Research Article, J Immunol Tech Infect Dis Vol: 14 Issue: 1
A Pattern of Cytokine Response in Suspected HIV-Negative Tuberculous Meningitis Patients, a Study from Tertiary Neurocenter in India
Priyanka Wakode1*, Veenakumari Haradara Bahubali1, Manjunatha Venkataswamy2, Nagarathna Chandrashekar1 and Netravathi Manjunath3
1Department of Neuromicrobiology, National Institute of Mental Health and Neurosciences, Hosur Road, Bengaluru, Karnataka, India
2Department of Neurovirology, National Institute of Mental Health and Neurosciences, Hosur Road, Bengaluru, Karnataka, India
3Department of Neurology, National Institute of Mental Health and Neurosciences, Hosur Road, Bengaluru, Karnataka, India
*Corresponding Author: Priyanka Wakode
Department of Neuromicrobiology, National Institute of Mental Health and Neurosciences, Hosur Road, Bengaluru, Karnataka, India
E-mail: priyanka.wakode@gmail.com
Received date: 06 January, 2024, Manuscript No. JIDIT-24-124612;
Editor assigned date: 08 January, 2024, PreQC No. JIDIT-24-124612 (PQ);
Reviewed date: 22 January, 2024, QC No. JIDIT-24-124612;
Revised date: 07 February, 2025, Manuscript No. JIDIT-24-124612 (R);
Published date: 14 February, 2025, DOI: 10.4172/2329-9541.1000396
Citation:Wakode P, Bahubali VH, Venkataswamy M, Chandrashekar N, Manjunath N (2025) A Pattern of Cytokine Response in Suspected HIV-Negative Tuberculous Meningitis Patients, a Study from Tertiary Neurocenter in India. J Immunol Tech Infect Dis 14:1.
Abstract
Background: Tuberculous Meningitis (TBM) is the most severe and chronic illness. Studying the association between mycobacterial burden and Cerebrospinal Fluid (CSF) parameters is crucial for understanding the cytokine-driven inflammatory response and disease severity. The primary objective of this study is to quantify cytokine levels in CSF and establish correlations with CSF parameters among patients with TBM, grouped by disease severity.
Materials and methods: A study included 50 TBM cases (25- Definite and 25-Probable) and 20 controls. The IL2, IL6, IFNγ, TNFα, and IL10 levels were estimated by Cytometric Bead Array (CBA) and compared between patients and controls.
Results: TBM patients exhibited significantly elevated levels of IL2, IL6, IFNγ, TNFα, and IL10 compared to controls. A significant correlation was identified among, (a) TBM with tuberculoma cases i) A correlation between CSF lymphocyte count and IL10 levels and ii) A correlation between PMN leukocyte count and IL10 levels; and (c) TBM with hydrocephalus cases displayed a correlation between PMN leukocyte count and IL10 levels. Notably, in TBM group, only confirmed TBM cases demonstrated an altered cytokine pattern with varying levels of cellularity.
Conclusion: These findings offer valuable insights into the immunological mechanisms involved in TBM, highlighting the importance of mycobacterial load and immune cell count in intensifying cytokine response. Moreover, they shed light on the intricate interplay of these factors in influencing disease severity and prognosis. The implications of these observations are significant and underscore the need for further research in this area.
Keywords: Cytokines; Cellularity; Hydrocephalus; Mycobacterium tuberculosis; Tuberculous meningitis; Tuberculoma
Abbreviations
TBM: Tuberculous Meningitis; CSF: Cerebrospinal Fluid; CNS: Central Nervous System; BBB: Blood- Brain Barrier; ATT: Anti-Tuberculosis Treatment; HIV: Human Immunodeficiency Virus; MTB: Mycobacterium tuberculosis; MRI: Magnetic Resonance Imaging; CBA: Cytokine Bead Array; TNFα: Tumor Necrosis Factor Alpha; IFNγ: Interferon Gamma; IL: Interleukins
Introduction
Tuberculous Meningitis (TBM) is the most lethal, chronic illness and often life-threatening with significant mortality and morbidity [1]. The reported mortality rate is 25-40% in non-Human Immunodeficiency Virus (HIV) TBM cases. Conversely, the mortality rate is further elevated among individuals co-infected with HIV and those infected with mycobacterial strains resistant to treatment [2-6]. Moreover, a delay in diagnosis results in severe neurological disabilities in 20-30% of survivors. Therefore, early diagnosis and timely initiation of appropriate treatment stand as the most crucial prognostic factors, but it's often challenging and subject to delays in many cases [5]. Preliminary TBM diagnosis is established through the comprehensive evaluation of patient history, clinical assessment, radiological findings, biochemical and microbiological analysis of Cerebrospinal Fluid (CSF). However, the early identification and initiation of treatment are often challenging as the clinical manifestations and imaging features are not very predictive because of their similarity with other Central Nervous System (CNS) infections [7-8]. Disease confirmation requires isolation of M. tuberculosis (MTB), the infecting pathogen from CSF of TBM patients. Although microbiological assays, such as smear microscopy, are rapid and costeffective, their sensitivity in CSF is very low (10-20%) due to the paucibacillary nature of the disease [3]. Isolation of MTB from CSF by culture is the gold standard, but it is a slow and labor-intensive process with variable diagnostic yield (0-80%). The molecular assays, including GeneXpert (40-50%), and GeneXpert ultra (70-95%) are comparatively more sensitive but importantly Negative Predictive Value (NPV) of these techniques are not adequate to rule out TBM [3,9-11]. Routine biochemical parameters such as increased White Blood Cell (WBC) count with lymphocytic pleocytosis, raised protein levels, and low glucose concentration are not sensitive enough for the early detection of the pathological processes. The presence of neutrophil predominance in the initial phases of the disease, however, is associated with better survival outcomes [1,7,12,13].
The host immune response contributes significantly to the disease outcome. An insufficient immune response may facilitate MTB survival; while an exaggerated inflammatory response initiates a cascade of pathological events which can lead to tissue damage [14,15]. An imbalance in pro-inflammatory and anti-inflammatory cytokine response can lead to clinical symptoms like focal neurological deficits, seizures, unconsciousness, hemiparesis, and the development of dense basal exudates resulting in adhesions, obliterative vasculitis, and hydrocephalus [2,16]. Despite best supportive care, the prognosis in TBM patients is terrible primarily due to this severe neurological sequela that contributes to a high mortality rate. Therefore, along with Anti-Tuberculosis Treatment (ATT), the inclusion of host-directed therapies with anti-inflammatory properties, such as corticosteroids and aspirin, can be beneficial [17-19]. These therapies help regulate the excessive inflammation induced by the MTB infection. Moreover, the concentration of other elements in CSF can offer valuable insights into overall health and the potential presence of co-existing illnesses. Hence, the identification of disease-specific, novel, and more reliable markers with increased sensitivity can streamline the diagnostic process and help reveal the underling pathological processes.
