Case Report, J Athl Enhanc Vol: 9 Issue: 1
A Personalized Low Intensity Exercise Prescription based on an Index of Non-Linear Heart Rate Variability: A Case Report
Rogers B*
College of Medicine, University of Central Florida, Orlando, Florida, USA
*Corresponding Author: Bruce Rogers
Assistant Professor of Internal Medicine
University of Central Florida College of Medicine
Orlando, Florida, USA
E-mail: bjrmd@knights.ucf.edu
Received: February 07, 2020 Accepted: February 18, 2020 Published: February 25, 2020
Citation: Rogers B (2020) A Personalized Low Intensity Exercise Prescription based on an Index of Non-Linear Heart Rate Variability: A Case Report. J Athl Enhanc 9:1. DOI: 10.37532/jae.2020.9(1).327
Abstract
The potential of an index of cardiac interbeat fractal complexity (DFA a1) to demarcate low intensity training was undertaken in a recreational athlete. The influence of absolute heart rate elevation versus work rate as factors responsible for loss of interbeat complexity was also examined via the usage of beta adrenergic blockade. Incremental cycling ramps were performed with and without the beta adrenergic blocking agent Atenolol 25 mg measuring DFA a1 during the last 2 minutes of each stage. No difference was seen between control and Atenolol trials for lactate thresholds, ventilation rates, rectus femoris muscle O2 desaturation and DFA a1 despite a 15 to 20 beat decrease in heart rate across all stages in the Atenolol trial. In both studies, DFA a1 progressively declined with cycling power reaching a value consistent with white noise at 25 Watts above the first ventilatory threshold. In conclusion, DFA a1 transition to an uncorrelated low complexity state occurred just above the VT1. In addition, the complexity index was related to cycling power, ventilation and presumably VO2 rather than the absolute heart rate. Longer constant power intervals near VT1 did not show additional or progressive complexity loss. DFA a1 may be a promising guide for low intensity training zone demarcation.
Keywords: Exercise prescription; Endurance training; Heart rate variability; DFA a1; Ventilatory threshold; Lactate threshold; Training intensity distribution; Cycling
Introduction
Proper modulation of endurance exercise intensity is essential for optimal training and rehabilitation [1,2]. Typical training intensity ranges are defined as zone 1 (below the first ventilatory threshold), zone 2 (between the first and second ventilatory threshold) and zone 3 (above the second ventilatory threshold) [3]. Attention has been recently focused on the need for the vast majority of endurance training to be at “low intensity” or zone 1 for marathon running, cycling and cross country skiing [4]. A recent publication showed that marathon runner’s performance scores were strongly predicted by the volume of easy continuous runs during the first seven years of their sports careers [5]. However, the exact upper limit of this low intensity zone is contentious [6] but may involve work rates at or below the first ventilatory or lactate thresholds. In addition, various formulas derived from power or heart rate relationships have also been advocated for zone definition but without knowing VO2max, ventilatory or lactate breakpoints, may not correspond to a given individuals low intensity zone [7-10]. Although training zone relationships may be more physiologically appropriate using gas exchange derived ventilatory thresholds or blood lactate studies [10], these methods are less attractive given the equipment needed, cost, invasiveness and need for near maximal efforts. Unfortunately, there has not been a reliable, objective, non-invasive, submaximal technique to delineate the transition out of an easy effort, into higher workloads.
The short term index of fractal complexity of the cardiac RR interbeat interval (DFA a1) has received attention for providing insights into both normal physiology as well as various disease states [11]. Although beyond the scope of this report, Goldberger et al. review points out that a cardiac interbeat DFA a1 near 0.5 represents an uncorrelated random distribution of values (white noise), whereas DFA a1 at rest in a normal individual is generally above 1 (either ordered Brownian noise or having long term fractal correlations). Several studies have shown that when measured during activity, the DFA a1 decline toward randomness is related to exercise load [12-16]. This is an attractive relationship to explore as an alternative to simple heart rate or power guidelines for endurance training intensity prescription.
The purpose of this case report is twofold. Given the importance of limiting training intensity to below the first ventilatory or lactate threshold, can interbeat complexity loss be used as a surrogate marker? Since the first ventilatory threshold may correspond to a different workload than the first lactate threshold [17,18], utilizing an appropriate index of heart rate availability could be helpful. To help answer this, a study involving one subject (author BR) will be presented with physiologic measurements derived from gas exchange, lactate testing along with the corresponding behavior of DFA a1. A second issue to be addressed is whether DFA a1 decline is a function of work load or simply related to exercise induced heart rate elevation? Although exercise intensity is generally related to heart rate [19], it may be misleading to assume that complexity loss is a simple consequence of a higher heart rate with shorter interbeat intervals. It is certainly plausible that complexity loss is more closely related to VO2, work rate or cardiac output rather than absolute heart rate [20,21].
