Review Article, Vector Biol J Vol: 4 Issue: 1
Adaptation of Anopheles coluzzii Larvae to Polluted Breeding Sites in Cotonou: A Strengthening in Urban Malaria Transmission in Benin
Razaki A Ossè1,2*, Sahabi Bio Bangana2,3, Rock Aïkpon2,4, Jeniffer Kintonou2, Hermann Sagbohan2,5, Aboubakar Sidick2, Wilfrid Sèwadé2,5, Casimir Kpanou2,5, Sidoine Nounagnon2, André Sominahouin2,6, Nwangwu Udoka7 and Martin Akogbéto2
1Ecole de Gestion et d’Exploitation des Systèmes d’Elevage, Université Nationale d’Agriculture, BP 43 Kétou, Bénin
2Centre de Recherche Entomologique de Cotonou (CREC), 06 BP 2604 Cotonou, Bénin
3Ecole d’Aquaculture de la Vallée, Université Nationale d’Agriculture, Bénin
4Ecole Normale Supérieure de Natitingou, Université Nationale des Sciences, Technologies, Ingénierie et Mathématiques, Bénin
5Faculté des Sciences et Techniques, Université d’Abomey Calavi, Bénin
6Département de Géographie et Aménagement du Territoire, Université d’Abomey-Calavi, Benin
7National Arbovirus and Vectors Research Centre, Enugu, Nigeria
*Corresponding Author : Razaki A Ossè
Ecole de Gestion et d’Exploitation des Systèmes d’Elevage, Université Nationale d’Agriculture, BP 43 Kétou, Benin
E-mail: ossraz@yahoo.fr
Received: April 02, 2019 Accepted: April 22, 2019 Published: May 22, 2019
Citation: Ossè RA, Bangana SB, Aïkpon R, Kintonou J, Sagbohan H, et al. (2019) Adaptation of Anopheles coluzzii Larvae to Polluted Breeding Sites in Cotonou: A Strengthening in Urban Malaria Transmission in Benin. Vector Biol J 4:1. doi: 10.4172/2473-4810.1000134
Abstract
Objectives: Urban malaria is on the rise in Cotonou due to rapid urbanization and development activities. However, malaria transmission depends on several factors. Hydrology is a key factor that influences the presence of suitable habitats for the development of Anopheles larvae, thereby affecting survival of the malaria vector. For a long time, clean water bodies were thought to be the favorite Anopheles gambiae s.l. breeding sites. Nowadays, Anopheles gambiae s.l. is found in polluted waters, which used to be only favorable for the development of Culex quinquefaciatus. The current study was designed to assess both the water quality of Anopheles breeding sites and the ability of Anopheles larvae to develop in polluted water bodies. Methods: Larvae were collected in four districts in the city of Cotonou. All open water bodies were considered as potential breeding sites and investigated. Most of the breeding sites were shallow, exposed to sunlight and contained different qualities of water. Four breeding sites containing Anopheles larvae were identified and sampled. Two water samples were taken from each breeding site, in 1.5 L and 10 L containers for the measurement of physicochemical parameters and the monitoring of larval development, respectively. Results: Fourteen physicochemical parameters were measured, and all four breeding sites sampled had a very high level of pollution with high values of temperature, color, chemical oxygen demand, biological oxygen demand, turbidity, total phosphors and suspended particles. Laboratory breeding of A. coluzzii larvae collected from field using polluted water samples, has yielded a high pupae rate (90%) which was significantly different from that observed with a laboratory strain of A. gambiae Kisumu (78,67%). Conclusion: These results indicate that the adaptation of A. coluzzii to polluted waters in Cotonou is quite alarming especially for urban malaria control.