Cytokines, secreted by various CNS cells like astroglia, endothelial cells, microglia, and monocytes in response to TB bacilli, play an essential role in regulating the immune response [20]. Proinflammatory cytokines (IFNγ, TNFα, IL6, and ILβ) promote inflammatory response, while anti-inflammatory cytokines (IL10 and IL4) are crucial for host defense against the pathogen. The imbalance in pro and anti-inflammatory cytokine levels affect the immune system, influencing the course of the disease in multiple ways. Previous studies have reported the presence of these inflammatory mediators (cytokines and chemokines) and their upregulation in the CSF of TBM patients [20].
A protective and detrimental role of various cytokines (TNFα, IFNγ, and IL6) and their association with a worse outcome of the disease has been suggested. TNFα contributes to the development of TBM pathology by triggering the release of other cytokines, inducing fever response, and causing tissue necrosis and cachexia. The action of TNFα is influenced by other cytokines like IFNγ and IL6. An in vivo study showed that IFNγ triggers sustained microglia activation leading to lesion-induced neuronal injury and amplifying the response. Therefore, the action of TNFα in the presence of IFNγ may be detrimental to neuronal elements. Studies have reported a positive correlation between cytokine levels and disease severity, while others find no correlation.
The objective of the current study was to measure levels of IL2, IL6, TNFα, IFNγ and IL10 in CSF of patients diagnosed with TBM and other CNS diseases by Cytokine Bead Array (CBA). Cytokine-induced inflammation disrupts the Blood-Brain Barrier (BBB) permeability, allowing the infiltration of multiple systemic inflammatory mediators and cells into the CNS, resulting in increased cellularity and local inflammation. Therefore, we evaluated all of the measured cytokines in relation to cellularity In addition, to investigate the potential involvement of these cytokines in the pathogenesis of the disease; we conducted a correlation analysis between the levels of cytokines and different CSF parameters, including cellularity, low glucose levels, and high protein levels. We categorized patients with TBM into two distinct groups based on the severity of the disease: Those with only meningitis and those with tuberculoma or hydrocephalus. We then assessed the correlation between cytokine levels and CSF parameters in each group. Ultimately, research has highlighted the crucial role of IL10 in regulating the inflammatory response. Consequently, assessing cytokine levels in TBM patients may aid in identifying markers of disease severity and potential targets for future therapeutic approaches to improve prognosis in TBM patients.
Materials and Methods
Study design and participants
A prospective case-control study was conducted in the Department of Neuromicrobiology from June 2020 to December 2021. The study included 50 individuals suspected of having TBM, diagnosed on the basis of clinical, laboratory, and radiological findings. The suspected TBM group was categorized into definite, probable, possible and non- TBM groups according to the standardized clinical case definition criteria laid by Maraise and Thwaites. The 'Definite TBM' cases were identified if MTB is seen in CSF by smear microscopy or culture method or if detected by GeneXpert MTB/RIF assay. While the probable and possible TBM cases were identified using a diagnostic scoring system that requires the presence of clinical criteria, abnormal CSF findings, radiological features consistent with TBM, response to anti-TB treatment and exclusion of alternative diagnosis. Conversely, non-TBM cases were identified if alternative diagnosis was confirmed.
The control group comprised of 20 CSF samples from individuals within a similar age range diagnosed with either infectious or noninfectious neuro-diseases. The infectious group comprised of clinically and microbiologically cases of meningitis (n=6) caused by bacteria other than mycobacteria, fungal and viral pathogens. These cases were identified through positive test results in bacterial or fungal culture, Gram stain or India ink stain, viral PCR, or response to treatment without ATT. The non-infectious group included cases diagnosed with non-infectious inflammatory diseases (n=4) and some cases with no evidence of infection who received spinal anesthesia for surgery (n=10).
All the cases included in this study were selected based on the inclusion and exclusion criteria listed below. The study was approved by Institutes Ethics Committee and written consent was obtained from all the patients or their relatives (if the patient is unconscious) before including in the study. Additionally, patient’s details including medical history, demographics, and laboratory findings, were obtained from medical records.
Inclusion criteria
- Age 18 ≥ to 55 years.
- Patients willing to participate in the study.
Exclusion criteria
- Patients infected with HIV or other CNS infections.
- Pregnancy.
- Patients having past history or presently on ATT.
Sample collection and laboratory investigations
Approximately 3-5 ml of CSF was obtained in a sterile vial from each patient and then sent to the microbiology laboratory for routine diagnostic investigations. The leftover CSF was stored at 4°C. With the permission of the treating clinician and the patient's consent, a small amount (200-300 microliters) of each patient's CSF was separately stored for the study purpose. The aliquoted samples were then centrifuged at 3000 rpm for 10 mins at 4°C and the supernatant was collected and stored in sterile cryotubes at -80°C until the measurement of analytes.
As part of routine diagnostic process, each patients CSF was used for cell counting (the number of lymphocytes and polymorphs), biochemical analysis (measurement of total protein and glucose concentrations), and microbiological investigations (Gram stain, India ink stain, culture for bacterial/fungal pathogens and viral PCR). All these samples were tested for the presence of MTB by AFB staining, solid (LJ, Lowenstein Jensen) and liquid culture method (MGIT 960 by BD, Becton Dickinson), and GeneXpert MTB/RIF assay.
Cytokine estimation in TBM and non-TBM cases
The levels of IL2, IFNγ, TNFα, IL6, and IL10, were estimated in CSF obtained from TBM and non-TBM cases using a cytokine bead array technology (CBA, human inflammation kit, catalog no # 551809, BD Biosciences).
The assay was performed following the manufacturer's instructions using undiluted CSF. The samples were acquired on the FACSVerseTM flow cytometer (Becton Dickinson, BD), and the FCAP Array software was used to estimate standard curves for all the measured cytokines and values in unknown samples. The results are expressed in pg/ml.
Statistical analysis
Acquired cytokine data were analyzed using IBMSPSS version 22 for Windows and GraphPad Prism version 8. The data for categorical variables is presented as a number with a percentage, while continuous variables are expressed as a median with a range. To compare the differences in cytokine levels between the cases and controls, Mann-Whitney U test was performed. The results are then presented as a median with quartile ranges. A significant difference was determined using two-tailed P values of <0.05, <0.01, and <0.001 as significant, highly significant, and very highly significant, respectively. Based on the normality test result, Pearson’s or Spearman’s correlation test was applied to assess the correlation between cytokine levels and CSF parameters in TBM patients grouped by disease severity.
