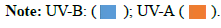Research Article, J Fashion Technol Textile Vol: 11 Issue: 1
A Comparative Study to Clarify the Effect of Different Nanoparticles on Improving the Functional Properties of Blended Cotton Fabrics
Hadeer l.A. Abdelkader, Manal E. E. Ahmad and Eman M. I. Elgendy*
Department of Home Economy, Mansoura University, Mansoura, Egypt
*Corresponding Author: Eman M. I. Elgendy
Department of Home Economy,
Mansoura University,
Mansoura,
Egypt
E-mail: eman.elgendy11@gmail.com
Received: 07-Dec-2022, Manuscript No. JFTTE-22-78308; Editor assigned: 09-Dec-2022, PreQC No. JFTTE-22-78308 (PQ); Reviewed: 23-Dec-2022, QC No JFTTE-22-78308; Revised: 30-Dec-2022, Manuscript No. JFTTE-22-78308 (R); Published: 09-Jan-2023 DOI: 10. 4172/2329-9568.1000280.
Citation: Hadeer lAA, Manal EEA, Eman MIE (2023) A Comparative Study to Clarify the Effect of Different Nanoparticles on Improving the Functional Properties of Blended Cotton Fabrics. J Fashion Technol Textile 11:1.
Abstract
Nanoparticles of metal and metal oxide received considerable attention as functional enhancers in the textile industry to provide unique multifunctional properties. The objective of this study is to compare the use of silver, zinc oxide, and titanium dioxide as coating agents for blended fabrics to improve the functional properties of these tissues. The silver and zinc oxide nanoparticles may be prepared with silver nitrate and zinc acetate, respectively. The scanning and transmitting electron microscopes were used to verify the size and form of the prepared nanoparticles. A comparison was made to study the effect of coating blended fabrics that have a 1/1 uniform weave composition, namely: Blended cotton (33% cotton, 67% polyester). This study showed good results in terms of high inhibition coefficient against harmful UV rays. The prepared and ready-to-use nanomaterials were used to test the results, which were carried out by standard textile test specifications. These tests involve tensile strength and elongation during cutting, air permeability test, UV resistance test, gram-positive and gram-negative bacterial resistance test, stain and dirt resistance test, and angle of contact measurement. This work concluded that the nanoparticles used have shown great potential to be used as a coating for medical and athletic tissues.
Keywords: Keywords: Metal oxide; Nanoparticles; Blended cotton; Ultraviolet; Electron microscope
Introduction
The textile industry has recently expanded and its functional properties have improved. These properties, in terms of protection against ultraviolet rays, as well as anti-bacterial, stain-resistant, waterproof, and anti-static force. Therefore, researchers were interested in developing textiles and giving them high functional properties [1].
Due to the importance of cotton fabrics, there are demands for them to acquire functional properties, making this one of the challenges of the last decades. The most important features include health and physiology. These properties are sought using modern weaving techniques, including spinning, weaving, fiber forming, and finishing processes [2].
Nanotechnology has real market potential for texture manufacturing. This is basically due to the reality that traditional methods used to provide different features to fabrics often do not lead to lasting effects. They will lose their functionality after washing or wearing. On the contrary, due to the large surface area of nanoparticles, so that nanotechnology can supply better affinity and high durability to fabrics. In addition, the coating of nanoparticles on the fabrics will not affect their ability to breathe or feel on their hands. As a consequence, interest in the use of nanotechnology in the textile industry is growing [3]. There are many conventional organic, inorganic, and inorganic hybrids used in the application processes [4]. Silver has long been used as an antimicrobial agent in wound recovery, both in a solid state and with saline solutions for cleaning wounds. Saturated AgNO3 dressings are available at this time [5]. Silver displays very delightful features due to catalytic activity, chemical settlement, perfect conductivity, and antibacterial action. In addition, silver nanoparticles (silver nanoparticles, AgNPs) are one of the farthest great studied particles at present [6]. There are many classic organic, hybrid organic, inorganic compounds, and inorganic compounds used in application processes [7]. Silver has long been used as an antimicrobial agent in wound recovery, both in its solid state and with saline solutions for cleaning wounds. At present, dressings saturated with AgNO3 can be found [8]. Among the inorganic compounds, Titanium dioxide (TiO2) has already been based as a chemical factor for textile recruitment due to its unparalleled natural and chemical features, environmental familiarities, biocompatibilities, and lower values [9]. Generally, zinc oxide can be used in several fields, such as zinc salts, through the internal preparation of nanoparticles in the existence of a textile substrate, or used as a previously prepared suspension solution [10,11]. ZnO particle has been utilized in the fabric to produce thermal stability, as well as produce heat insulation, control the proportion of moisture, hydrophobicity, and the degree of electrical connection [12-15]. TiO2 is considered one of the metal oxides that are used to develop the textile industry due to its many features. As it can block ultraviolet rays, its high chemical stability, the ability to resist bacteria and microbes, and has photocatalytic properties [16,17]. This paper focused on the high potentialities of the application of nano-inorganic elements or their oxides in the textile industry, especially for producing high-performance textiles. Few studies have compared the use of silver nanoparticles, zinc oxide, and titanium dioxide to produce high-performance fabrics. These fabrics can resist Gram-positive and Gram-negative bacteria, in addition to blocking ultraviolet rays and their ability to resist dirt and stains. This is done without the fabric losing its ability to absorb water and air permeability, as well as without affecting the tensile and elongation forces.