Since beta adrenergic blocking agents reduce heart rate during exercise but maintain similar VO2 max, lactate thresholds [22] and cardiac output [23], they are ideally suited to answering this question. Although heart rate is diminished after beta blockade treatment, there is a compensatory rise in stroke volume, maintaining cardiac output at usual levels [24]. Taking advantage of the similar workrate to VO2 relation but at lower heart rates, a comparison of DFA a1 during a progressive exercise test, with and without beta blockade will be done in the same individual.
Case Report
Participant
The subject was a 62 year old male, recreational cyclist (author BR) with no past medical history. Training consisted of approximately 150 miles of mixed intensity road riding each week.
Height was 185 cm and weight 80 kg. There were no regular medications, significant past medical history, no tobacco, alcohol, caffeine or recreational drug use. Ethical approval of this study was waived in accordance with the recommendations of the 1995 Data Protection Directive and local ethics review board because the author was the subject of his own research and consented freely to the study and data sharing.
VO2 max testing was performed at the University of Florida Sports Performance Center (Gainesville, FL, USA) using a standard metabolic cart (Viasys, CareFusion; Yorba Linda, CA; USA). After a 30 minute warm up consisting of intermittent, variable power cycling, the subject began cycling at 100 Watts, increasing 30 Watts every 3 minutes to exhaustion. The test was performed with the subject’s own bicycle on an electrically controlled trainer. A cadence of about 80 RPM was maintained throughout the test. Gas exchange testing was done with the metabolic cart and power monitoring done by both the test center’s electrically controlled trainer as well as the subjects own power meter. Lactate measurements were taken at the end of each ramp stage by the testing staff using the Lactate Plus monitors (Nova Biomedical, Waltham, MA). The lactate thresholds during the VO2 max testing were calculated using the template software of Newell [25] and confirmed by visual inspection by the Center director. Ventilatory threshold evaluation was done by metabolic cart software using both V slope and PetO2 methods.
Study design
The study was composed of two parts. The initial section was that of investigating DFA a1 behavior during progressive, 5 minute constant power cycling intervals with and without beta blockade. The second part was to determine if the DFA a1 index remained stable over longer, 10 minute cycling intervals just below and above the first ventilatory threshold (outlined in simulated training ride).
DFA a1 performance relative to cycling power and heart rate Non exhaustive cycling ramps with and without beta blockade were done with the subjects own bicycle on a resistance trainer (Elite Muin, Fontaniva, Italy). Each submaximal ramp session was done at the same time (8 am), same ambient temperature of 23 degrees C, and 1 hour after the ingestion of 100 ml of grapefruit juice. Two sessions were done, a control ramp (Ctrl) followed one week later by a similar ramp done 60 minutes after ingesting 25 mg atenolol (Aten). Atenolol 25 mg was chosen based on Chou et al. [23]. Before each ramp session the subject cycled for 15 minutes at 80 to 120 Watts. The ramp commenced at approximately 110 Watts with an incremental rise of 25-30 Watts every 5 minutes. Between ramps stages, pedaling were temporarily stopped, the fingers washed and the lactate measured (60- 90 seconds from stopping) by fingerstick. The test was terminated when lactate rose above 5 mmol. Cadence varied between 60 to 80 rpm.
Simulated training ride
To investigate the possibility that DFA a1 would continue to change after a longer time of exercise, a series of simulated training rides were done. Each 10 minute test segment was done on a different day and were performed 1-2 weeks after the last ramp session under similar conditions with the same equipment (at 8 am, 23 degrees C and 1 hour post ingestion of 100 ml grapefruit juice). After 45 minutes of continuous cycling on the indoor trainer (at or below VT1 power), 10 minute intervals at 10 Watts below VT1, near VT1 and 25 Watts above VT1 power was done (163w, 183w, 197w). Cadence varied between 68 and 72 rpm.
Measuring tools
Power was measured by the Assomia Duo pedal device (Favero Electronics srl, Arcade, Italy) with a reported accuracy of 1% and a sample rate of 1 Hz. Manufacturer recommended calibration was done prior to each session.