Results
Demographic details of TBM patients
Cohorts of 50 patients with suspected TBM were evaluated for the presence of MTB in CSF. Among these patients, MTB was detected in CSF of 25 patients (50%), and they were classified as 'Definite TBM' cases. Of these 25 patients, 22 were culture-positive, 17 were GeneXpert positive, and 14 were positive for both tests. The remaining 25 patients were classified as 'probable TBM' cases. Among the 20 non- TBM subjects, four were diagnosed with viral meningitis (Herpes Simplex Virus, n=4), and two were diagnosed with fungal meningitis (Cryptococcal meningitis, n=2). Therefore, they were categorized under the infectious group. In addition, the non-infectious group included patients diagnosed with Neuromyelitis Optica (n=3), Myasthenia Gravis (n=1), and a number of cases with no evidence of CNS infection, but undergoing spinal anesthesia for surgical procedures (n=10). The details of patients in each group are described in below given Table 1.
| Groups | Sub-groups | Gender, n (%) | Age (mean ± SD) | |
| Male | Female | |||
| Cases | Definite TBM | 15 (60%) | 10 (40%) | 29.6 ± 7.5 |
| Probable TBM | 12 (48%) | 13 (52%) | 34 ± 11.77 | |
| Controls | Infectious | 3 (50%) | 3 (50%) | 39.50 ± 11.20 |
| Non-infectious | 6 (43%) | 8 (57%) | 36.14 ± 13.42 | |
| Note: TBM: Tuberculous Meningitis; SD: Standard Deviation | ||||
Table 1: Basic characteristics of patients in cases and controls.
Clinical, CSF and MRI findings of TBM patients
The clinical criteria were fever, headache, vomiting, seizure, loss of appetite, and altered sensorium lasting more than five days. The patient’s details with these clinical features are summarized in Table 2. The Mean ± SD duration of illness was 21.68 ± 26.69 (6-120 days). Among the TBM cases, one patient presented with a family history of TB, while two had a contact history with TB patients. The occurrence of other unusual symptoms suggestive of TBM, including papilloedema, speech disturbance, diplopia, and blurring of vision, was observed in 10 (20%), 9 (18%), 8 (16%), and 11 (22%) respectively. Among these, 3 (6%) and 1 (2%) patients had VIth and IIIrd cranial nerve palsy, respectively. Risk factors associated with TBM, such as smoking, alcoholism, and diabetes, were present in 11 (22%), 4 (8%), and 4 (8%) cases, respectively. Additionally, Hyponatremia (HTN) was reported in 8 (14%) patients.
CSF analysis showed >10 cells in all TBM patients with lymphocyte predominance observed in 42 (84%) patients, and the cell count ranged from 16 to 890 cells/mm3. The mean CSF protein, glucose, and chloride concentrations were 187.4 mg/dl, 36.84 mg/dl, and 101.6 mg/dl, respectively. All the TBM patients underwent cranial CT/MRI; it was normal in 10 (20%) and abnormal in 40 (80%) patients. Some patients showed the signs of meningeal enhancement, neck stiffness, Kernig’s and Brudzinski’s signs (Table 2). The hydrocephalus was present in 7 (14%) cases in mild form (dilation of temporal horns only) and in 4 (8%) cases moderate form (dilation of third and fourth ventricles), respectively. In addition, MRI scans showed other radiological findings, such as single or multiple tuberculomas in the left or right temporal or occipital regions, infarction, and basal exudates in some cases (Table 2).
| Clinical parameters | Number of patients, n=50 (%) |
| Fever | 43 (86%) |
| Headache | 43 (86%) |
| Vomiting | 35 (70%) |
| Seizure | 9 (18%) |
| Altered sensorium | 35 (70%) |
| Kernig’s/Brudzinski sign | 12 (24%)/3 (6%) |
| Neck rigidity | 33 (66%) |
| Past/Family history of TB | 0/3 (6%) |
| Photophobia | 3 (6%) |
| Weakness (Hemiparesis) | 9 (18%) |
| CSF parameters | |
| CSF pleocytosis >10 cells | 50 (100%) |
| CSF cells >250 cells | 26 (52%) |
| Lymphocyte predominance | 42 (84%) |
| PMNL predominance | 8 (16%) |
| Raised protein, mg/dl | 48 (96%) |
| Low glucose, mg/dl | 34 (68%) |
| Glucose ≤ 30 mg/dl | 25 (30%) |
| Radiological features | Number of patients, n=50 (%) |
| Meningeal enhancement | 27 (54%) |
| Tuberculoma | 12 (24%) |
| Infarct | 5 (10%) |
| Basal exudate | 5 (10%) |
| Hydrocephalus | 13 (26%) |
| Note: TB: Tuberculosis; MRI: Magnetic Resonance Imaging; PMNL: Polymorphonuclear Leukocyte | |
Table 2: Clinical, CSF and radiological features of TBM patients.
Comparative expression levels of cytokines in cases and controls
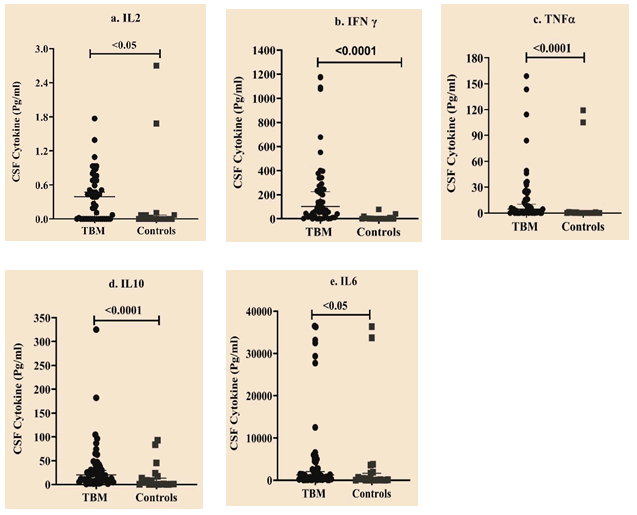
The concentration of Th1 and Th2 cytokines in CSF were compared between cases and controls. Results showed significantly elevated levels of IL2, IFNγ, TNFα, IL6, and IL10 in TBM (P<0.001) compared to controls (Table 3 and Figure 1).
| Cytokines | Cases (n=50) | Controls (n=20) | Mann-Whitney test (U) | P-value |
| Median (Q1, Q3) | ||||
| IL2 | 0.39 (0, 0.68 ) | 0 (0, 0.07 ) | 344 | <0.05 |
| IFNγ | 102.8 (19.57, 277.66 ) | 0 (0, 4.64 ) | 89 | <0.0001 |
| TNFα | 4.52 (0.33, 23.24 ) | 0 (0, 0.79 ) | 254 | <0.001 |
| IL10 | 20.05 (9.07, 41.57 ) | 0.97 (0.14, 16.12 ) | 229 | <0.001 |
| IL6 | 1266.2 (266., 4586.23 ) | 187.71 (8.39, 1830.31 ) | 307 | <0.05 |
| Note: Q1, Q3=Interquartile range; IL: Interleukin; TNF:Tumor Necrosis Factor; IFN: Interferon | ||||
Table 3: Cytokine levels in cases and controls.