Materials and Methods
Escherichia coli strain F220 was obtained from the laboratory of the Genetic Engineering Unit at the Faculty of Science, Mansoura University. The chemicals such as ascorbic acid, silver nitrate, ammonia, acetic acid, and Sodium Dodecyl Sulfate (SDS) were bought from Sigma firm.
Preparation of nanoparticles Silver and Zinc oxide on blend fabric
Preparation of silver nanoparticles: It was prepared using an aqueous solution of AgNO3 according to the method of Sondi I and Salopek-Sondi after modification [18]. 0.01 mol of silver nitrate was added to ammonium hydroxide dropwise until the pH of the solution becomes pH 12.5% of SDS (Sodium Dodecyl Sulfate) was prepared. SDS solution was added to the silver nitrate solution dropwise for half an hour with constant stirring at 70°C. 0.01 mol of ascorbic acid was prepared, then this solution was added for half an hour with continuous stirring at 70°C. Blend fabric (33% cotton + 67% polyester (was cut 4 cm by 4 cm and weighed about 0.29 gm. The blended fabric was added to the solution at the same temperature with stirring for an hour. The fabric was removed from the solution and dried at room temperature as sample 1.
Preparation of Zinc oxide nanoparticles: It was synthesized based on the method of d’ A´gua, et al. after some modifications [19]. A solution of 0.08 mol. of zinc acetate was prepared. Blend fabric (33% cotton + 67% polyester (was cut 4 cm by 4 cm and weighed about 0.29 gm. It was placed in the zinc acetate solution while stirring. A solution of 4% of sodium hydroxide has been prepared, then the temperature is raised to 50°C, and it is added drop by drop for around 15 min, stirring continually for an hour. Allow the solution to cool at room temperature and then remove the fabric, rinse and leave to dry at room temperature as sample 2.
Processing Titanium dioxide nanoparticles: A solution of 0.08 mol. of titanium dioxide nanoparticles was prepared by dissolving 2 gm of nano titanium dioxide (degosa) in 250 ml of distilled water. It was stirred for half an hour at 50°C. Blend fabric was placed in the solution while stirring. Allow the solution to cool at room temperature, then remove the fabric, and rinse. The coated fabric was placed in the drying oven at a temperature of 100°C, to dry and stabilize the particles as sample 3.
Characterization of nanosilver: The size and morphology of the solution of nanoparticles were observed by transmission electron microscope (TEM, Hitachi, H-800). Samples for TEM imaging were prepared on 100-mesh Formvar-coated copper grids (Electron Microscopy Sciences). The other technique SEM is in which the coated fabrics are photographed on the surface of the tissue. The sample size was carried out from transmission electron micrographs using the software Image Tool for Windows (Version 2.0). Data were analyzed using software Microcal Origin 7.0.
Measurement methods
Physical and mechanical measurements on the raw cloth to identify the properties of the cloth using a different set of devices, at the National Institute of Measurement and Calibration in Giza. Microscopically tests using a Transmission Electron Microscope (TEM) for the prepared nano-solutions and Scanning Electron Microscope (SEM) for nano-processed cloth pieces in the Electron Microscope Unit at the Faculty of Agriculture, Mansoura University. Microbiological tests using gram-positive (Staphylococcus aureus) and gram-negative (Escherichia coli) bacteria in the Genetic Engineering Unit at the Faculty of Science, Mansoura University. Physical and mechanical measurements on the treated fabric to study the effect of the treatment materials on the properties of the fabrics used in the study. The test of resistance to ultraviolet radiation penetration has been measured at the photometric unit at the National Institute for Measurement and Calibration in Giza.