Heart rate and R-R intervals were recorded with the Hexoskin vest at a sampling frequency of 256 Hz (Carre Technologies Inc., Montreal, Canada). The RR intervals were extracted and analyzed by Kubios software (Kubios HRV 3.3, Biosignal Analysis and Medical Imaging Group, Department of Physics, University of Kuopio, Kuopio, Finland). Artifacts were less than 1% and most intervals had zero artifacts. DFA a1 metrics were determined for the last 2 minutes of each ramp stage (4 and 5 minutes).
Ventilation rate was measured with the Hexoskin garment (sample rate 128 Hz). This has been shown to provide reasonable accuracy with 5.3-7.9% error [24] during cycling.
Lactate assessment was performed by the Lactate Scout handheld meter (EKF Diagnostics, Cardiff, England). Control solution testing was done prior to each session. Log-log and fixed 4 mmol lactate thresholds were calculated as per software written by Newell [25].
Desaturation kinetics of the rectus femoris were recorded with an NIRS device, the Humon Hex (Dynometrics, Inc., Boston, Ma). This device has been shown to agree closely with a benchtop NIRS measurement system [25] for the measurement of quadriceps muscle oxygen saturation.
Perceived effort was graded according to the Borg scale for each ramp stage.
Statistical technique and data interpretation
Average power was calculated for each ramp interval by spreadsheet (Apache OpenOffice). Both average heart rate and DFA a1 were assessed for the last 2 minutes of each 5 minute ramp level or for every 2 minute window during the simulated training ride by the Kubios software. Ventilation rate and Rectus Femoris O2 saturation were also averaged for the last 2 minutes of each ramp stage from the raw Hexoskin data and the Humon Hex sensor respectively.
Results
VO2 max test
Peak heart rate was 179 bpm, first ventilatory threshold occurred at 58% of maximum stable VO2, which corresponded to the 172 Watt stage of the test. The first lactate threshold occurred at 223 Watts (via log-log plot) with an OBLA (4.0 mmol lactate) of 285 Watts. Peak stable oxygen consumption was 55 ml/kg/min which occurred during the final 330 Watt stage.
DFA a1 change relative to cycling power and heart rate with and without Aten
Power: Beta blockade ramp intervals were matched to control intervals for power as closely as possible. The mean power of each Aten stage was within 5% of control (Table 1).
Ctrl-AtenDFA a1
Ctrl-AtenArtifacts (%)
Ctrl-AtenLac (mmol)
Ctrl-AtenRF O2 sat (%)
Ctrl-AtenVe (L/min)
Ctrl-AtenRPE
Ctrl-Aten110110-941.37-1.350-01.4-1.653-5259-522-2136115-1011.11-1.270-01.4-1.750-5161-582-2157121-1080.994-0.9170-01.4-2.050-5263-633-3175127-1140.723-0.6660-01.3-1.749-5171-714-4197134-1190.542-0.5030-01.4-1.849-5079-795-5225143-1250.343-0.3360-02.0-2.047-4997-956-6254151-1310.269-0.2970-0.762.4-3.544-47117-1177-7290160-1400.243-0.2410-0.715.8-7.137-42170-1808-8
Table 1: Results per ramp stage for Control (Ctrl) and Atenolol (Aten) trials. From left to right, the first column is average 5 minute power in watts, heart rate in bpm, DFA a1, Artifacts in percent, Lactate in mmol/L, rectus femoris muscle O2 saturation in percent, ventilation average over last 2 minutes of ramp stage in L/min and RPE at each stage end.
Ve: Ventilation rates for each Aten stage were generally within 5% of control. Several intervals were matched exactly.
Lactate thresholds: The first lactate threshold was 211 Watts for the control and 219 Watts for the Aten study. Estimated OBLA (4 mmol) was 271 Watts for the control and 263 Watts for the Aten trial. Given the variability with lactate testing in general, it was felt that there was no significant difference in either the first or second thresholds with respect to Aten, control or VO2 max ramps.
Heart rate: Heart rate was between 15 and 20 bpm lower during the Aten session at each stage of power.
Rectus Femoris NIRS: Desaturation patterns of the Rectus Femoris were similar between control and Aten ramp trials.
Perceived effort: There was no major difference in perceived effort between the control and Aten studies.
Interbeat complexity: DFA a1 did decrease with rising cycling power in both Aten and control trials to a similar degree (Figure 1b). DFA a1 decline also was similar across both control and Aten ramp studies when compared to Ve (Figure 1c), which is logical considering the close relation between work rate, VO2 and Ve [19]. For instance, at the power associated with LT1 (220w), Ve and DFA a1 were virtually identical (97 L/min/0.343 vs. 95 L/min/0.336) in the control and Atenolol session respectively. Heart rate rose during both the control and Aten progressive cycling ramps with the expected shift of the heart rate to power curve seen with beta blockade (Figure 1) [23].