Figure 1: Scatter plot representing data for cytokine expression between TBM compared to controls. Horizontal bars depict median with range. X-axis indicates study groups. Y-axis indicates cytokine levels (Pg/ml). a) IL2 b) IFNγ c) TNFα d) IL10 e) IL6.
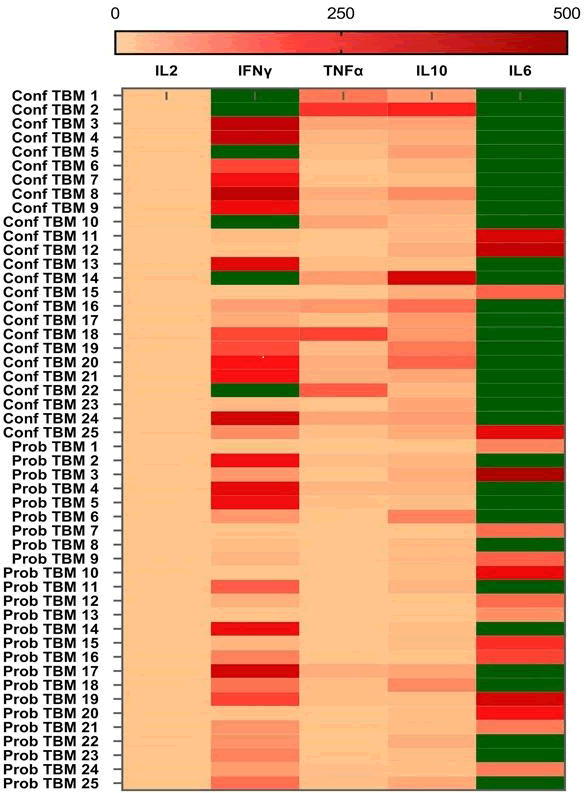
Cytokine expression in confirmed and probable TBM cases
The expression levels of cytokines IL2, TNFα, IFNγ, IL6, and IL10, were measured using cytometric bead array in an equal number of confirmed and probable TBM cases by cytometric bead array. In all TBM cases, the level of IL6 was found to be exceptionally high, whereas the expression level of IL2 was remarkably low (Figure 2).
Figure 2: A heat map illustrating cytokine profiles in confirmed and probable TBM cases. The levels of IL2, IFNγ, TNFα, IL10, and IL6 were estimated by multiplex cytokine assay, with a detection limit of 0-500 pg/ml. The concentrations above the detection threshold limit are indicated in green.
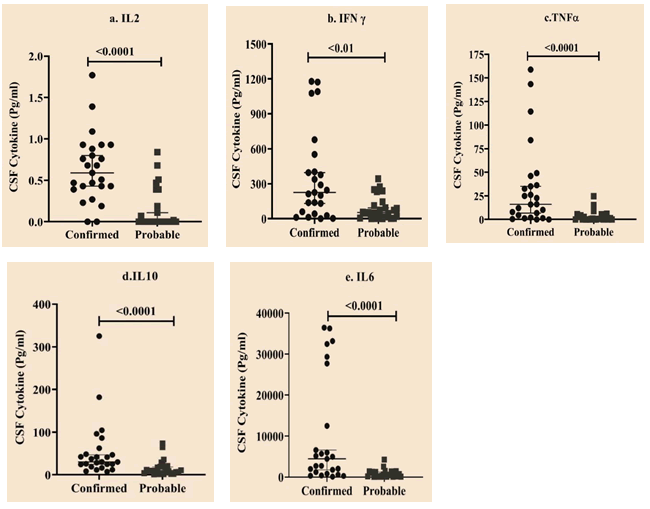
The Mann-Whitney U test was performed to compare the cytokine expression levels between confirmed and probable TBM cases. The findings revealed that the expression levels were significantly higher in the confirmed TBM group than in the probable TBM group (Table 4 and Figure 3). This suggests that a high mycobacterial load triggers a prominent intrathecal cytokine storm in confirmed TBM cases.
| Cytokine | CTBM (n=25) | PTBM (n=25) | Mann-Whitney U test | P-value |
| Median (Q1, Q3) | ||||
| IL2 | 0.59 (0.41, 0.91) | 0 (0, 0.29) | 88 | <0.0001 |
| IFNγ | 225 (50.68, 476.76) | 53.13 (10.07, 128.55) | 163 | 0.003 |
| TNFα | 16.15 (3.37, 41.25) | 0.63 (0, 5) | 101.5 | <0.0001 |
| IL10 | 30.43 (21.62, 55.46) | 9.86 (3.91, 20.05) | 107 | <0.0001 |
| IL6 | 4459 (1015, 20097) | 452.16 (105.28, 1341.52) | 97 | <0.0001 |
| Note: CTBM: Confirmed TBM; PTBM: Probable TBM; Q1,Q3=Inter quartile range | ||||
Table 4: Cytokine expression levels between confirmed and probable TBM cases.
Figure 3: Scatter plot representing data for cytokine expression levels between confirmed and probable TBM groups. Horizontal bars depict median with range. X-axis indicates study groups. Y-axis indicates cytokine levels (Pg/ml) a) IL2, b) IFNγ, c) TNFα, d) IL10, e) IL6.
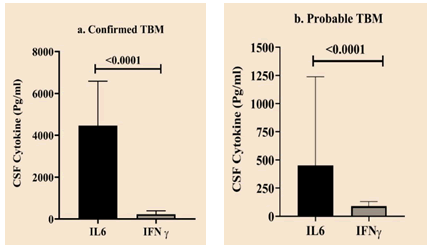
Comparative expression levels of IL6 and IFNγ in confirmed and probable TBM groups
Both confirmed and probable TBM groups showed a significant difference in the expression levels of IL6 and IFNγ (Figure 4).