The natural and mechanical measurements were as follows: ASTM D629-15 standard test methods for quantitative analysis of textiles to identify the type of material used and the mixing ratio in it. This test was carried out according to the specification.
The number of warp and weft threads test: The test measured the number of warp and weft threads for each of the materials used in the study before and after nano processing. This test was carried out according to the standard method of Egyptian standard specification No. 294: determination of the number of threads in the length unit of fabric.
Square meter weight test: The weight was measured according to the standard method ASTM D3776/D3776M-09a standard test methods for mass per unit area (weight) of fabric.
The thickness test: The test was carried out according to the specification ASTM D1777-standard test method for thickness of textile material.
Hardness test: The hardness was obtained according to the standard method ASTM D 1833-standard test method for: stiffness of fabrics 1. The equation for the stiffness modulus is G=W × c3, where G=flexural rigidity, mg cm, (bending stiffness), W=fabric mass per unit area, mg/cm2, (fabric mass per unit area) and c=bending length, cm (length bending).
Air permeability test: The air permeability can be measured according to the ASTM D 737-standard test method for air permeability of textile fabrics.
The tensile strength and elongation test: This test was carried out according to the method Textiles-tensile properties of fabrics ISO 13934. The tensile strength is measured in kg and the elongation at cut is measured in percentage (%).
The contact angle test: The test was carried out according to the standard method. ASTM D5725: Standard test method for surface wettability and absorbency of sheeted materials using an automated contact angle tester.
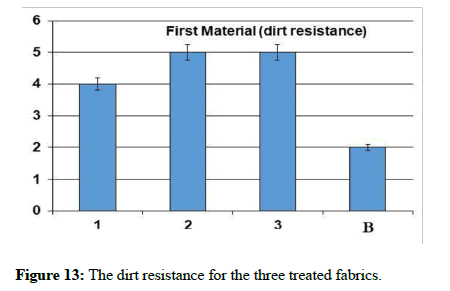
Dirt resistance test: The test was carried out according to the standard method AATCC Test method 130 soil release oily stain release method. The result was calculated using the indicator of the degree of resistance to the stain, as shown in Figure 1.
Figure 1: Shows the indicator of the degree of soiling.
Note: As each of these numbers has a specific significance, namely:
The number (1.0) means that the material is not resistant to stains. The
number (2.0) means that the material is resistant to stains acceptable.
If the stain appears as in the figure specified in (3.0) this means that
the material is resistant to stains well. Whereas, number (4.0) denotes
that the material is resistant to stains very well. Finally, the number
(5.0) means that the material has excellent stain resistance.
Antibacterial assays: Both gram-negative bacteria (Escherichia coli ATCC8739) and Gram-positive bacteria (Staphylococcus aureus ATCC6538) were examined against the blend fabrics. In broth containing glycerol (15% v/v), bacteria were preserved frozen at -70°C.
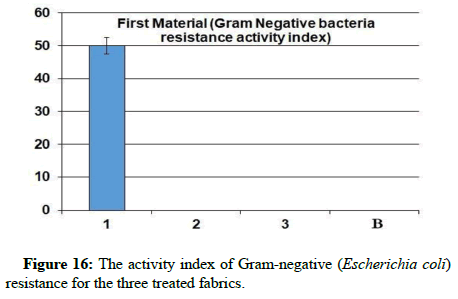
The agar diffusion method can be used to determine the antibacterial activity. Firstly, the blended fabrics were sheared with a laser into round disks with a diameter of 5 mm [20]. An autoclave can be used to sterilize the disks (at 120°C. for 15 min.). In Muller Hinton Agar (Allonne, France, MHA–Biokar Diagnostics), frozen microorganisms were vaccinated overnight at 37°C for aerobic bacteria. By measuring the diameter of the widening inhibition zone around the fabric disks, Antibacterial activity was measured. Negative control was measured through the untreated fabric. Each treatment was repeated three times. The standard antibiotic ampicillin was used to measure the antibacterial activity as a control, using the same method, the same concentration, and solvents [21]. By the formula as under, the percentage of activity index was calculated.