Keywords: A. coluzzii; Polluted water; Physicochemical parameters; Urban malaria; Cotonou
Introduction
Malaria has been considered a predominantly rural disease in Africa, mainly because it is adapted to breeding sites for Anopheles mosquitoes, which are few in densely populated urban areas [1]. Malaria transmission is dependent on a number of environmental and hydrological factors that affect vector survival, including the presence of suitable habitats for the development of Anopheles larvae [2]. In urban centers, water pollution is considered to be a major factor that generally reduces the growth of Anopheles larvae. The intensity of malaria transmission is on average eight times higher in rural areas than in urban areas [3]. Aquatic habitats are an important component of the process that leads to malaria transmission. Part of the life cycle of mosquitoes including egg-laying, larval development, pupation and emergence occur in aquatic habitats. These habitats are essential for defining the types of malaria vectors present in an area, their abundance and the dynamics of emerging adult mosquitoes [4]. Immature stages of malaria vectors prefer different types of habitat that differ in their biological and physicochemical characteristics [5-7]. Anopheles gambiae s.s., a major vector of malaria in Africa, is known to breed in small, relatively clean, clear, sunny, temporary water bodies, and most of the time without overhanging vegetation [8]. Other studies have also reported the development of A. gambiae s.s. larvae in large permanent and polluted water bodies [9,10]. However, the clean or polluted characterization of an aquatic habitat is mainly based on visual examination and is not well defined.
Assessment of water quality of Anopheles breeding sites may provide insight into the reproductive demand of Anopheles larvae, particularly in urban areas. The city of Cotonou, like many cities in sub-Saharan Africa, is subject to rapid and uncontrolled urbanization that has had a great impact on natural ecosystems. This is believed to have favored the adaptation of Anopheles to various xenobiotics and the expansion of their niche to polluted habitats [2,11] and cultivated agricultural sites [12]. In addition, the amount of pollutants released by domestic or industrial activities into the natural environment has increased in recent decades [13]. Most of these compounds accumulate in rivers or stagnant water bodies. Despite the high toxicity of some of these compounds, their impact on aquatic fauna and the natural ecosystem is poorly understood. There is indeed a fear that the exposure of mosquito larvae to these substances could reduce their level of susceptibility to insecticides used for vector control. Similarly, poverty, deterioration of infrastructure and overcrowding are some other factors that contribute to the development of conditions that modify Anopheles breeding sites. Mismanagement of urban environments and industrialization in Cotonou has become a serious development issue and has increased the scale of urban malaria. Malaria programs in most parts of the country focus on rural communities. As a result, the bio-ecology of Anopheles in breeding sites in urban areas has received very little attention. Since the availability of suitable habitats for egg-laying favors Anopheles and possibly malaria transmission, the characterization of the Anopheles breeding sites is of a major importance for the control of malaria. It is in this perspective that the present work was designed to study the adaptation of Anopheles coluzzii larvae to polluted water bodies in the city of Cotonou in order to determine adequate methods to control their development.
Materials and Methods
Study zone
The study was conducted from July to September 2016 in Cotonou. The city of Cotonou is situated on the low, sandy littoral plain and is located at the intersection of parallels 6°20 and 6°24 north latitude, and meridians 2°20 and 2°29 east longitudes. It is located at the southern end of Benin, bordering the Atlantic Ocean. It is bounded on the west by the district of Abomey-Calavi, on the east by the district of Sèmè- Kpodji, on the north by Lake Nokoué and on the south by the Atlantic Ocean. Nearly 33% of the Cotonou area consists of wetlands and 67% of urbanized areas [14]. The city of Cotonou has a surface area of 79 km2 and is divided into thirteen districts (Figure 1). The relief of Cotonou is relatively flat with an altitude that oscillates between 0.3 m and 6 m. The climate is hot and humid with two rainy seasons and two dry seasons including: a big rainy season from April to July, a small dry season from August to September, a small rainy season from October to November and a long dry season from December to March. The average annual rainfall is about 1320 mm. The relief, the climatic, edaphic and hydrological conditions, the size of the population, and human factors promote the proliferation and the pollution of mosquito breeding sites in Cotonou. In the study area, the level of malaria transmission is high throughout the year [15] and most breeding sites are polluted.
Breeding sites sampling and larval prospecting
After investigation of breeding sites in the different districts of Cotonou, four (4) Anopheles breeding sites were selected in four (4) different zones including Agla, Houéyiho, Ladji and Tanto. Water samples were taken in 1.5-liter plastic bottles from each breeding site for physicochemical analyzes (Figure 2). For this purpose, the bottles were rinsed several times with the water to be taken before filling them completely without trapping air bubbles. The identity of the sampler, the date, the location and the sample number were marked on each bottle. The bottles were then placed in a box containing ice and were sent to the Laboratory of Quality Control of Water and Food (LQCWF) which is under the National Direction of Public Health of the Ministry of Health for analysis. Water samples were also taken from the different breeding sites into cans of ten liters and labeled. These were used for larval development at the CREC insectarium. Finally, larvae or nymphs of the Anopheles of these breeding sites were collected using a ladle and filters then transported in labeled bins to the CREC insectarium.