Figure 4: Comparative expression levels of IFNγ and IL6 in confirmed and probable TBM groups. Statistically significant differences indicated as a bar with p value.
Cytokine levels in TBM patients with and without radiological findings
The cytokine levels in TBM cases with no MRI features (meningitis only) were evaluated against patients with radiological features of disease severity, i.e., TBM with tuberculoma in one group and TBM with hydrocephalus in another group. Statistical analysis showed no significant difference in cytokine levels between these groups (Table 5). However, we were unable to assess the changes in cytokine levels in patients presenting with basal exudate and infarct due to the insufficient number of patients with these features. These findings suggest that cytokine levels may not differ significantly between TBM cases with and without radiological abnormalities, although further studies are required to confirm these results.
| Cytokines | Cytokine levels in pg/ml (mean ± SD) | P-value | ||
| Meningitis only (n=29) | TBM with tuberculoma (n=10) | TBM with hydrocephalus (n=9) | ||
| IFNγ | 253.4 ± 346.9 | 238.8 ± 345.4 | 160.1 ± 124.6 | 0.91 |
| TNFα | 24.6 ± 43.9 | 14.22 ± 18.11 | 8.435 ± 10.79 | 0.87 |
| IL2 | 0.38 ± 0.42 | 0.483 ± 0.4669 | 0.4011 ± 0.3671 | 0.72 |
| IL6 | 5217 ± 9985 | 8187 ± 13993 | 4736 ± 8796 | 0.88 |
| IL10 | 39.57 ± 66.1 | 36.26 ± 35.11 | 28.88 ± 19.19 | 0.6 |
| Note: SD: Standard Deviation. Based on the normality test results (Shapiro-Wilk test) data were compared by parametric or non-parametric test to determine the significant difference (p value <0.05). | ||||
Table 5: Comparative assessment of cytokine levels in TBM patients (Only meningitis) and those with tuberculoma or hydrocephalus.
The evaluation of cytokine levels and CSF parameters in TBM patients
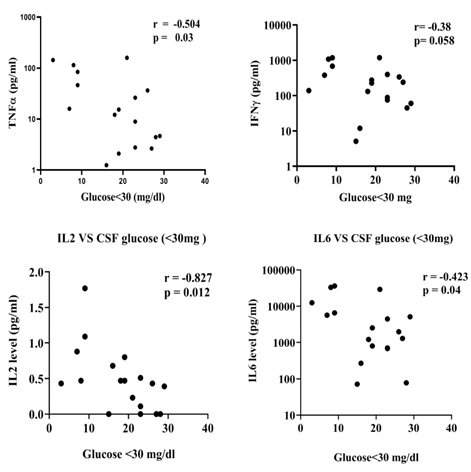
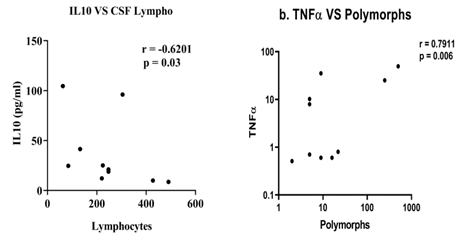
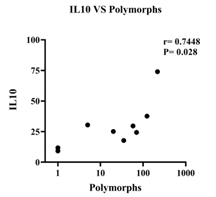
The study investigated the correlation between cytokine levels and common CSF parameters of TBM, such as low CSF glucose, raised protein, lymphocyte count, and PMN leukocyte count, in TBM patients with meningitis alone compared to those TBM patients presented with tuberculoma or hydrocephalus. In addition, the results showed a negative correlation between low CSF glucose (<30 mg) and levels of TNFα, IFNγ, IL2, and IL6 in TBM patients with meningitis alone. In contrast, no such association was observed in TBM patients presented with tuberculoma and hydrocephalus (Figure 5). Furthermore, TBM with tuberculoma patients showed a negative correlation between levels of IL10 and CSF lymphocyte count and a positive correlation between levels of TNFα and PMN leukocyte count (Figure 6). Finally, TBM patients with hydrocephalus revealed a positive correlation between levels of IL10 and PMN leukocyte count (Figure 7).
Figure 5: Correlation between cytokine levels and low CSF glucose (<30 mg) in TBM patients (Only meningitis). Low CSF glucose negatively correlated with, a) TNFα (r=-0.504, p=0.03). b) IFNγ (r=-0.38, p=0.058). c) IL2 (r=0.827, p=0.012) d. IL6 (r=-0.423, p=0.04).
Note: TNF: Tumour Necrosis Factor; IFN: Interferon; IL: Interleukin.
Figure 6: Correlation between cytokine levels and CSF cells (lymphocyte and PMN leukocyte count) in TBM with tuberculoma patients. a) CSF lymphocyte count correlated with IL10 level (r =-0.6201, p=0.03). b) CSF PMN leukocyte count positively correlated with TNFα level (r=0.7911, p=0.006).
Note: PMN leukocyte=Polymorphonuclear leukocyte.
Figure 7: Correlation between levels of IL10 and PMN leukocyte count in TBM with hydrocephalus patients. PMN leukocyte count positively correlated with levels of IL10 (r=0.7448, p=0.02).
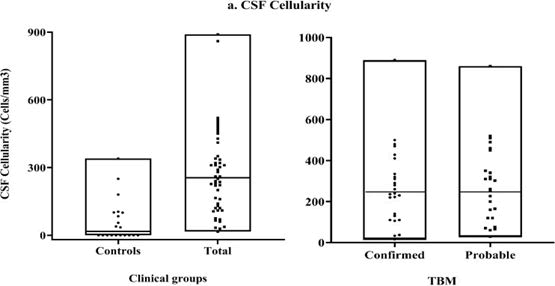
Evaluation of cytokine levels and CSF cellularity in cases and controls
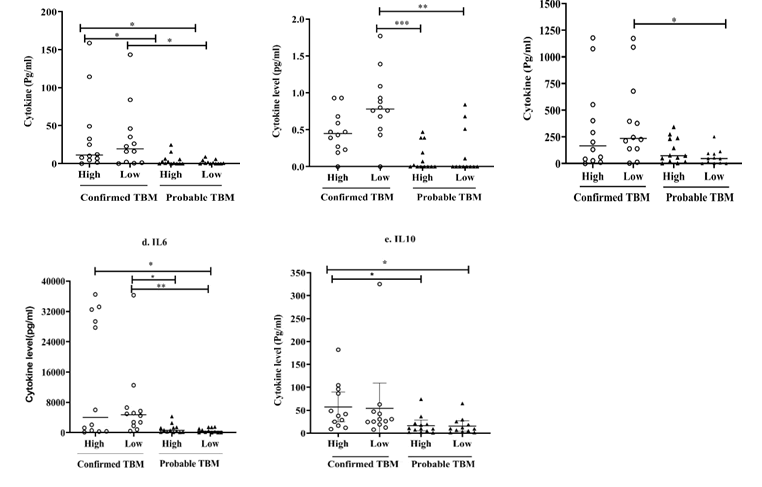
As shown in Figure 8, CSF cellularity was evaluated in both cases and controls. An internal cut-off value was estimated based on a global median value of total number of cells present in all samples of TBM cases. All the samples in the control group had cellularity below this limit value. Further, based on the same cut-off value, the confirmed and probable TBM cases were classified into high and low cellularity groups to assess the relationship between CSF cellularity and cytokine levels. The results showed that the percentage of patients with high cellularity was higher in the probable TBM group (52%) compared to the confirmed TBM group (48%).
