Case Report, Clin Oncol Case Rep Vol: 6 Issue: 9
Acute interstitial nephritis after treatment with pembrolizumab in a patient with triple negative breast cancer: a case report
Youssef Elhaitmy1,2*, Soukaina El Anssari1,2, Lamiae Amaadour1 , Karima Oualla1 , Zineb Benbrahim1 , Samia Arifi1 , Nawfel Mellas1
1Department of Medical Oncology, University Hospital Center Hassan II, City of Fez, Morocco.
2Department of Medical Oncology, North Hospital, University Hospital of SaintEtienne, Saint-Etienne, France.
*Corresponding Author:Youssef Elhaitmy ,
Department of Medical Oncology University Hospital Center Hassan II, City of Fez, Morocco.
E-mail: elhaitmy@gmail.com
Received: September 05, 2023; Manuscript No: COCR-23-113032;
Editor Assigned: September 07, 2023; PreQC Id: COCR-23-113032 (PQ);
Reviewed: September 16, 2023; QC No: COCR-23-113032 (Q);
Revised: September 18, 2023; Manuscript No: COCR-23-113032 (R);
Published: September 21, 2023; DOI: 10.4172/cocr.6(9).309
Citation: Elhaitmy Y, Anssari SE, Amaadour L, Oualla K, Benbrahim Z, et al. (2023) Acute Interstitial Nephritis After Treatment with Pembrolizumab in a Patient with Triple Negative Breast Cancer: A Case Report. Clin Oncol Case Rep 6:9
Abstract
Pembrolizumab is a humanized monoclonal antibody targeting the anti-Programmed Cell Death 1 (PD-1) receptor expressed on the surface of cytotoxic T lymphocytes. Currently the introduction of immunotherapy in a neoadjuvant setting has improved the management of these types of tumors with the arrival of the keynote 522 study. We present here the case of a patient with breast cancer treated with Pembrolizumab and chemotherapy in the setting of neoadjuvant treatment complicated with nephritis acute interstitial related to anti-PD-1. Permanent discontinuation of Pembrolizumab, treatment with corticosteroids intravenous and the use of dialysis have gradually normalized the function patient's kidney. Nephritis is one of the rare side effects that can occur when the use of immunotherapy. Through this case report we will discuss the clinicbiological and histopathological characteristic of Pembrolizumab related nephropathy
Keywords: Pembrolizumab,Triple negative, Acute interstitial nephritis
Introduction
Triple-Negative Breast Cancer (TNBC), as defined by the absence of estrogen and progesterone receptor expression, as well as the lack of HER-2 over expression/amplification, corresponds to 15% of breast cancer and represents an aggressive form of the disease. TNBC are frequently confounded with basal subtype in the molecular classification of breast cancer and also share some similarities with BRCA1-mutated tumors. Epidemiological and clinical characteristics are distinct from other subtypes, including a younger age at diagnosis, a higher risk of relapse in spite of increased chemo sensitivity, and a higher incidence of lung and brain metastatic relapses [1-4]. Conventional cytotoxics remain the mainstay of current systemic management but recent evaluation of more targeted therapeutics, including specific cytotoxics (such as the use of platinum salts), poly (ADP-ribose) polymerase (PARP) and Epithelial Growth Factor Receptor (EGFR) inhibition, and antiangiogenics have been performed, providing contrasted but rather disappointing results. Herein, we present the case of caucasian women treated with immunotherapy and chemotherapy in the setting of neoadjuvant treatment complicated with acute interstitial nephritis related to immunotherapy. We aimed to describe the therapeutic management in front of nephropathy related to Pembrolizumab.