Physicochemical parameters of Anopheles breeding sites surveyed in the city of Cotonou
Physicochemical parameters were measured using the standard methods described by American Public Health Association [16]. The parameters measured were temperature, pH, redox, electrical conductivity (EC), total dissolved solids (TDS), salinity, turbidity, hardness, color, nitrogen (NTK), suspended solids (SS), total phosphorus (P-PO43-), biological oxygen demand (BOD) and chemical oxygen demand (COD). The temperature was taken in situ at each breeding site using a thermometer. pH and redox were measured using a pH-meter, conductivity, TDS and salinity using a conductivity meter, and turbidity using a turbidity meter.
Monitoring the impact of polluted breeding sites on larval development and emergence of Anopheles
In order to monitor the ability of Anopheles larvae to develop in polluted waters, 1st and 2nd instar larvae of two populations of Anopheles were placed in water collected from the breeding sites, including samples of A. gambiae Kisumu strain, which is maintained in the CREC insectarium and wild populations of A. gambiae s.l. collected from the field during sampling. A series of three bins containing 1 L of polluted water each received 50 larvae L1-2 from the field. The same operation was carried out with Kisumu larvae. At the same time, control bins containing tap water were set up with 50 L1-2 larvae from each Anopheles population. The experiment was repeated a second time under the same conditions. During the experiments, the larvae were fed with cat croquettes finely ground as a powder. For each series of bins, larval mortality and adult emergence were determined and recorded.
Molecular characterization and determination of detoxifying enzymes of Anopheles gambiae collected from the field
A sample of 100 A. gambiae s.l. that emerged from larvae collected from the field underwent PCR analysis. The PCR identification followed the protocol of Santomalazza et al. [17] and the presence of the Kdr West mutation followed the method of Martinez-Torres et al. [18]. The assay of the various enzymatic activities such as esterases, oxidase and glutathione S transferase was carried out on a sample of 60 A. gambiae s.l. from the field, using the techniques described by Hemingway et al. [19].
Data analysis
Mortality and arrival at the pupal stage proportions were calculated and the graphs were made in the Microsoft Excel. Statistical analysis was performed using the computing environment R, version 2.11.1 [20].
Results
Physicochemical parameters of breeding site waters
A total of 14 parameters were measured in four water samples collected from four A. gambiae s.l. breeding sites (Table 1). Turbidity was high in Tanto (217 NTU) and Agla (345 NTU) breeding sites and moderate in Ladji (16.76 NTU) and Houéyiho (16.51 NTU) breeding sites. The measures of the temperature, color, COD and BOD of all breeding sites were significantly elevated compared to values for fresh water out of a tap (p<0.05). In addition, the total dissolved solids (TDS) measures in waters from Ladji (464 mg/L) and Houéyiho (467 mg/L) were significantly higher than the standard value (150 mg/L) whereas those of Tanto (97.7 mg/L) and Agla (41.2 mg/L) were significantly lower than the standard value. Measures of conductivity and hardness in all four localities were significantly lower than their values for fresh water out of a tap (p<0.05). With regard to suspended solids (SS), the value recorded at Houéyiho was close to the standard value in contrast to the values recorded in other localities, which were very high. Total phosphorus in the waters of Tanto (4.79 mg/L), Ladji (5.68 mg/L) and Agla (4.89 mg/L) were not significantly different from the standard value (5 mg/L). In addition, the pH values of all localities were within the standard window. These results suggest a high level of water pollution in the breeding sites of the various localities surveyed.