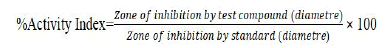
UV spectrophotometric method: During exposure to UV radiation, the UV penetration index can be measured through fabrics, by analyzing the absorption properties of fibers.
Estimation of the barrier features of woven fabrics during UV irradiation
As in the literature to determine barrier properties Sun Protection Factor (SPF) was explained which allows one to compare the preventive energy of various materials [22]. The Maximum Exposure Dose (MED) was known as the ratio of maximum exposure dose for unprotected skin exposure. It refers to how long the covered skin can be exposed to sunlight compared to the exposed skin. The data of literature declared a prolonged term of SPF, the so-called UPF index [23]. UPF means protection against UV radiation. Many publications reported different divisions of fabrics Exposure concerning barrier properties. For example, New Zealand and Australia suppose the following division of barrier properties of woven fabrics. PN-79/ T-06590 Protection against optical radiation instruments for measuring UV radiation:
UPF: <20 the fabric fails to meet protective properties
UPF: 21-30 protective properties at a low level
UPF: 32-40 medium protective properties
UPF: 41-50 good protective properties
UPF: >50 very good protective properties
UPF can be calculated from the formula: UPF=Σ Eλ. Sλ. Δλ/Σ Eλ. Sλ. Tλ. Δλ
Where:
Eλ: spectral intensity of the radiation (W/m2.nm),
Sλ: spectral relative biological efficiency,
Tλ: spectral permeability of the protective item-textile fabric,
Δλ: ranges of wavelength.
Measurements were carried out by the draft standard Draft EN-13758-1 Textiles–Protection against UV radiation part 1 test method for flat fabrics [24].
Results and Discussion
Preparing nanoparticles Silver and Zinc oxide nanoparticles
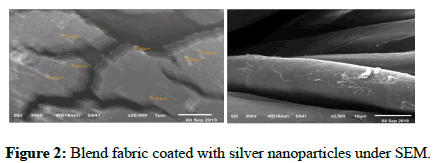
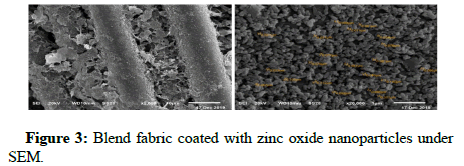
Silver nanoparticles with high-density coating were formed through pre-activation by ammonium hydroxide and in situ reductions of silver nitrate. Zinc oxide nanoparticles with high-density coating were formed through pre-activation by sodium hydroxide and in situ reductions of zinc acetate. Titanium dioxide nanoparticles in degosa crystalline phases were added on the surface of the blend fabric (33% cotton+67% polyester), which resulted in the formation of a low surface energy layer around the fabric. The fabrics were characterized by reflectance spectrophotometry, and scanning electron microscopy. Microscopy images showed the formation of uniform and high-density coating of silver, zinc oxide, and titanium dioxide on the surface of cotton fibers (Figures 2-4).
Measurement methods
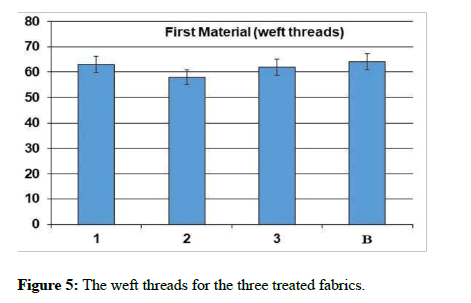
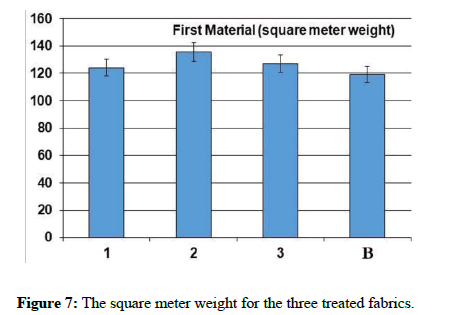
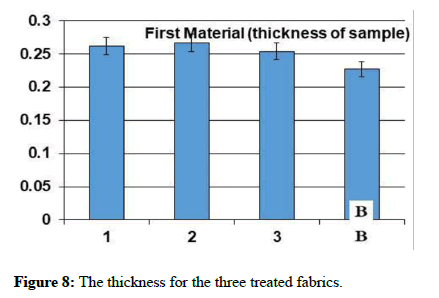
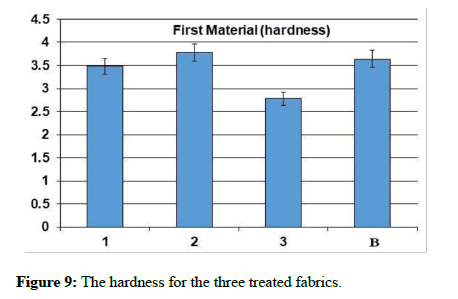
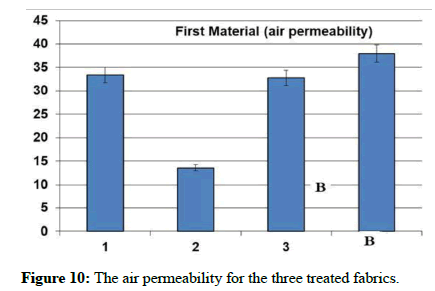
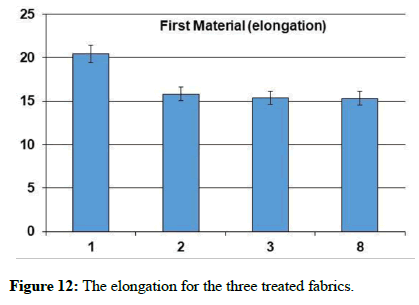