Figure 8: CSF cellularity-total CSF cells in clinical groups (cases and controls) and TBM groups (Confirmed and probable). Results expressed as cells/mm3.
According to the data, confirmed TBM patients with low cellularity exhibited significantly elevated TNFα and IFNγ levels compared to low cellularity subgroup in the probable TBM counterparts, along with elevated levels of IL2 and IL6 compared to both cellularity subgroups in the probable TBM group.
On the other hand, the confirmed TBM patients in the high cellularity sub-group displayed notably higher IL6 levels compared to low cellularity sub-group in the probable TBM group. In addition, TNFα and IL10 levels were also found to be significantly elevated compared to both low and high cellularity sub-groups in the probable TBM group. The probable TBM patients from both low and high cellularity sub-groups showed no significant difference in the cytokine levels (Figure 9).
Figure 9: Based on the threshold value, the confirmed and probable TBM groups were categorized into low and high cellularity subgroups and the cytokine levels were compared with respect to cellularity. The significant difference between subgroups displayed as connecting lines with *for p<0.05, **for p<0.01 and ***for p<0.001.
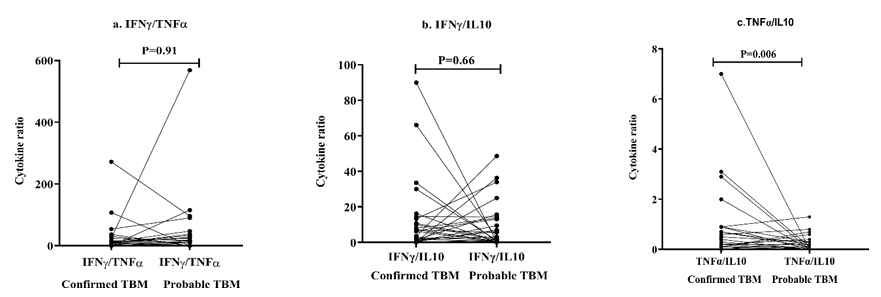
Th1/Th2 Cytokine ratios in confirmed and probable TBM groups
The Th1/Th2 cytokine ratios were evaluated between confirmed and probable TBM groups. The IFNγ/TNFα and IFNγ/IL10 ratios were seen altered in TBM patients but did not reach statistical significance. On the other hand, confirmed TBM patients showed significantly higher (p<0.01) TNFα/IL10 ratio compared to probable TBM patients (Table 6 and Figure 10).
| Cytokine ratio | Groups (Mean ± SD) | Mann-Whitney U test | P-value | |
| Confirmed TBM | Probable TBM | |||
| IFNγ/TNFα | 27.63 ± 55.61 | 45.35 ± 113.7 | 307 | 0.91 |
| IFNγ/IL10 | 13.36 ± 21.59 | 9.788 ± 13.1 | 290 | 0.66 |
| TNFα/IL10 | 0.912 ± 1.52 | 0.216 ± 0.32 | 176.5 | 0.006 |
| Note: Data were tested for normal distribution and analyzed using the Mann-Whitney U test. A p- value <0.05 indicates a significant difference between the two groups. SD: Standard deviation; TNF: Tumor Necrosis Factor; IFN: Interferon; IL: Interleukin |
||||
Table 6: Cytokine ratio in confirmed versus probable TBM groups.
Figure 10: Th1/Th2 cytokine ratio in confirmed versus probable TBM groups.
Discussion
The host's immune response to the pathogen plays a pivotal role in determining the disease outcome. This response is primarily mediated through leukocytosis, encompassing specific subsets of activated CD4 T cells. The activation of these cells triggers the secretion of inflammatory cytokines, contributing to BBB dysfunction through a crosstalk mechanism between cells infiltrating the CNS and cytokines.
Cytokines play a critical role in host defense against mycobacterial infection; however, an imbalance in the levels of pro and antiinflammatory cytokines can exacerbate the disease progression and severity. Effective management of the disease requires a comprehensive understanding of its immune-pathogenesis, characterized by immunemediated damage resulting from an exaggerated inflammatory response induced by mycobacterial infection, despite ATT treatment. The proinflammatory cytokines play a crucial role in sustaining the inflammatory response; however, their continuous release can worsen the situation. Moreover, the intensity of the immune response directly impacts the resulting clinical presentation and course of the disease. As a result, it can lead to several complications, like hydrocephalus, tuberculoma, basal exudates, and more. Therefore, along with the ATT and adjunctive corticosteroids, alternative host-directed therapies are required to improve the disease outcome.
As per previous research, we also noticed that patients with TBM have significantly higher levels of IL2, IFNγ, TNFα, IL6, and IL10 as compared to non-TBM patients. This indicates that these cytokines play a crucial role in the inflammation caused by mycobacterial pathogenicity in the CNS. Th1 cytokines help in inducing an immune response that eliminates pathogens and protects the host from infection. TNFα, a well-known Th1-type cytokine, plays a dual role in MTB infection as it controls the infection and also contributes to the disease pathogenesis. The findings of an animal study revealed that TNFα was associated with clinical progression and disease severity. In a study by Akalin et al., the expression of TNFα was reported in 42% of the TBM cases; however, in our study, it was detected in an even higher proportion, about 76% of the TBM cases. This suggests a significant involvement of this cytokine in the pathogenesis of TBM. Therefore, administering thalidomide, an anti-TNF agent, in combination with ATT, will help effectively lower TNFα levels. IFNγ, another crucial Th1 cytokine, plays an important role in preventing rapid progression of MTB infection to some extent, and thus the impaired production of IFNγ can increase the susceptibility to the disease. In our current study, IFNγ was detected in 98% of TBM patients, and the level was significantly higher compared to the controls (<0.001). Our study found that the IL6 level was remarkably elevated (266.7-4586.2 pg/ml) among all the cases of TBM. It is a pro-inflammatory cytokine that stimulates the production of acute-phase proteins and is also implicated in the pathology of many infectious and non- infectious disorders. Thus, in our study, patients from both cases and control group showed elevated expression of IL6, primarily triggered by TLR4 stimulation.