| Parameters | Units | Localities | Fresh water out of a tap | |||
|---|---|---|---|---|---|---|
| Tanto | Ladji | Agla | Houéyiho | |||
| Temperature | °C | 30 | 31 | 29 | 32 | 25 |
| pH | 8.04 | 7.88 | 8.77 | 7.99 | 6.5<pH<8.5 | |
| Redox | Mv | -56.7 | -54.2 | -103.7 | - 60.2 | <40 |
| Color | PtCo | 65.7 | 36.6 | 39.3 | 97.9 | 15 |
| Conductivity | US/cm | 232 | 1104 | 98.1 | 1111 | 2000 |
| TDS | mg/L | 97.7 | 464 | 41.2 | 467 | 150 |
| Hardness | mg/L of CaCO3 | 7 | 8.2 | 5.4 | 7.3 | 500 |
| Turbidity | NTU | 217 | 16.76 | 345 | 16.51 | 5 |
| Salinity | ‰ | 1.1 | 0.3 | 0.3 | 0.3 | - |
| SS | mg/L | 303.17 | 183.46 | 466.15 | 18.48 | <25 |
| NTK | mg/L | 0.38 | 18.54 | 17.4 | 6.68 | 1-3 |
| P-PO43- | mg/L | 4.79 | 5.68 | 4.89 | 7.1 | 5 |
| COD | mg/L of O2 | 314 | 146 | 316 | 100 | 30 |
| BOD | mg/L of O2 | 73 | 51 | 65 | 26 | 8 |
Table 1: Physicochemical parameters of breeding site waters.
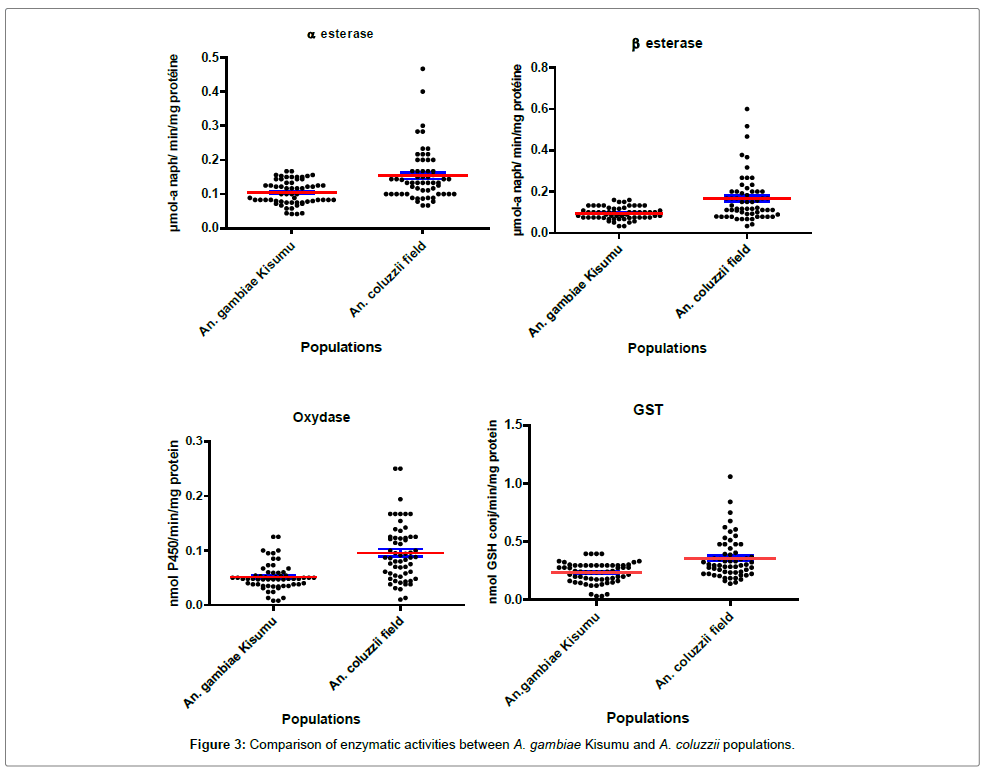
Molecular and biochemical characteristics of Anopheles gambiae collected from the field the results of the PCR showed that 100% of A. gambiae s.l. that emerged from larvae collected from the field were A.coluzzii. The Kdr West mutation was recorded in this population of A. coluzzii with a very high allelic frequency of 97.46%. Biochemical tests showed significantly elevated enzymatic activities of esterase, oxidase and GST in A. coluzzii compared to the Kisumu reference strain (p<0.001) (Figure 3).