The natural and mechanical measurements: Physical and mechanical measurements can be measured. The wettability property was measured by the contact angle goniometry technique. The study showed that the fabric increased its water absorption. From the results of the analysis of the air permeability test, it is clear that the air permeability of the sample 1 and 3 was a little lower than the raw sample, while for sample 2, the air permeability was much less than the raw sample. As for the square meter weight test, it has been found that the treated samples have increased in weight than the raw sample. It is clear from the graph that the thickness of treated samples has increased to the raw sample. As for the hardness test, it has been found that the hardness of sample 2 has become higher than the raw sample. Whereas, sample 1 decreased by a small percentage than the raw sample, while sample 3 decreased by a greater percentage than the raw sample. In addition, It is clear from the previous graphs for the treated samples that the tensile strength of samples 1 and 2 increased than the raw sample, while sample 3 increased by a greater percentage. As for the test of the elongation ratio at the cut, it has been found that sample 1 has the highest elongation ratio than the raw sample, followed by sample 2, while sample 3 did not exceed a raw sample.
On the other hand, it is clear from the previous graph that the treated samples became more resistant to dirt than the raw sample, as it became excellent resistance to dirt. The two samples 2 and 3 treated with zinc oxide and titanium dioxide nanoparticles achieved a high resistance to dirt by 100%, followed by sample 1 treated with nanosilver. The nanoparticles achieved a higher resistance to soiling than silver nanoparticles for the blended cotton material. The ZnO and TiO2 electronic structures provide photocatalytic activity, giving them unique multifunctional properties. When they are excited by radiation of energy equal to or greater than the band gap (Eg), excited electrons move to the conduction band (e-CB), leaving behind holes in the valence band (h+VB). In the presence of water and oxygen, at least two photochemical reactions occur simultaneously. The first is oxidation, in which photo-induced positive holes are involved, and the second is reduction, in which photo-induced negative electrons are involved.
In these reactions, highly reactive oxygen species react, with oxygen and water, to form superoxide anion (•O−2) and hydroxyl (•OH) radicals. These species react with dirt and microorganisms degrading them to water and carbon dioxide [25-27]. The results were subjected in Table 1 and Figures 5-13.
| The sample | Tensile strength | Hardness | Air permeability | Square meter weight | Fabric thickness | warp thread count | Weft thread count | Resistant to stains and dirt | contact angle |
|---|---|---|---|---|---|---|---|---|---|
| Blank | 52.4 | 3.64 | 37.94 | 119.12 | 0.231 | 92 | 64 | 2 | zero |
| 1 | 55.1 | 3.48 | 33.36 | 124.2 | 0,276 | 76 | 63 | 4 | zero |
| 2 | 55.1 | 3.78 | 13.56 | 135.46 | 0.268 | 85 | 58 | 5 | zero |
| 3 | 63.2 | 2.78 | 32.78 | 126.99 | 0.261 | 78 | 62 | 5 | zero |
Table 1: The natural and mechanical measurements for the three treated fabrics.