Case Presentation
We present the case of a 40-years-old patient admitted for management of mammary carcinoma of the left breast triple negative unmutated. Breast Magnetic Resonance Imaging (MRI) showed a 34 mm nodule without adenopathy. Positron Emission Tomography (PET) has found an intense hyper metabolism with a plurifocal lesion in the left breast, and at the level of the axillary lymph nodes. After discussion of the file in the multidisciplinary team meetings the patient started a neoadjuvant treatment combining Pembrolizumab /Carboplatin/Paclitaxel and Pembrolizumab/Adriamicyn/ Cyclophosphamide. After 03 cycles of Pembrolizumab/Carboplatin/ Paclitaxel, the patient presented an acute renal failure associated with proteinuria at 2g/24h and bilateral nephromegaly (Figure1). The creatinine level was increased to 465 Umol/l with a clearance reaching 08 ml/min (clearance CKD EPI = 08ml/ min). Renal biopsy puncture were performed and concluded of no acute tubular necrosis and confirmed the diagnosis of acute interstitial nephritis (figure 2). The patient benefited from several sessions of dialysis with administration of corticosteroids (1mg/kg). A comparative PET scan showed an excellent metabolic response for left breast lesions .She therefore benefited from a left partial mastectomy with lymph nodes dissection, the anatomopathological results were in favor of a presence of residual carcinomatous patches at the level of the resection. A revision surgery was done. Biologically, there was an improvement in renal function with an increase in clearance reaching 77ml/min .The collegial decision was in favor of a continuation of chemotherapy based on Adriamycin and Cyclophosphamide as adjuvant treatment without immunotherapy.
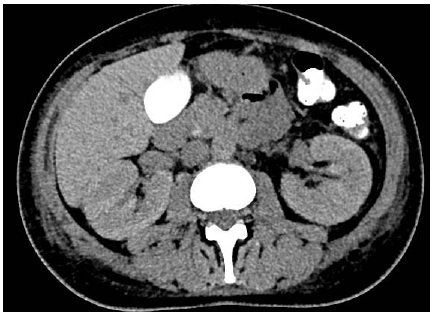
Figure 1: Computed Tomography (CT) showing bilateral nephromegaly (15 cm on the right et 14 cm on the left) in a patient with acute interstitial nephritis.
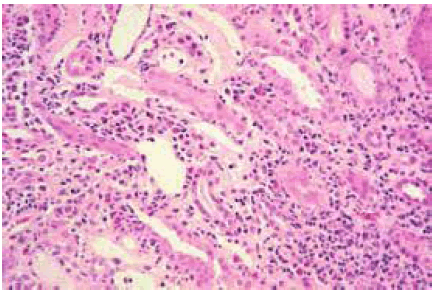
Figure 2: Histopathological image of acute interstitial nephritis.
Discussion
Pembrolizumab is a humanized monoclonal antibody targeting the PD-1 receptor expressed on the surface of cytotoxic T lymphocytes. With other specific antibodies, it is part of the Immune Checkpoint Inhibitors (ICI). Immunotherapy-related nephritis are immuno-allergic events graded according to the severity of the impairment of renal function. A dosage of serum creatinine, greater than 6 times the norm, as is the case in our patient, allows us to conclude that the severity is grade 4 according to the scale of severity of side effects linked to immunotherapy created by the American Society for Clinical Oncology (ASCO) and taken over by the Belgian (BSMO) and European (ESMO) societies [5,6]. The mechanisms of this immuno-allergic reaction have not yet been fully described. It is possible that in patients treated with drugs described as responsible for acute interstitial nephritis, treatment with immune checkpoint inhibitors raises the immune tolerance that normally protects the kidneys from these drugs. Although the first studies concerning immunotherapy in cancer only describe rare cases of kidney damage linked to immunotherapy, more recent research seems to show the opposite. These new data demonstrate that the most frequently retained post-biopsy histological diagnoses are those of acute interstitial nephritis and acute tubular necrosis, with more rarely cases of glomerulonephritis with minimal lesions [7,8]. Given the severity of the renal function impairment, our approach was to treat the patient directly with intravenous corticosteroids. According to the opinion of the nephrologist contacted by our care, a renal biopsy would have been indicated to allow the diagnosis of certainty if the patient presented serious symptoms related to his acute renal failure. According to the American guidelines for the management of side effects linked to immunotherapy, such impairment of renal function requires treatment with intravenous corticosteroids in a center where dialysis of the patient is possible. Such severe damage unfortunately contraindicates the reintroduction of immunotherapy in the treatment of the patient. A less effective second-line treatment with chemotherapy will be offered to the patient when the disease progresses because studies show that a response to immunotherapy can persist after stopping it [9,10]. In our case, the first oncological evaluation by imaging after stopping pembrolizumab did not show any pejorative evolution of the cancerous lesions, on the contrary it was a response. Attempts to reintroduce immunotherapy after renal side effects have been published and show an even more severe recurrence of renal damage sometimes leading to the death of the patient.