Many other bacteria, including Escherichia coli and Neisseria species, also induce the significant production of IL6 by activating TLR4 signaling. Similar to these bacterial infections, elevated IL6 levels have no effect on the progression of MTB infection.
MTB, an intracellular pathogen, can lead to chronic infection, and it has developed various strategies to ensure its survival. This includes impeding phagosome maturation, acidification, and phagosome lysosome fusion. In addition, previously, it was reported that the pathogen ensures its survival during the robust cellular immune response by inducing increased expression of IL6, and it also plays an inhibitory role in IFNγ signaling. In the present study, both confirmed, and probable TBM cases showed a significant reduction in the expression of IFNγ (<0.0001), which could be attributed to the inhibitory effect of IL6. In the active stage of the disease, the elevated levels of IL4 and IL10 derive a Th2 response that suppresses the Th1 pathway to inhibit the production of pro-inflammatory cytokines, thus enabling the development of a protective response. In the previous study, pretreatment TBM cases exhibited an absence of IL10 expression. Conversely, our study revealed a notable upregulation of IL10 in TBM cases compared to IL2 (p<0.0001) and TNFα (p<0.0001). Detailed data on this expression are not presented herein.
The group analysis of TBM has indicated that confirmed TBM cases exhibit higher expression levels of all the measured cytokines compared to probable TBM cases (<0.05). This difference in cytokine expression can be attributed to the high mycobacterial load in cultureconfirmed TBM cases. However, analysis of cytokine levels between TBM cases and infectious controls was inconclusive due to an insufficient number of cases in the infectious control group. A study conducted by Sharma, et al., reported that TBM patients with hydrocephalus, exudates, and infarcts showed elevated levels of ILβ, TNFα, and IFNγ in Cerebrospinal Fluid (CSF), respectively. Conversely, another study by Mishra et al. failed to find any correlation between CSF cytokine levels and radiological findings in TBM. Despite more TBM cases in our study compared to a study by Mishra et al., statistically, we found no difference in cytokine levels between TBM patients with no radiological features (meningitis only) and TBM with tuberculoma or hydrocephalus. Additionally, analyzing cytokine levels and classic CSF parameters showed a different association pattern in TBM patients (meningitis only) compared to those presented with tuberculoma or hydrocephalus. The classic CSF parameters of TBM typically include elevated protein level, low glucose level, and CSF pleocytosis with lymphocyte predominance in over >90% of the cases. In TBM patients (meningitis only), all the measured pro-inflammatory cytokines (IL2, TNFα, IFNγ, and IL6) exhibited a negative correlation with low CSF glucose. In contrast, no such association was observed in TBM patients presented with tuberculoma or hydrocephalus. The low concentration of CSF glucose during infection is an indication of the anaerobic metabolic activity in the cerebral tissues. Our findings suggest that further studies are necessary to investigate the association between cytokine levels and CSF parameters in TBM patients with different clinical presentations. Furthermore, TBM with tuberculoma patients showed a negative correlation between lymphocyte predominance and IL10 level, and a positive correlation between increased PMN leukocyte count and TNFα level. These findings suggest the potential involvement of elevated lymphocytes and PMN leukocytes, increased TNFα level, and reduced IL10 level in the severity and structure of granulomas. Finally, TBM with hydrocephalus patients showed a positive correlation between levels of IL10 and PMN leukocyte count. The influx of PMN leukocytes at the site of infection in humans is a sign of active TB disease. PMN leukocytes have been reported to act as a "Trojan horse" for mycobacteria, contributing to TB pathology rather than protecting the host. However, the protective or detrimental effect of PMN leukocytes also depends on the virulence of the MTB strain. Further investigations are required to address these findings.
In both confirmed and probable cases of TBM, the cytokine levels were evaluated in relation to CSF cellularity. The findings revealed that despite a higher percentage of cells in the probable TBM group (52%), the cytokine levels were elevated in the confirmed TBM group, suggesting that the increased mycobacterial load plays a significant role in cytokine production in TBM. Moreover, the confirmed TBM group, regardless of cellularity level showed a significant expression of TNFα and IL6, but only the low cellularity subgroup displayed significantly higher expression levels of IL2 and IFNγ. This data probably indicates that the production of these cytokines in TBM is independent of cell influx into the CNS. Further, a significant expression level of IL10 was only demonstrated in the high cellularity sub-group of the confirmed TBM group. The IL10 has been identified as an important clinical biomarker of disease progression in active TB disease. It was also reported that increased IL10 levels during MTB infection reduce Th1 immunity, promote mycobacterial growth, and aggravate the disease severity. Therefore, in the present study, increased expression level of IL10 in confirmed TBM patients may be indicative of disease progression.
A balance between pro and anti-inflammatory cytokine responses is crucial in the effective management of disease. Therefore, evaluating the ratio of Th1 and Th2 cytokine levels in TBM patients may help understand the difference between pathological processes and local inflammatory response during infection. The present study observed no significant difference in IFNγ/TNFα and IFNγ/IL10 ratios in the probable and confirmed TBM groups. However, TNFα/IL10 ratio was found to be significantly higher in the confirmed TBM group compared to the probable TBM group (p=0.006), indicating a predominant Th1 response in these patients resulting in poor prognosis due to low Th2 cytokine response and high mycobacterial load. The role of IL10 has been reported in the treatment of CNS related illnesses. One of the main limitations of our study is small number of samples in the infectious control group because of which we could not assess the difference in the cytokine levels between TBM and other infectious CNS disorders to identify markers specific to TBM or for the differential diagnosis of TBM from other infectious CNS diseases presenting with similar clinical and radiological features.