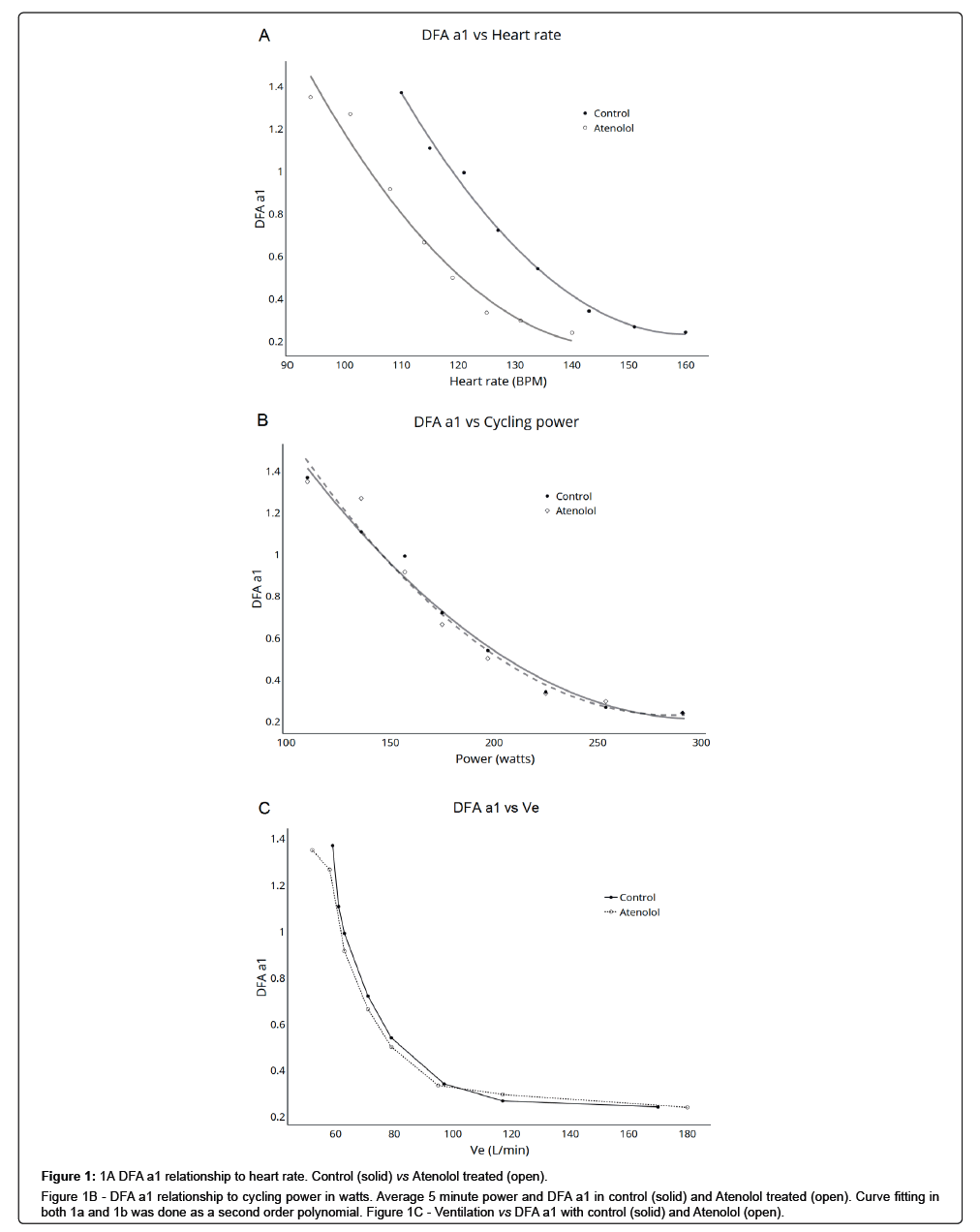
Figure 1: 1A DFA a1 relationship to heart rate. Control (solid) vs Atenolol treated (open).
Figure 1B - DFA a1 relationship to cycling power in watts. Average 5 minute power and DFA a1 in control (solid) and Atenolol treated (open). Curve fitting in both 1a and 1b was done as a second order polynomial. Figure 1C - Ventilation vs DFA a1 with control (solid) and Atenolol (open).