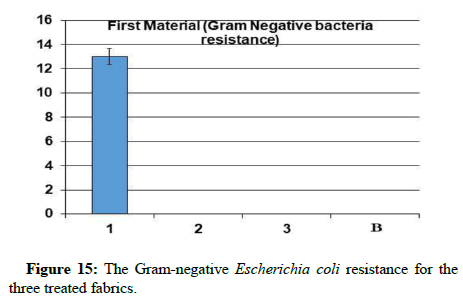
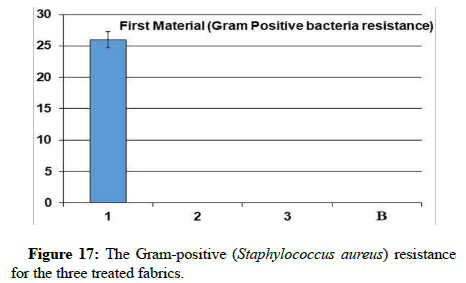
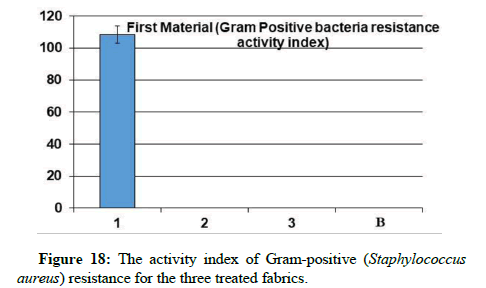
Antibacterial assays: Through the results, it is clear that sample 1 became resistant to Gram-positive and Gram-negative bacteria, but the percentage of resistance to positive bacteria is higher than the resistance of the sample to negative bacteria. While it has been found that samples 2 and 3 did not show resistance to gram-positive and gram-negative bacteria from this, it is clear that the use of nano-silver treatment with blend fabric is the most effective in resisting grampositive and gram-negative bacteria.
A promising method to obtain antibacterial fabrics was afforded; some hypotheses indicated that catalytic oxidation of silver ions, with nascent oxygen, reacts with bacterial cell membranes, leading to cell death. More recently, Li’s research showed that the silver bactericidal effect was caused by silver (I) chelation preventing DNA from unwinding [28]. Antibacterial activity was determined against grampositive Staphylococcus aureus and gram-negative Escherichia coli. The fabric demonstrated high antibacterial activity against two bacterial challenges, as shown via the formation of the inhibition zone in Table 2, (Figures 14-18).
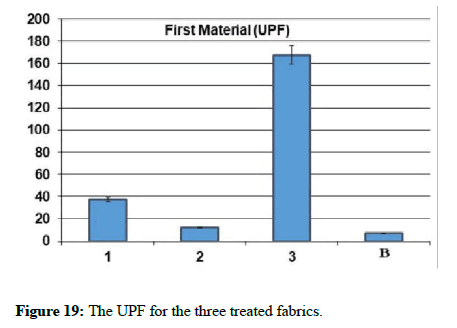
| The sample | Gram-positive Staphylococcus aureus | Gram-negative Escherichia coli | UV Resistance |
|---|---|---|---|
| Blank | - | - | 6.85 |
| 1 | 26 | 11 | 37.5 |
| 2 | - | - | 12.2 |
| 3 | - | - | 167.69 |
Table 2: The antibacterial assays and UV penetration index for the three treated fabrics.
