.
- Atwal D, Joshi KP, Ravilla R, Mahmoud F (2017) Pembrolizumab-induced pancytopenia: A case report. Perm J 21: 17-004.
 Spanish
Spanish  Chinese
Chinese  Russian
Russian  German
German  French
French  Japanese
Japanese  Portuguese
Portuguese  Hindi
Hindi 