The Aten study showed a drop of DFA a1 into low complexity values at heart rates typically associated with normal complexity (Figure 1a). For example, comparing ramp trials at a similar heart rate of about 125 bpm (but with different average power), DFA a1 during Aten cycling was 0.336 whereas that of the control ramp was 0.723. In summary, DFA a1 values of the Aten and control sessions were similar across workloads and Ve but not heart rates. Furthermore, in both control and Aten ramps, the workload at which DFA a1 transitioned from correlated to uncorrelated RR complexity properties (white noise) was near the first ventilatory threshold. There was a continued decrease of DFA a1 as cycling power and ventilation increased Table 1.
Simulated training ride: As seen in Table 2 and Figure 2, after an initial 45 minute segment at or below VT1 power, DFA a1 and heart rate remained relatively stable from minute 4 through the 10 minute continuous test interval. Each DFA a1 value was based on the prior 120 seconds and there were zero artifacts in each session. There was no systematic decline in DFA a1 complexity over time Figure 1.
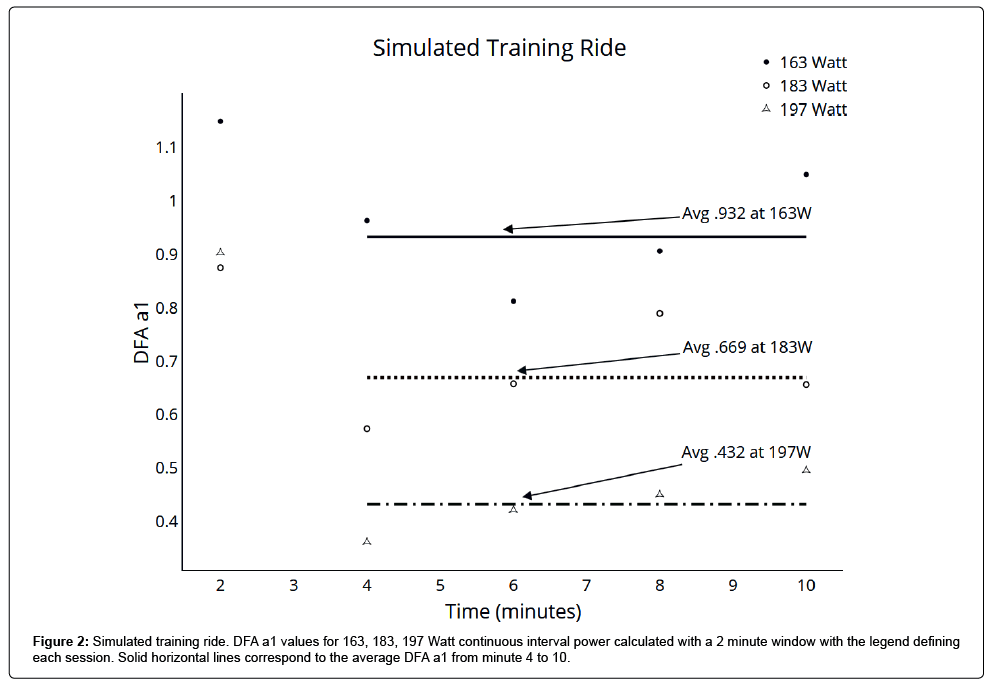
Figure 2: Simulated training ride. DFA a1 values for 163, 183, 197 Watt continuous interval power calculated with a 2 minute window with the legend defining each session. Solid horizontal lines correspond to the average DFA a1 from minute 4 to 10.
Table 2: After 45 minutes of cycling at or below VT1 power, ten minute constant power intervals were done at 163, 183 and 197 Watts (VT1 minus 10w, VT1 plus 10w and VT1 plus 25w). DFA a1 and average heart rate for each 2 minute window of continuous power is shown at 2, 4, 6, 8 and 10 minutes elapsed time.