Influence of polluted breeding sites on larval development
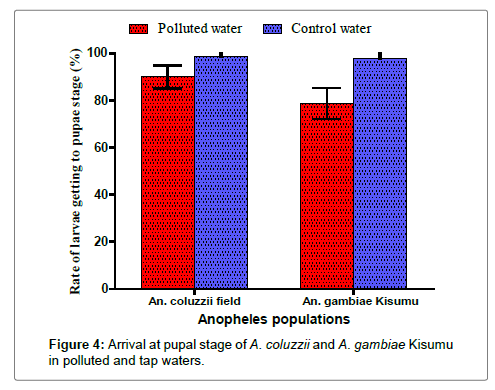
The analysis of the arrival at pupal stage of A. gambiae Kisumu and A. coluzzii samples showed that 90% of A. coluzzii pupated in the bins containing polluted breeding site water compared to 78.67% of A. gambiae Kisumu. These two proportions were significantly different (p=0.011) (Figure 4). In the control bins (tap water), there was no significant difference between the two species. The proportions of A. coluzzii and A. gambiae Kisumu that pupated were respectively 98.67% and 98%. In addition, arrival at pupal stage of A. coluzzii in tap water was significantly higher than in polluted water (p=0.003). Similarly, arrival at pupal stage of A. gambiae Kisumu in tap water was significantly higher than in polluted water (p<0.0001).
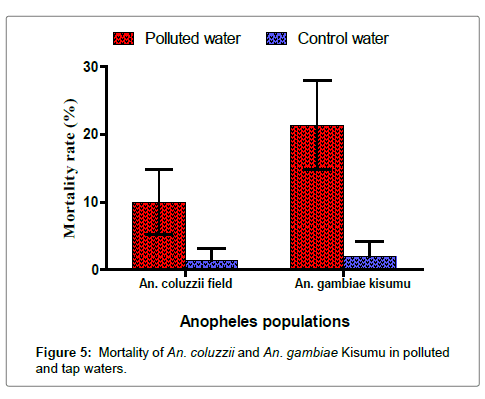
In addition, mortality of A. gambiae Kisumu and A. coluzzii during larval development in polluted water were respectively 21.33% and 10% (Figure 5). There was a significant difference between these two proportions (p=0.011). In addition, a significant difference was observed between the mortality of A. coluzzii in polluted versus tap water (p=0.003). However, in tap water, no significant difference (p=1) was recorded between A. coluzzii (1.33%) and A. gambiae Kisumu (2%).
Discussion
The ecology of mosquito breeding sites is an important factor that influences the transmission of malaria. Understanding the ecology of breeding sites is therefore important in the design of malaria control programs. The results of the present study confirmed the assertion that there is a problem with pollution of Anopheles breeding sites in the city of Cotonou. Of the 14 parameters measured, only the pH and total phosphorus showed no significant differences with the standard values. The pH depends on the concentration of the anions, cations, salts and synthetic compounds that indicate its acidic or basic character [21]. Therefore, it can be a direct or indirect determinant of any aquatic life. Apart from the direct effect that is not clearly known, anions and cations may also indirectly affect mosquito breeding by promoting certain types of aquatic vegetation or organisms that feed on mosquito larvae or by affecting potential biological control agents of mosquito larvae [21]. In addition, a high level of other parameters were recorded in waters collected from the breeding sites and provides a good indication of the high level of pollution of the breeding sites at these four localities. This observation of the heavy pollution of these breeding sites indicates that the contamination of these urban waters was not principally due to industrial activities but rather to municipal activities, market gardening or the inappropriate disposal of solid household waste. This is alarming for public health and aquatic environment safety in the city of Cotonou.
The present study has also identified A. coluzzii as the only species of the A. gambiae complex encountered in the four study zones. In addition, the Kdr-West gene was found in A. coluzzii at a very high frequency (97.46%). The Kdr gene plays a very important role in the resistance of malaria vectors to pyrethroids and DDT. In addition, esterase, oxidase and GST activities were significantly elevated in this species, suggesting their involvement in metabolic resistance in A. coluzzii. Thus, the high level of resistance observed in A. coluzzii in our study area could be related not only to the bio ecology of the vector but also to the intensity of the selection pressure exerted by the use of xenobiotics and insecticides in public health and market gardening by the human population.