Conclusion
Early detection and rapid management of immunotherapy-related toxicities, with a multidisciplinary approach, offers greater chances of recovery of initial function for the patient and thus the possibility of continuing treatment later. Depending on the grade of severity of the side effects, immunotherapy must sometimes be temporarily suspended or even completely stopped. The increasing use of immunotherapy, particularly in combination with chemotherapy or even radiotherapy, will probably bring out new side effects. Significant vigilance on the part of health professionals is therefore necessary.
Conflicts of Interest
The authors declare no conflicts of interest regarding the publication of this paper.
References
- Cortazar P, Zhang L, Untch M, Mehta K, Costantino JP, et al. (2014) Pathological complete response and long-term clinical benefit in breast cancer: The CTNeoBC pooled analysis. Lancet 384: 164-172. [Google Scholar] [Cross Ref]
- Huang M, Qi CZ, Ramsey S, Briggs A, Zhao J, et al. (2019) Evaluation of pathological complete response as a trial-level surrogate for long-term survival outcomes among triple-negative breast cancer patients receiving neoadjuvant therapy. Ann Oncol 30: 34-35. [Google Scholar] [Cross Ref]
- Sikov WM, Polley MY, Twohy E, Perou CM, Singh B, et al. (2019) CALGB (Alliance) 40603: Long-term outcomes (LTOs) after neoadjuvant chemotherapy (NACT)+/-carboplatin (Cb) and bevacizumab (Bev) in triple-negative breast cancer (TNBC). J Clin Oncol 37: 591-591. [Google Scholar] [Cross Ref]
- Spring LM, Fell G, Arfe A, Sharma C, Greenup R, et al. (2020) Pathologic complete response after neoadjuvant chemotherapy and impact on breast cancer recurrence and survival: A comprehensive meta-analysis. Clin Cancer Res 26: 2838-2848. [Google Scholar] [Cross Ref]
- Brahmer JR, Lacchetti C, Schneider BJ, Atkins MB, Brassil KJ, et al. (2018) Management of immune-related adverse events in patients treated with immune checkpoint inhibitor therapy: American society of clinical oncology clinical practice guideline. J Clin Oncol 36: 1714-1768. [Google Scholar] [Cross Ref]
- Haanen JB, Carbonnel F, Robert C, Kerr KM, Peters S, et al. (2017) Management of toxicities from immunotherapy: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol 28: iv119-iv142. [Google Scholar] [Cross Ref]
- Wanchoo R, Karam S, Uppal NN, Barta VS, Deray G, et al. (2017) Adverse renal effects of immune checkpoint inhibitors: A narrative review. Am J Nephrol 45: 160-169. [Google Scholar] [Cross Ref]
- Izzedine H, Mathian A, Champiat S, Picard C, Mateus C, et al. (2019) Renal toxicities associated with pembrolizumab. Clin Kidney J 12: 81-88. [Google Scholar] [Cross Ref]
- Marron TU, Ryan AE, Reddy SM, Kaczanowska S, Younis RH, et al. (2021) Considerations for treatment duration in responders to immune checkpoint inhibitors. J Immunoth Cancer 9: e001901. [Google Scholar] [Cross Ref]
- Borcoman E, Nandikolla A, Long G, Goel S, Le Tourneau C (2018) Patterns of response and progression to immunotherapy. Am Soc Clin Oncol Edu Book 38: 169-178. [Google Scholar] [Cross Ref]
 Spanish
Spanish  Chinese
Chinese  Russian
Russian  German
German  French
French  Japanese
Japanese  Portuguese
Portuguese  Hindi
Hindi 