Discussion
This report explores the utility of a nonlinear index of heart rate variability, namely DFA a1, to demarcate the zone of low intensity endurance training. Additionally, an evaluation of absolute heart rate versus workload, as factors driving interbeat complexity was done. DFA a1 complexity loss appears more a function of physical workload or VO2 rather than absolute heart rate elevation. The rise in heart rate with progressive cycling power was blunted by Atenolol treatment; however the interbeat complexity index was not affected to a noticeable degree. Moreover, as a surrogate for VO2, both cycling power and Ve were both related to DFA a1 values in a similar fashion during each ramp condition. There have been several studies showing that DFA a1 values will decrease with progressive exercise loading [12,16,20,21]. One could say this is due to heart rate rise with shorter interbeat intervals that simply don’t have the time related bandwidth to maintain complexity. In this case report, the stroke volume, heart rate relationship was altered by utilizing Atenolol, with the final cardiac output unchanged. Although cardiac output nor VO2 were directly measured, the use of Ve as a surrogate for VO2 [19], along with vasti desaturation kinetics as an index for arterial-venous O2 extraction [26,27], provides a reasonable premise for similarity of VO2 in control and Atenolol treatment conditions. In view of the proposal to link complexity loss with rising VO2, let us also consider the study by Gronwald regarding effects of cycling cadence and DFA a1 [14]. Here, although mechanical power was fixed, a cadence of 120 rpm induced higher RPE, lactate, heart rate and lower DFA a1 compared to standard cadences of 60-90. Given the major loss of cycling efficiency at cadences at or above 120 rpm [16], it would be expected that the subjects were operating at a higher VO2 for the same net cycling power compared to the usual cadence. This was also supported by the higher lactate values and RPE in the high cadence group. In this situation, DFA a1 was still tied to VO2 rise (with heart rate elevation part of the VO2 relationship) rather than the power measured mechanically. Mechanisms revolving around DFA a1 behavior may relate to “organismic stress” [14,16] as well as models of central fatigue [28,29]. The close relation of complexity decline with workload and VO2 rather than absolute heart rate is further evidence of this concept.
Another goal of this study was to determine if there was a physiologic breakpoint where DFA a1 transitioned from correlated to uncorrelated states as a surrogate for obtaining VT1 by lactate testing or gas exchange. The underlying basis of the lactate and ventilatory threshold is a continuing matter of debate in the sports literature [5,15,16,29]. Even proponents of a tight association between VT1 and LT1 show only a moderate correlation coefficient when examined statistically [5]. The measurement of lactate thresholds obtained by ramp testing is common but do we choose log-log, linear spline intersections or fixed levels such as 2 mmol for our zone ceiling [29]? Obtaining VT1 related training zone information by gas exchange testing may not provide a more accurate guideline. VT1 derived from computer software regression methods may yield variable results [30], making the VT1 obtained in this study potentially inexact. Visual determination, even by triple criteria methods, may not be detectable in up to 10% of subjects [31] lastly; gas exchange testing is costly, equipment intensive and usually requires high intensity efforts.
Therefore, in many situations athletes may be left with markedly different work rate recommendations for low intensity training as illustrated in this subject whose VT1 by gas exchange was 40 Watts below their measured first lactate threshold by log-log methods despite both being done at a major university sports lab. If the subject were to do the bulk of their “easy” (zone 1) training just below the LT1 measured via lactate blood testing, they would be spending significant amounts of time exposed to unwanted physiologic stress as expressed by the DFA a1 [32]. Since the DFA a1 index decline exhibited an incremental relation to workload in both control and Atenolol ramps, a logical extension is leveraging this for training intensity distribution purposes. For instance, at the subjects VT1 power, the DFA a1 was about 0.7, but at a power 25 Watts higher, dropped to 0.5 (white noise), and rose to near 1 at a power 20 Watts lower. This indicates a usable dynamic range for intensity monitoring purposes. Further, utilizing DFA a1 transitions as a means to define low level, high volume training is also supported by the relative stability over longer time frames. During the simulated training intervals, DFA a1 remained stable over a 10 minute continuous segment, despite the subject already having spent 45 minutes at or below VT1 power, without a trend toward loss of complexity over time. At power levels 10 Watts below VT1, there were never any values below .8 but at a power 25 Watts above VT1, all values were uncorrelated (white noise). During a work rate several Watts above VT1, DFA a1 varied from 0.57 to near 0.8, with an average similar to that during the 5 minute control and Atenolol ramp trials. Based on this case report study, using a cycling power that avoids DFA a1 complexity loss seems to be a reasonable training prescription guideline for low intensity sessions.
If this metric is to be pursued further, a comment on the importance of avoiding ECG artifact is appropriate. In many HRV studies, an artifact rate below 5% is considered acceptable [33,34]. However, there is evidence showing that nonlinear indexes of HRV are affected by even low levels of artifact [35]. This is not the case with other indexes of HRV where artifact correction methods may preserve underlying data [36,37]. Even a low level of artifact correction tends to raise the degree of interbeat complexity, creating a misleading result. The artifact percent at each ramp stage was clearly stated to highlight the quality of the ECG measuring, and resultant validity of nonlinear parameters.