The larvae of A. gambiae s.l. live normally in small water bodies that are often temporary, scattered, sunny and close to homes [22]. These habitats are distinguished by physical as well as biological characteristics, which directly influence the distribution and abundance of mosquito larvae in the wild. The adaptation of A. coluzzii in the polluted aquatic habitats found in this study is quite alarming especially for the fight against urban malaria. These results confirm those of several authors who have shown the tolerance of A. gambiae s.l. larvae to polluted breeding sites [23-25].
This adaptation may result in the appearance of malaria transmission throughout the year as opposed to its normal seasonal urban transmission pattern, due to the abundance of appropriate breeding sites as a result of on-going urbanization in many neighborhoods throughout the year. Therefore, the use of Bacillus thuringiensis var israelensis (Bti) or other larvicides is recommended for larval control in these breeding sites in a cost-effective manner. In any case, it is justified to have an appropriate vector control measure by the Ministry of Health. In addition, pollution of breeding sites by domestic waste can affect the resistance of malaria vectors to insecticides. Indeed, several studies, thanks to the technique of microarrays, have been able to show that the expression of many genes predominantly Cytochrome P450 monooxygenases was induced following the exposure of larvae to xenobiotics [26-28].
The arrival at the pupal stage recorded between A. coluzzii (90%) and A. gambiae Kisumu (78.67%) suggest that adaptation of A. coluzzii to polluted waters may be influenced by their insecticide resistance status and vice versa. A study must therefore be carried out on the relationship between the physicochemical properties of mosquito breeding site waters and the resistance of malaria vectors to insecticides in urban areas. Since mosquito larval habitats have an influence on the expression of detoxification enzymes as an adaptive response in mosquitoes that in turn can influence their ability to withstand exposure to insecticides, we recommend that such a study to be carried out to determine enzymatic activity profiles in relation to the physical, chemical and biological parameters of water. In addition, other environmental factors such as bacterial composition, chlorophyll, water hyacinth can influence the impact of some water bodies on the larval development of Anopheles. Furthermore, there is a need for comparing the physicochemical properties of “potential” breeding sites in which no larvae were found, with those used in this study.
Conclusion
This study highlights the capacity of A. coluzzii populations to develop in breeding sites containing polluted water, which could have serious consequences on the epidemiology of urban malaria in Cotonou. This requires entomological surveillance in urban areas.
References
- Keating J, Macintyre K, Mbogo CM, Githure JI, Beier JC (2004) Characterisation of potential larval habitats for Anopheles mosquitoes in relation to urban land-use in Malindi, Kenya. Intl J Hlth Geograph 3:9.
- Awolola TS, Oduola AO, Obansa JB, Chukwurar NJ, Unyimadu JP (2007) Anopheles gambiae s.s. breeding in polluted water bodies in urban Lagos, south western Nigeria. J Vector Borne Dis 44: 241 -244.
- Robert V, Macintyre K, Keating J, McWilson W, Trappe JP, et al. (2003) Malaria transmission in urban subSaharan Africa. Am J Trop Med Hyg 68: 169–176.
- Ndenga B, Githeko A, Omukunda E, Munyekenye G, Atieli H, et al. (2006) Population dynamics of malaria vectors in western Kenya highlands. J Med Entomol 43: 200–206.
- Edillo FC, Tripet F, Toure YT, Lanzaro GC, Dolo G, et al. (2006) Water quality and immature of the M and S forms of Anopheles gambiae ss and An. arabiensis in a Malian village. Malaria J 5:35.
- Munga S, Minakawa N, Zhou G, Mushinzimana E, Barrack O, et al. (2006) Association between land cover and habitat productivity of malaria
vectors in western Kenyan highlands. Am J Trop Med Hyg 74: 69–75. - Ndenga B, Simbauni J, Mbugi J, Githeko A, Fillinger U (2011) Productivity of malaria vectors from different habitat types in the western Kenya highlands. PLoS One 6: 4.
- Service MW (1971) Studies on sampling larval population of Anopheles gambiae complex. Bull WHO 45:169–180.
- Sattler MA, Mtasiwa D, Kiama M, Premji Z, Tanner M, et al. (2005) Habitat characterisation and spatial distribution of Anopheles sp mosquito larvae in Dar es Salaam (Tanzania) during the extended dry season. Malar J 4: 4.