Conclusion
Significantly elevated levels of all the measured cytokines in CSF of TBM patients highlight that these cytokines play a major role in the inflammatory response induced at the site of infection. The data suggest that the confirmed TBM cases exhibit higher cytokine levels, correlating with mycobacterial load and are probably unrelated to cell influx in the CNS. Associations with clinical presentations and CSF parameters vary, highlighting the need for further investigations. The study suggests a link between cytokines, immune cell types, and disease progression. An association between immune cells and cytokines in TBM with tuberculoma patients suggests that elevated lymphocytes and PMN leukocytes, high TNFα, and low IL10 levels may contribute to the formation and severity of granulomas in TBM. In addition, the elevated TNFα/IL10 ratio observed in these patients suggests a likely correlation between a low Th2 cytokine response, a high mycobacterial load, and a poor prognosis. Overall, these findings provide valuable insights into the immunological mechanisms at play in TBM, highlighting the role of mycobacterial load and immune cell count in exacerbating cytokine response and their interplay in causing disease severity and prognosis.
Acknowledgments
The authors express gratitude to the Director of Institute for granting permission to use the Flow cytometry machine, the central facility, at the institute for conducting the study. Furthermore, we would also like to extend our sincere appreciation to the Neurology Faculty for their valuable assistance in selecting cases and providing samples for our study after the Ethics approval. This research did not receive any specific grant from external funding agencies.
Conflicts of Interest
The authors declare no conflict of interest.
Author’s Contribution
All authors have read and approved the final version of the manuscript.
References
- Ahlawat S, Chaudhary R, Dangi M, Bala K, Singh M, et al. (2020) Advances in tuberculous meningitis diagnosis. Expert Rev Mol Diagn 20: 1229-1241.
- Thwaites GE, Bang ND, Dung NH, Quy HT, Oanh DT, et al. (2004) Dexamethasone for the treatment of tuberculous meningitis in adolescents and adults. N Engl J Med 351: 1741-1751.
[Google Scholar] [PubMed]
- Thwaites G, Chau TT, Mai NT, Drobniewski F, McAdam K, et al. (2000) Tuberculous meningitis. J Neurol Neurosurg Psychiatry 68: 289-299.
[Crossref] [Google Scholar] [PubMed]
- van Laarhoven A, Dian S, Ruesen C, Hayati E, Damen MS, et al. (2017) Clinical parameters, routine inflammatory markers, and LTA4H genotype as predictors of mortality among 608 patients with tuberculous meningitis in Indonesia. J Infect Dis 215: 1029-1039.
[Crossref] [Google Scholar] [PubMed]
- Tho DQ, Torok ME, Yen NT, Bang ND, Lan NT, et al. (2012) Influence of anti-tuberculosis drug resistance and Mycobacterium tuberculosis lineage on outcome in HIV-associated tuberculous meningitis. Antimicrob Agents Chemother 56: 3074-3079.
[Crossref] [Google Scholar] [PubMed]
- Whitworth LJ, Troll R, Pagan AJ, Roca FJ, Edelstein PH, et al. (2021) Elevated cerebrospinal fluid cytokine levels in tuberculous meningitis predict survival in response to dexamethasone. Proc Natl Acad Sci USA 118: e2024852118.
[Crossref] [Google Scholar] [PubMed]
- Ho J, Marais BJ, Gilbert GL, Ralph AP (2013) Diagnosing tuberculous meningitis–have we made any progress?. Trop Med Int Health 18: 783-793.
[Crossref] [Google Scholar] [PubMed]
- de Almeida SM, Furlan SM, Cretella AM, Lapinski B, Nogueira K, et al. (2020) Comparison of cerebrospinal fluid biomarkers for differential diagnosis of acute bacterial and viral meningitis with atypical cerebrospinal fluid characteristics. Med Princ Pract 29: 244-254.
[Crossref] [Google Scholar] [PubMed]
- Bahr NC, Boulware DR (2014) Methods of rapid diagnosis for the etiology of meningitis in adults. Biomark Med 8: 1085-1103.
[Crossref] [Google Scholar] [PubMed]
- Nhu NT, Heemskerk D, Thu DD, Chau TT, Mai NT, et al. (2014) Evaluation of GeneXpert MTB/RIF for diagnosis of tuberculous meningitis. J Clin Microbiol 52: 226-233.
[Google Scholar] [PubMed]
- Vinny PW, Vishnu VY (2019) Tuberculous meningitis: A narrative review. J Curr Res Scientific Med 5: 13-22.
- Palacios CF, Saleeb PG (2020) Challenges in the diagnosis of tuberculous meningitis. J Clin Tuberc Other Mycobact Dis 20: 100164.
[Crossref] [Google Scholar] [PubMed]
- Jeren T, Beus I (1982) Characteristics of cerebrospinal fluid in tuberculous meningitis. Acta Cytol 26: 678-680.
[Google Scholar] [PubMed]
- Stadelman AM, Ellis J, Samuels TH, Mutengesa E, Dobbin J, et al. (2020) Treatment outcomes in adult tuberculous meningitis: A systematic review and meta-analysis. Open Forum Infect Dis 7: ofaa257.
[Crossref] [Google Scholar] [PubMed]
- Wilkinson RJ, Rohlwink U, Misra UK, van Crevel R, Mai NT, et al. (2017) Tuberculous meningitis. Nat Rev Neurol 13: 581-598.
[Crossref] [Google Scholar] [PubMed]
- Sharma S, Goyal MK, Sharma K, Modi M, Sharma M, et al. (2017) Cytokines do play a role in pathogenesis of tuberculous meningitis: A prospective study from a tertiary care center in India. J Neurol Sci 379: 131-136.
[Crossref] [Google Scholar] [PubMed]
- Rohilla R, Shafiq N, Malhotra S (2021) Efficacy and safety of aspirin as an adjunctive therapy in tubercular meningitis: A systematic review and meta-analysis. EClinicalMedicine 34.
[Crossref] [Google Scholar] [PubMed]
- Prasad K, Singh MB, Ryan H (2016) Corticosteroids for managing tuberculous meningitis. Cochrane Database Syst Rev. 2016;4.
[Crossref] [Google Scholar] [PubMed]
- Schoeman JF, Elshof JW, Laubscher JA, van Rensburg AJ, Donald PR (2001) The effect of adjuvant steroid treatment on serial cerebrospinal fluid changes in tuberculous meningitis. Ann Trop Paediatr 21: 299-305.
[Crossref] [Google Scholar] [PubMed]
- Misra UK, Kalita J, Srivastava R, Nair PP, Mishra MK, et al. (2010) A study of cytokines in tuberculous meningitis: Clinical and MRI correlation. Neurosci Lett 483: 6-10.
[Crossref] [Google Scholar] [PubMed]
 Spanish
Spanish  Chinese
Chinese  Russian
Russian  German
German  French
French  Japanese
Japanese  Portuguese
Portuguese  Hindi
Hindi