In summary, DFA a1 correlation properties were reduced with increasing exercise effort as shown in prior studies [11-14]. Both cycling power and ventilation correlated with DFA a1 decline in a similar fashion with or without the use of the beta blocker. Atenolol shifted the heart rate to power relationship but did not disturb the association of interbeat complexity with workload. Given that both the cardiac output and VO2 should have been similar between control and Atenolol conditions [21], it can be inferred that the DFA a1 index is related more to the subject's overall organismic demands rather than absolute heart rate elevation. The transition to an uncorrelated DFA a1 state occurred near the measured first ventilatory and lactate thresholds. This association with an early physiologic breakpoint seems to be of interest for training zone manipulation and fitness status, and further research should more deeply explore these relationships. Along this line, if these results were to be replicated on broader panels, it could be suggested that with current endurance exercise recommendations (centering on spending large amounts of time in a low-intensity state), utilization of a low cost, readily available metric that can be obtained without maximal effort would be particularly attractive.
Author Contributions Statement
BR was responsible for the entirety of this report, as well as being the test subject. The raw data supporting the conclusions of this manuscript will be made available by the author, without undue reservation, to any qualified researcher.
Acknowledgments
The author would like to thank Dr. Thomas Gronwald for both the pioneering research on the usage of DFA a1 during dynamic exercise as well for the encouragement in writing this article.
References
- Esteve-Lanao J, Foster C, Seiler S, Lucia A (2007) Impact of training intensity distribution on performance in endurance athletes. J Strength Cond Res 21: 943-949.
- Garber CE, Blissmer B, Deschenes MR, Franklin BA, Lamonte MJ, et al. (2011) American College of Sports Medicine position stand. Quantity and quality of exercise for developing and maintaining cardiorespiratory, musculoskeletal, and neuromotor fitness in apparently healthy adults: guidance for prescribing exercise. Med Sci Sports Exerc 43: 1334-1359.
- Hofmann P, Tschakert G (2017) Intensity and duration-based options to regulate endurance training. Front Physiol 8: 337.
- Stoggl TL, Sperlich B (2019) Training intensity, volume and recovery distribution among Elite and recreational endurance athletes. Front Physiol 10: 592.
- casado A, Hanley B, Santos-Concejero J, Ruiz-Perez LM (2019) world-class long-distance running performances are best predicted by volume of easy runs and deliberate practice of short-interval and tempo runs. J Strength Cond Res.
- Pallares JG, Moran-Navarro R, Ortega Jf, Fernandez-Elias Ve, Mora-Rodriguez R (2016) Validity and reliability of ventilatory and blood lactate thresholds in well-trained cyclists. Plos one 11: e0163389.
- Mann T, Lamberts RP, Lambert MI (2013) Methods of prescribing relative exercise intensity: physiological and practical considerations. Sports Med 43: 613-625.
- Bellinger P, Arnold B, Minahan C (2019) Quantifying the Training-Intensity Distribution in Middle-Distance Runners: The Influence of Different Methods of Training-Intensity Quantification. Int J Sports Physiol Perform. 12: 1-20.
- Hansen D, Bonn K, Alders T, Hermans A, Copermans K (2019) Exercise training intensity determination in cardiovascular rehabilitation: Should the guidelines be reconsidered? Eur J Prev Cardiol 26: 1921-1928.
- Iannetta D, Inglis EC, Mattu AT, Fontana FY, Pogliaghi S, et al. (2019) A Critical Evaluation of Current Methods for Exercise Prescription in Women and Men. Med Sci Sports Exerc.
- Goldberger AL, Amaral LA, Hausdorff JM, Ivanov PCh, Peng CK, et al. (2002) Fractal dynamics in physiology: alterations with disease and aging. Proc Natl Acad Sci U S A. 99: 2466-2472.
- Casties JF, Mottet D, Le Gallais D (2006) Non-linear analyses of heart rate variability during heavy exercise and recovery in cyclists. Int J Sports Med 27: 780-785.
- Gronwald T, Ludyga S, Hoos O, Hottenrott K (2018) Non-linear dynamics of cardiac autonomic activity during cycling exercise with varied cadence. Hum Mov Sci 60: 225-233.
- Gronwald T, Hoos O, Hottenrott K (2019) Effects of Acute Normobaric Hypoxia on Non-linear Dynamics of Cardiac Autonomic Activity During Constant Workload Cycling Exercise. Front Physiol 10: 999.
- Blake OM, Wakeling JM (2015) Muscle coordination limits efficiency and power output of human limb movement under a wide range of mechanical demands. J Neurophysiol 114: 3283-3295.
- Gronwald T, Hoos O, Hottenrott K (2019) Effects of a Short-Term Cycling Interval Session and Active Recovery on Non-Linear Dynamics of Cardiac Autonomic Activity in Endurance Trained Cyclists. J Clin Med 8:2.