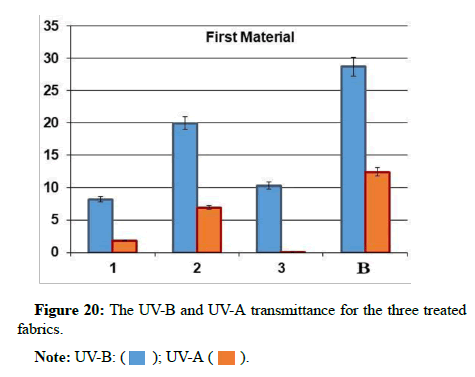
UV spectrophotometric method: The UV penetration index can be measured through fabrics. Ultraviolet blocking was measured via transmittance data in the range of 290-400 nm. UV-A Transmittance (mean transmittance percentage in the range 315-400 nm). UV-B Transmittance (mean transmittance percentage in the range 290-315 nm) shown in Table 2 and Figures 19-21. The results are as the followings:
The UPF of s-Blank=6.85
UV-B Transmittance (mean transmittance percentage in the range 290-315 nm)=12.39
UV-A Transmittance (mean transmittance percentage in the range 315-400 nm)=28.69
The UPF of s-1=37.50
UV-B Transmittance (mean transmittance percentage in the range 290-315 nm)=1.86
UV-A Transmittance (mean transmittance percentage in the range 315-400 nm)=8.22
The UPF of s-2=12.20
UV-B Transmittance (mean transmittance percentage in the range 290-315 nm)=6.93
UV-A Transmittance (mean transmittance percentage in the range 315-400 nm)=19.96
The UPF of s-3=167.69
UV-B Transmittance (mean transmittance percentage in the range 290-315 nm)=0.08
UV-A Transmittance (mean transmittance percentage in the range 315-400 nm)=10.30
From the results above, it has been found that sample 3 has very good ultraviolet protection of the fabric by ultraviolet protection factor value=167.69. UV-B Transmittance=0.08 this means that sample 3 has great blocking of ultraviolet rays, especially of the type UVB. Hence, it is clear that the proposed coverage provides a great blocking of ultraviolet rays, especially of the type UVB, which represents a dangerous source for human health and the body.
Conclusion
All treated fabrics have high resistance to dirt and stain removal. The treated fabric with nano titanium dioxide particles showed excellent resistance to dirt and stain removal. It also provides great blocking of ultraviolet rays, especially of the type UVB followed by the treated fabric with nano silver particles. The treated fabric with nano silver particles has high resistance against gram-positive Staphylococcus aureus and gram-negative Escherichia coli and has good resistance to dirt and stain removal. The treated fabric with nano zinc oxide particles also provides high resistance to dirt and stain removal higher than in the case of nano silver particles. On the other hand, the fabric retained its water absorption and air permeability, and the tensile and elongation forces were not affected.
References
- Gokarneshan N, Velumani K (2018) Significant trends in nano finishes for improvement of functional properties of fabrics. Handbook of Renewable Materials for Coloration and Finishing 16(1):387-434.
- Perelshtein I, Lipovsky A, Perkas N, Tzanov T, Gedanken A (2016) Sonochemical co-deposition of antibacterial nanoparticles and dyes on textiles. Beilstein J Nanotechnol 7(1):1-8.
- Verbič A, Gorjanc M, Simončič B (2019) Zinc oxide for functional textile coatings: Recent advances. Coatings 9(9):550.
- Alvarez J, Lipp-Symonowicz B (2000) Examination of the absorption properties of various fibres in relation to UV radiation. Autex Res J 3(2):72-77.
- Sánchez-López E, Gomes D, Esteruelas G, Bonilla L, Lopez-Machado AL et al (2020) Metal-based nanoparticles as antimicrobial agents: An overview. Nanomaterials 10(2):292.
[Crossref] [Google Scholar] [PubMed]
- Aderibigbe BA (2017). Metal-based nanoparticles for the treatment of infectious diseases. Molecules 22(8):1370.
[Crossref] [Google Scholar] [PubMed]
- Zhang XF, Zhi-Guo liu, Wei shen, Sangiliyandi Gurunathan (2016) Silver nanoparticles: Synthesis, characterization, properties, applications, and therapeutic approaches. Int J Mol Sci 17:1534.
[Crossref] [Google Scholar] [PubMed]
- Hackenberg S, Scherzed A, Technau A, Froelich K, Hagen R, et al. (2013) Functional responses of human adipose tissue-derived mesenchymal stem cells to metal oxide nanoparticles in vitro. J Biomed Nanotechnol 9(1):86-95.
[Crossref] [Google Scholar] [PubMed]
- Li H, Deng H, Zhao J (2009) Performance research of polyester fabric treated by nano titanium dioxide (nano-TiO2) anti-ultraviolet finishing. Int J Chem 1(1):57-62.
- Kołodziejczak-Radzimska A, Jesionowski T (2014) Zinc oxide−from synthesis to application: A review. Materials 9(7):2833-2881.
[Crossref] [Google Scholar] [PubMed]
- Parihar V, Raja M, Paulose R (2018) A brief review of structural, electrical and electrochemical properties of zinc oxide nanoparticles. Adv Mater 53(2):119-130.
- Cai L, Song AY, Li W, Hsu PC, Lin D, et al. (2018) Spectrally selective nanocomposite textile for outdoor personal cooling. Adv Mater. 30(35):1802152.