- Kamdem C, Tene Fossog B, Simard F, Etouna J, Ndo C, et al. (2012) Anthropogenic habitat disturbance and ecological divergence between incipient species of the malaria mosquito Anopheles gambiae. PLoS One 7: e39453.
- Djouaka RF, Bakare AA, Bankole HS (2007) Does the spillage of petroleum products in Anopheles breeding sites have an impact on the pyrethroid resistance? Malar J 6:159.
- Mireji PO, Keating J, Kenya E (2006) Differential induction of proteins in Anopheles gambiae sensu stricto(Diptera: Culicidae) larvae in response to heavy metal selection. Int J Trop Insect Sci 26: 214–226.
- Biney C, Amuzu AT, Calamari D (1994) Review of heavy metals in the African aquatic environment. Ecotox Environ Safe 28: 134–215.
- INSAE (2002) Cahiers des quartiers de villes de commune de Cotonou. p: 48.
- Akogbéto M, Modiano D, Bosman A (1992) Malaria transmission in the lagunar area of Cotonou (Benin). Parassitologia 34:147–154.
- American Public Health Association (1998). American Water Works & Water Environment Federation. Standard Methods for the Examination of Water and Wastewater. 20th ed. United Book Press, Inc., Baltimore, Maryland. 541p.
- Santolamazza F, Mancini E, Simard F, Qi Y, Tu Z, et al. (2008) Insertion polymorphisms of SINE200 retrotranposons within speciation island of Anopheles gambiae molecular forms. Malar J 7:163.
- Martinez-Torres D, Chandre F, Williamson MS, Darriet F, Berge JB, et al (1998) Molecular characterization of pyrethroids knockdown resistance (kdr) in the major malaria vector Anopheles gambiae s.s. Insect Mol Biol 7:179–184.
- Hemingway J, Hawkes N, Prapanthadara L, Jayawardenal KGI, Ranson H (1998) The role of gene splicing, gene amplification and regulation in mosquito insecticide resistance. Philos Trans R Soc Lond B Biol Sci 353: 1695–1699.
- R Development Core Team (2005) R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria, www.R-project.org.
- Bos R (1991) Water Quality, Disease and Human Health. Paper presented at the Water Quality, Conference, Bratislava.
- Gimnig JE, Ombok M, Kamau L, Hawley WA (2001) Characteristics of larval Anophelinae (Diptera: Culicidae) habitats in Western Kenya. J Med Entomol38: 282-288.
- Kabula BI, Attah PK, Wilson MD, Boakye D (2011) Characterization of Anopheles gambiae s.l and insecticide resistance profile relative to physicochemical properties of breeding habitats within Accra Metropolis, Ghana. Tanzania J Health Res 13:3.
- Tene Fossog B, Antonio-Nkondjio C, Kengne P, Njiokou F, Besansky NJ, et al. (2013) Physiological correlates of ecological divergence along an urbanization gradient: Differential tolerance to ammonia among molecular forms of the malaria mosquito Anopheles gambiae. BMC Ecol 13: 1.
- Kudom AA (2015) Larval ecology of Anopheles coluzzii in Cape Coast, Ghana: Water quality, nature of habitat and implication for larval control. Malar J 14:447.
- Nkya TE, Akhouayri I, Poupardin R, Batengana B, Mosha F, et al. (2014) Insecticide resistance mechanisms associated with different environments in the malaria vector Anopheles gambiae: A case study in Tanzania. Malar J 13:28.
- Poupardin R, Riaz MA, Jones CM, Chandor-Proust A, Reynaud S, et al. (2012) Do pollutants affect insecticide-driven gene selection in mosquitoes? Experimental evidence from transcriptomics. Aquatic toxicol 114: 49-57.
- Poupardin R, Riaz MA, Vontas J, David JP, Reynaud S (2010) Transcription profiling of eleven cytochrome P450s potentially involved in xenobiotic metabolism in the mosquito Aedes aegypti. Insect Mol Biol 19: 185-193
 Spanish
Spanish  Chinese
Chinese  Russian
Russian  German
German  French
French  Japanese
Japanese  Portuguese
Portuguese  Hindi
Hindi