- Hopker JG, Jobson SA, Pandit JJ (2011) Controversies in the physiological basis of the 'anaerobic threshold' and their implications for clinical cardiopulmonary exercise testing. Anaesthesia 66: 111-123.
- Plowman and Smith (2017) Exercise Physiology for Health, Fitness, and Performance. Baltimore, Md. Lippincott Williams & Wilkins 74-76.
- Gastinger S, Sorel A, Nicolas G, Gratas-Delamarche A, Prioux J (2010) A comparison between ventilation and heart rate as indicator of oxygen uptake during different intensities of exercise. J Sports Sci Med 9: 110-118.
- Blasco-Lafarga C, Camarena B, Mateo-March M (2017) Cardiovascular and Autonomic Responses to a Maximal Exercise Test in Elite Youngsters. Int J Sports Med 38: 666-674.
- Gronwald T, Hoos O, Ludyga S, Hottenrott K (2019) Non-linear dynamics of heart rate variability during incremental cycling exercise. Res Sports Med 27: 88-98.
- Wonisch M, Hofmann P, Fruhwald FM, Hoedl R, Schwaberger G (2002) Effect of beta(1)-selective adrenergic blockade on maximal blood lactate steady state in healthy men. Eur J Appl Physiol 87: 66-71.
- Chou TH, Akins JD, Crawford CK, Allen JR, Coyle EF (2019) Low Stroke Volume during Exercise with Hot Skin Is Due to Elevated Heart Rate. Med Sci Sports Exerc 51: 2025-2032.
- Elliot CA, Hamlin MJ, Lizamore CA (2019) Validity and Reliability of the Hexoskin Wearable Biometric Vest During Maximal Aerobic Power Testing in Elite Cyclists. J Strength Cond Res 33: 1437-1444.
- Newell J, Higgins D, Madden N, Cruickshank J, Einbeck J, et al. (2007) Software for calculating blood lactate endurance markers. J Sports Sci 25: 1403-1409.
- Farzam P, Starkweather Z, Franceschini MA (2018) Validation of a novel wearable, wireless technology to estimate oxygen levels and lactate threshold power in the exercising muscle. Physiol Rep 6: e13664.
- Grassi B, Quaresima V (2016) Near-infrared spectroscopy and skeletal muscle oxidative function in vivo in health and disease: a review from an exercise physiology perspective. J Biomed Opt 21: 091313.
- Iannetta D, Inglis EC, Fullerton C, Passfield L, Murias JM (2018) Metabolic and performance-related consequences of exercising at and slightly above MLSS. Scand J Med Sci Sports 28: 2481-2493.
- Noakes TD, St Clair Gibson A, Lambert EV (2005) From catastrophe to complexity: a novel model of integrative central neural regulation of effort and fatigue during exercise in humans: summary and conclusions. Br J Sports Med 39: 120-124.
- Faude O, Kindermann W, Meyer T (2009) Lactate threshold concepts: how valid are they?. Sports Med 39: 469-490.
- Gaskill SE, Ruby BC, Walker AJ, Sanchez OA, Serfass RC, et al. (2001) Validity and reliability of combining three methods to determine ventilatory threshold. Med Sci Sports Exerc 33: 1841-1848.
- Gronwald T, Hoos O (2020) Correlation properties of heart rate variability during endurance exercise: A systematic review. Ann Noninvasive Electrocardiol 25: e12697.
- Ekkekakis P, Lind E, Hall EE, Petruzzello SJ (2008) Do regression-based computer algorithms for determining the ventilatory threshold agree? J Sports Sci 26: 967-976.
- Quintana DS, Alvares GA, Heathers JA (2016) Guidelines for Reporting Articles on Psychiatry and Heart rate variability (GRAPH): recommendations to advance research communication. Transl Psychiatry 6: e803.
- Raleigh C, Donne B, Fleming N (2018) Association between different Non-Invasively Derived Thresholds with Lactate Threshold during graded incremental exercise. Int J Exerc Sci 11: 391-403
- Giles DA, Draper N (2018) Heart Rate Variability during Exercise: A Comparison of Artefact Correction Methods. J Strength Cond Res 32: 726-735.
- Rincon Soler AI, Silva LEV, Fazan R Jr, Murta LO Jr (2018) The impact of artifact correction methods of RR series on heart rate variability parameters. J Appl Physiol (1985) 124: 646-652.
 Spanish
Spanish  Chinese
Chinese  Russian
Russian  German
German  French
French  Japanese
Japanese  Portuguese
Portuguese  Hindi
Hindi