[Crossref] [Google Scholar] [PubMed]
- Karthik S, Siva P, Balu KS, Suriyaprabha R, Rajendran V, et al. (2017). Acalypha indica–mediated green synthesis of ZnO nanostructures under differential thermal treatment: Effect on textile coating, hydrophobicity, UV resistance, and antibacterial activity. Adv Powder Technol 28(12):3184-3194.
- Pal S, Mondal S, Maity J (2018) Synthesis, characterization and photocatalytic properties of ZnO nanoparticles and cotton fabric modified with ZnO nanoparticles via in-situ hydrothermal coating technique: Dual response. Mater Technol 33(14):884-891.
- Thi VH, Lee BK (2017) Development of multifunctional self-cleaning and UV blocking cotton fabric with modification of photoactive ZnO coating via microwave method. J Photochem Photobiol A Chem 338:13-22.
[Crossref] [Google Scholar] [PubMed]
- Kaseem M, Hamad K, Ur Rehman Z (2019) Review of recent advances in polylactic acid/TiO2 composites. Materials 12(22):3659.
[Crossref] [Google Scholar] [PubMed]
- Sukthavorn K, Nootsuwan N, Wuttisarn R, Jongrungruangchok S, Veranitisagul C (2021) Golden glittering biocomposite fibers from Poly (lactic acid) and Nanosilver-Coated Titanium Dioxide with unique properties; antimicrobial, photocatalytic, and ion-sensing Properties. ACS omega. 6(25):16307-16315.
[Crossref] [Google Scholar] [PubMed]
- Sondi I, Salopek-Sondi B (2004) Silver nanopartiklar som antimikrobiellt medel: En fallstudie pa E. coli som modell for gramnegativa bakterier. J Colloid Interface Sci. 275(1):177-82.
[Crossref] [Google Scholar] [PubMed]
- d’Água RB, Branquinho R, Duarte MP, Maurício E, Fernando AL, et al. (2018) Efficient coverage of ZnO nanoparticles on cotton fibres for antibacterial finishing using a rapid and low cost in situ synthesis. New J Chem 42(2):1052-60.
[Crossref] [Google Scholar] [PubMed]
- Stylianakis I, Kolocouris A, Kolocouris N, Fytas G, Foscolos GB (2003) Spiro [pyrrolidine-2, 2′-adamantanes]: synthesis, anti-influenza virus activity and conformational properties. Med Chem Lett 13(10):1699-1703.
- Hanko VP, Rohrer JS (2004) Determination of sucralose in splenda and a sugar-free beverage using high-performance anion-exchange chromatography with pulsed amperometric detection. J Agric Food Chem 52(14):4375-4379.
[Crossref] [Google Scholar] [PubMed]
- Zimnicki J, Krysiak K, Kaźmierska M (2000) Dyes as a barrier to UV radiation. Part 2. “Objective assessment of protective properties of fabrics.
- Alvarez J, Lipp-Symonowicz B (2003) Examination of the absorption properties of various fibres in relation to UV radiation. Autex Research Journal. 3(2):72-77.
- Rabiei H, Dehghan SF, Montazer M, Khaloo SS, Koozekonan AG (2022) UV protection properties of workwear fabrics coated with TiO2 nanoparticles. Front Public Health.
[Crossref] [Google Scholar] [PubMed]
- Ghayempour S, Montazer M (2017) Ultrasound irradiation based in-situ synthesis of star-like Tragacanth gum/zinc oxide nanoparticles on cotton fabric. Ultrason Sonochem. 34:458-465.
[Crossref ] [Google Scholar] [PubMed]
- Markov SL, Vidaković AM (2014) Testing methods for antimicrobial activity of TiO2 photocatalyst. Acta Periodica Technologica 45:141-152.
- Ibrahim SF, Essa DM, Osman EM (2016) Spectral evaluation studies on titanium dioxide nano-particles and their add-mixtures on textile fabrics. Egypt J Chem 59(6):1069-1093.
- Li P, Li J, Wu Ch, Wu Q (2005) Synergistic antibacterial effects of β-lactam antibiotic combined with silver nanoparticles. Nanotechnol 16(9):1912–1917.
 Spanish
Spanish  Chinese
Chinese  Russian
Russian  German
German  French
French  Japanese
Japanese  Portuguese
Portuguese  Hindi
Hindi